The Balance between Energy, Environmental Security, and Technical Performance: The Regulatory Challenge of Nanofluids
Abstract
:1. Introduction
2. Analysis of Existing Regulations
- Annex I: General provisions for assessing substances and preparing chemical safety reports;
- Annex II: A guide to the compilation of safety data sheets [35];
- Annex III: Criteria for substances registered in quantities between 1 and 10 tonnes;
- Annex VI: Information requirements referred to in article 10 [36];
- Annex VII: Standard information requirements for substances manufactured or imported in quantities of one tonne or more;
- Annex VIII: Standard information requirements for substances manufactured or imported in quantities of 10 tonnes or more;
- Annex IX: Standard information requirements for substances manufactured or imported in quantities of 100 tonnes or more;
- Annex X: Standard information requirements for substances manufactured or imported in quantities of 1000 tonnes or more;
- Annex XI: General rules for adaptation of the standard testing regime set out in annexes VII to X;
- Annex XII: General provisions for downstream users to assess substances and prepare chemical safety reports.
- Names or other identifiers of the nanoforms or sets of similar nanoforms of the substance;
- Number based particle size distribution with the indication of the number fraction of constituent particles in the size range within 1 nm–100 nm;
- Description of surface functionalization or treatment and identification of each agent including IUPAC name and CAS or EC number;
- Shape, aspect ratio and other morphological characterization: crystallinity, information on assembly structure including, e.g., shell-like structures or hollow structures, if appropriate;
- Surface area (specific surface area by volume, specific surface area by mass, or both).
- Chapter R.6: QSARs and grouping of chemicals;
- Chapters R.7a; R.7b; R.7c: Endpoint specific guidance;
- Chapter R.8: Characterization of dose (concentration)—concentration for human health;
- Chapter R.10: Characterization of dose (concentration)—concentration for environment;
- Chapter R.14: Occupational exposure assessment.
3. Nanofluids and IoNanofluids
4. Nanofluid Selection Strategy
5. Heat Transfer Pilot Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murshed, S.M.S.; Nieto de Castro, C.A. (Eds.) Nanofluids: Synthesis, Properties and Applications; NOVA Science Publishers, Inc.: New York, NY, USA, 2014; ISBN 978-1-63321-677-8. Available online: https://novapublishers.com/shop/nanofluids-synthesis-properties-and-applications/ (accessed on 21 May 2021).
- Angayarkanni, S.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Lourenço, M.J.V. Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids. Energies 2020, 13, 99. [Google Scholar] [CrossRef] [Green Version]
- Sezer, N.; Atieh, M.A.; Koҫ, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431. [Google Scholar] [CrossRef]
- Ramaye, Y.; Kestens, V.; Braun, K.A.; Linsinger, T.; Held, A.; Roebben, G. The Certification of Equivalent Diameters of Silica Nanoparticles in Aqueous Solution, Certified Reference Material ERM®—FD101b, JRC 105046. Luxembourg. 2017. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC105046 (accessed on 17 March 2021).
- Colloids are classified in Sol (dispersion of a solid in a liquid or a solid in a solid), Aerosol (dispersion of a solid or a liquid in gas), Emulsion (dispersion of a liquid in a liquid) and Foams (dispersion of a gas in a liquid) and Gel (dispersion of a liquid in a solid).
- Nanotechnologies—Vocabulary—Part 1: Core Terms. ISO/TS 80004-1:2010. Available online: https://www.iso.org/standard/51240.html; revised by ISO/TS 80004-1:2015; https://www.iso.org/standard/68058.html (accessed on 26 April 2021).
- Dental Vocabulary—Part 2: Dental Materials. ISO 1942-2-1989. Available online: https://www.iso.org/standard/6654.html; revised by Dentistry—Vocabulary; ISO 1942:2020; https://www.iso.org/standard/72249.html (accessed on 26 April 2021).
- Hunter, R.J. Foundations of Colloid Science, 2nd ed.; Oxford University Press: Oxford, UK, 2001; ISBN 978-0198505020. [Google Scholar]
- Domínguez, M.S.; Abreu, C.R. (Eds.) Nanocolloids. In A Meeting Point for Scientists and Technologists; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-801578-0. [Google Scholar] [CrossRef]
- Rauscher, H.; Roebben, G.; Mech, A.; Gibson, N.; Kestens, V.; Linsinger, T.P.J.; Sintes, J.R. An Overview of Concepts and Terms Used in the European Commission’s Definition of Nanomaterial; Joint Research Centre (JRC), Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-79-99660-3. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC113469 (accessed on 27 April 2021).
- Babick, F.; Ullmann, C. Error propagation at the conversion of particle size distributions. Powder Technol. 2016, 301, 503–510. [Google Scholar] [CrossRef]
- Madavan, R.; Kumar, S.S.; Iruthyarajan, M.W. A Comparative Investigation on Effects of Nanoparticles on Characteristics of Natural Esters—Based Nanofluids. Colloids Surf. A 2018, 556, 30–36. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faizan, M.; Ahmed, R.; Ali, H.M. A critical review on thermophysical and electrochemical properties of Ionanofluids (nanoparticles dispersed in ionic liquids) and their applications. J. Taiwan Inst. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Commission Staff Working Paper SWD. Types and Uses of Nanomaterials, Including Safety Aspects; European Commission: Brussels, Belgium, 2012; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=SWD:2012:0288:FIN:EN:PDF (accessed on 23 March 2021).
- Mordor Intelligence. Nanomaterials Market—Growth, Trends, COVID-19 Impact, and Forecast (2021–2026). Retrieved from Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/nanomaterials-market (accessed on 23 March 2021).
- Transparency Market Research. Research, Nanomaterials in Cosmetic and Personal Care Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2016–2024. Retrieved from Transparency Market Research. Available online: https://www.transparencymarketresearch.com/nanomaterials-cosmetic-personal-care.html (accessed on 25 March 2021).
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. In Proceedings of the International Conference on Modern Trends in Manufacturing Technologies and Equipment (ICMTMTE 2017), MATEC Web Conference, Sevastopol, Russia, 11–15 September 2017; pp. 129–133. [Google Scholar] [CrossRef]
- Zion Market Research. Nanomaterials Market to Report Impressive Growth, Revenue to Surge to US$16.8 Billion By 2022. Retrieved from Zion Market Research. Available online: https://www.zionmarketresearch.com/news/nanomaterials-market (accessed on 23 March 2021).
- Allied Market Research. Europe Nanomaterials Market. Retrieved from Allied Market Research. Available online: https://www.alliedmarketresearch.com/europe-nanomaterials-market (accessed on 23 March 2021).
- Ansen, S.F.; Baun, A. European regulation affecting nanomaterials-Review of limitations and future. Dose Response 2012, 10, 364–383. [Google Scholar] [CrossRef] [Green Version]
- Directive 2009/126/CE, Directive 2006/21/CE, Directive 2003/40/CE, Directive 2000/55/CE, Directive 98/70/CE and Directive 92/42/CE, related with the energy sector do not have specific requirements related to the nanotechnologies.
- ECHA. REACH Regulation. Retrieved from ECHA European Chemicals Agency. Available online: https://echa.europa.eu/regulations/reach/legislation (accessed on 12 January 2021).
- ECHA. CLP Legislation. Retrieved from ECHA European Chemicals Agency. Available online: https://echa.europa.eu/regulations/clp/legislation (accessed on 12 January 2021).
- Directives like the Water Framework Directive, the Directive on the Protection of Workers from the Risks Related to Exposure to Carcinogens and Mutagens at Work and the Directive on the Safety of Toys aim to protect the environment, workers or consumers, do not have specific rules for nanomaterials. The respective requirements apply in the same way for all substance’s forms.
- European Chemicals Agency. New OECD Guidance Documents for the Risk Assessment of Nanomaterials. Retrieved from EUON—European Union Observatory for Nanomaterials. Available online: https://euon.echa.europa.eu/view-article/-/journal_content/title/new-oecd-guidance-documents-for-the-risk-assessment-of-nanomaterials (accessed on 20 February 2021).
- European Chemicals Agency. Regulation. Retrieved from EUON European Observatory for Nanomaterials. Available online: https://euon.echa.europa.eu/regulation (accessed on 20 February 2021).
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chem. Inginieur. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- EU SCIENCE HUB. How are Nanomaterials Regulated in the EU? Retrieved from EU Science Hub. Available online: https://ec.europa.eu/jrc/en/science-update/how-are-nanomaterials-regulated-eu (accessed on 22 February 2021).
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Moniz, F.B.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Pesudo, L.Q.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee. Retrieved from Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/ae5ada03-0dc3-48f8-9a32-0460e65ba7ed/language-en (accessed on 27 April 2021).
- Rauscher, H.; Roebben, G.; Sanfeliu, A.B.; Emons, H.; Gibson, N.; Koeber, R.; Linsinger, T.; Rasmussen, K.; Sintes, J.R.; Sokull-Klüttgen, B.; et al. Towards a Review of the EC Recommendation for a Definition of the Term “Nanomaterial”. Institute for Health and Consumer Protection (Joint Research Centre). EU Publications. 2014. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC95675/towards%20review%20ec%20rec%20def%20nanomaterial%20-%20part%203_report_online%20id.pdf (accessed on 26 April 2021).
- European Union. Commission Regulation (EU) 2018/1881. Official Journal L 308. European Union: Brussels, Belgian, 2018. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32018R1881 (accessed on 26 April 2021).
- Amended According to the Commission Regulation (EU) 2020/878. Revised Requirements for EU Safety Data Sheets. Available online: https://www.chemsafetypro.com/Topics/EU/EU_regulation_2020_878_new_SDS_requirements_EU.html (accessed on 13 April 2021).
- Article 10 of REACH is related with the information to be submitted for general registration purposes in the scope of REACH.
- Although the European Commission adopted a recommendation for a definition of the expression “nanomaterial”, this term is currently not defined in a unique, regulatory binding way. Its definition and implementation depend on the specific regulatory context.
- Commission Recommendation 2011/696/EU, OJ L 275, 20.10.2011. Commission Recommendation 2011/696/EU. OJ L. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:275:0038:0040:en:PDF (accessed on 17 February 2021).
- A set of similar nanoforms is also defined as a group of nanoforms characterized by the required parameters “where the clearly defined boundaries in the parameters of the individual nanoforms within the set still allow to conclude that the hazard assessment, exposure assessment and risk assessment of these nanoforms can be performed jointly.
- Rauscher, H.; Mech, A.; Gibson, N.; Gilliland, D.; Held, A.; Kestens, V.; Koeber, R.; Linsinger, T.P.J.; Stefaniak, E.A. Identification of Nanomaterials through Measurements; Publications Office of the European Union: Luxemburg, 2019; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC118158 (accessed on 18 February 2021).
- ECHA’s Guides. Available online: https://echa.europa.eu/guidance-documents/guidance-on-reach (accessed on 18 February 2021).
- European Chemicals Agency. Guidance for Identification and Naming of Substances under REACH and CLP; ECHA: Helsinki, Finland, 2017; Available online: https://echa.europa.eu/documents/10162/23036412/substance_id_en.pdf/ee696bad-49f6-4fec-b8b7-2c3706113c7d (accessed on 12 April 2021).
- European Chemicals Agency. Appendix for Nanoforms Applicable to the Guidance on Registration and Substance Identification. 2019. Available online: https://echa.europa.eu/documents/10162/13655/how_to_register_nano_en.pdf/f8c046ec-f60b-4349-492b-e915fd9e3ca0 (accessed on 2 March 2021).
- Global Harmonizing System, GPS, Guidance Documents. Available online: http://guidance.echa.europa.eu or https://euon.echa.europa.eu/safety or https://osha.europa.eu/; www.unece.org (accessed on 6 March 2021).
- Lovestam, G.; Lövestam, R.G.-K.G.; Rauscher, H.; Roebben, G.; Klüttgen, B.S.; Gibson, N.; Putaud, J.; Stamm, H. Considerations on a Definition of Nanomaterial for Regulatory Purposes. Joint Research Centre of the European Commission, 2010. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/jrc_reference_report_201007_nanomaterials.pdf (accessed on 2 March 2021).
- Scientific Committee of Emerging and Newly Identified Health Risks. Scientific Basis for the Definition of the Term “Nanomaterial”. European Commission, 2010. Available online: https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_032.pdf (accessed on 3 March 2021).
- Rauscher, H.; Roebben, G.; Mech, A.; Gibson, N.; Kestens, V.; Linsinger, T.P.J.; Sintes, J.R. An Overview of Concepts and Terms Used in the European Commission’s Definition of Nanomaterial; Publications Office of the European Union: Luxembourg, 2019; Available online: https://op.europa.eu/en/publication-detail/-/publication/62b6ebc2-2f43-11e9-8d04-01aa75ed71a1/language-en (accessed on 22 March 2021).
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Revs. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Engineered Nanoparticles: Review of Health and Environmental Safety (ENRHES), Grant Grant Agreement ID: 218433, FP7-NMP—Specific Programme “Cooperation”: Nanosciences, Nanotechnologies, Materials and New Production Technologies. Final-Report; Conclusions on p. 359–360, and EC4SafeNano: European Centre for Risk Management and Safe Innovation in Nanomaterials Nanotechnologies. 2008. Available online: http://ec4safenano.eu/ (accessed on 26 February 2021).
- Rickerby, D. Nanotechnology for Sustainable Manufacturing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482214826. Available online: https://www.routledge.com/Nanotechnology-for-Sustainable-Manufacturing/Rickerby/p/book/9781482214826 (accessed on 27 February 2021).
- Gonçalves, A.R.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kuhlbusch, T.A.J.; Schnekenburger, J.; Lehr, C.-M. Safety of Nanomaterials along Their Lifecycle: Release, Exposure, and Human Hazards; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781466567863. Available online: https://www.routledge.com/Safety-of-Nanomaterials-along-Their-Lifecycle-Release-Exposure-and-Human/Wohlleben-Kuhlbusch-Schnekenburger-Lehr/p/book/9781466567863 (accessed on 27 February 2021).
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132−7189. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Ribeiro, A.P.C.; Vieira, S.I.C.; França, J.P.M.; Lourenço, M.J.V.; Santos, F.J.V.; Murshed, S.M.S.; Goodrich, P.; Hardacre, C. Synthesis, Properties and physical applications of IoNanofluids, Chap. 2. In Ionic Liquids: New Aspects for the Future; Kadokawa, J.-I., Ed.; Intech: London, UK, 2013; pp. 165–193. Available online: http://cdn.intechopen.com/pdfs/13912/InTech-Thermal_properties_of_ionic_liquids_and_ionanofluids.pdf (accessed on 22 April 2021).
- Nieto de Castro, C.A.; Paredes, X.; Vieira, S.I.C.; Murshed, S.M.S.; Lourenço, M.J.V.; Santos, F.J.V. IoNanofluids: Innovative Agents for Sustainable Development, Chap. 37. In Nanotechnology for Energy Sustainability, Part IV; Baldev, R., Van de Voorde, M., Yashwant, M., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 3, pp. 911–936. ISBN 978-3-527-34014-9. Available online: https://www.wiley-vch.de/en?option=com_eshop&view=product&isbn=9783527340149&title=Nanotechnology%20for%20Energy%20Sustainability (accessed on 22 April 2021).
- Queirós, C.S.G.P. Lignocellulosic Biomass for a New Generation of Thermal Fluids. Ph.D. Thesis, University of Lisbon, Lisboa, Portugal, 2019. Available online: https://www.repository.utl.pt/handle/10400.5/18319 (accessed on 12 May 2021).
- Queirós, C.S.G.P.; Sofia, C.; Lourenço, A.; Ferreira, J.; Miranda, I.; José, M.; Lourenço, V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Conv. Bioref. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Sofia, C.; Lourenço, A.; Ferreira, J.; Miranda, I.; José, M.; Lourenço, V.; Pereira, H. Characterization of Hakea sericea Fruits Regarding Chemical Composition and Extract Properties. Waste Biomass Valor. 2020, 11, 4859–4870. [Google Scholar] [CrossRef]
- Bobbo, S.; Buonomo, B.; Manca, O.; Vigna, S.; Fedele, L. Analysis of the Parameters Required to Properly Define Nanofluids for Heat Transfer Applications. Fluids 2021, 6, 65. [Google Scholar] [CrossRef]
- Paredes, X.; Queirós, C.S.G.P.; Santos, F.J.V.; Santos, A.F.; Santos, M.S.C.S.; Lourenço, M.J.V.; Nieto de Castro, C.A. Thermophysical Properties of 1-Hexyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide, [C6mim][(CF3SO2)2N]—New Data, Reference Data and Reference Correlations. J. Phys. Chem. Ref. Data 2020, 49, 043101. [Google Scholar] [CrossRef]
- Nanouptake—Overcoming Barriers to Nanofluids Market Uptake (COST Action CA15119). Available online: http://www.nanouptake.eu/ (accessed on 21 May 2021).
- NANOConVEX—Nanofluids for Convective Heat Transfer Devices. Available online: https://nanoconvex.eu/ (accessed on 21 May 2021).
- Antoniadis, K.D.; Tertsinidou, G.J.; Assael, M.J.; Wakeham, W.A. Necessary Conditions for Accurate, Transient Hot-Wire Measurements of the Apparent Thermal Conductivity of Nanofluids are Seldom Satisfied. Int. J. Thermophys. 2016, 37, 1–22. [Google Scholar] [CrossRef]
- Tertsinidou, G.J.; Tsolakidou, C.M.; Pantzali, M.; Assael, M.J.; Colla, L.; Fedele, L.; Bobbo, S.; Wakeham, W.A. New Measurements of the Apparent Thermal Conductivity of Nanofluids and Investigation of Their Heat Transfer Capabilities. J. Chem. Eng. Data 2016, 62, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Bioucas, F.E.B.; Vieira, S.I.C.; Lourenço, M.J.V.; Santos, F.J.V.; Lopes, M.L.M.; Nieto de Castro, C.A.; Massonne, K. [C2mim][CH3SO3]—A Suitable New Heat Transfer Fluid? Part 1. Thermophysical and Toxicological Properties. Ind. Eng. Chem. Res. 2018, 57, 8541–8551. [Google Scholar] [CrossRef]
- Vieira, S.I.C.; Araújo, M.; André, R.; Madeira, P.; Humanes, M.; Lourenço, M.J.V.; Nieto de Castro, C.A. Sepia melanin: A new class of nanomaterial with anomalously high heat storage capacity obtained from a natural nanofluid. J. Nanofluids 2013, 2, 104–111. [Google Scholar] [CrossRef]
- Massonne, K.; Vieira, S.; Lourenço, M.J.; de Castro, C.N. Use of Melanin or Melanin Particles for Solar Thermal Energy Conversion. Patent Number EP 3 228 192 A2, 11 October 2017. Available online: https://data.epo.org/publication-server/rest/v1.0/publication-dates/20171011/patents/EP3228192NWA2/document.pdf (accessed on 21 May 2021).
- Massonne, K.; Vieira, S.; Lourenço, M.J.; Nieto de Castro, C.A. Melanin Particles for Solar Thermal Energy Conversion. In Handbook on Industrial Applications of Nanofluids in Energy Sector; Matthias, H., Buschmann, L.H.L., Lucía, B., Eds.; D11 Nanouptake COST Action; Bubok Publishing S.L: Madrid, Spain, 2020; p. 53. [Google Scholar] [CrossRef]
- Bioucas, F.E.B.; Vieira, S.I.C.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Performance of heat transfer fluids with nanographene in a pilot solar collector. Sol. Energy 2018, 172, 171–176. [Google Scholar] [CrossRef]
- Pereira, L.V.; Paredes, X.; Nieto de Castro, C.A.; Lourenço, M.J.V. Influence of Nanofluids in the Performance of a Pilot Solar Collector. In Proceedings of the 1st International Conference on Nanofluids (ICNf2019) and 2nd European Symposium on Nanofluids (ESNf2019), Castelló, Spain, 26–28 June 2019; pp. 211–215. Available online: http://repositori.uji.es/xmlui/handle/10234/183448?locale-attribute=en (accessed on 17 February 2021).
- NIST Standard Reference Database 69: NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 12 January 2021).
- Engineeringtoolbox. Propylene Glycol based Heat-Transfer Fluids. Available online: https://www.engineeringtoolbox.com/propylene-glycol-d_363.html (accessed on 15 May 2021).
- Schliesser, J.M.; Smith, S.J.; Li, G.; Li, L.; Walker, T.F.; Parry, T.; Boerio-Goates, J.; Woodfield, B.F. Heat capacity and thermodynamic functions of nano-TiO2 rutile in relation to bulk-TiO2 rutile. J. Chem. Thermodyn. 2015, 81, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Shakel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health? Arch. Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
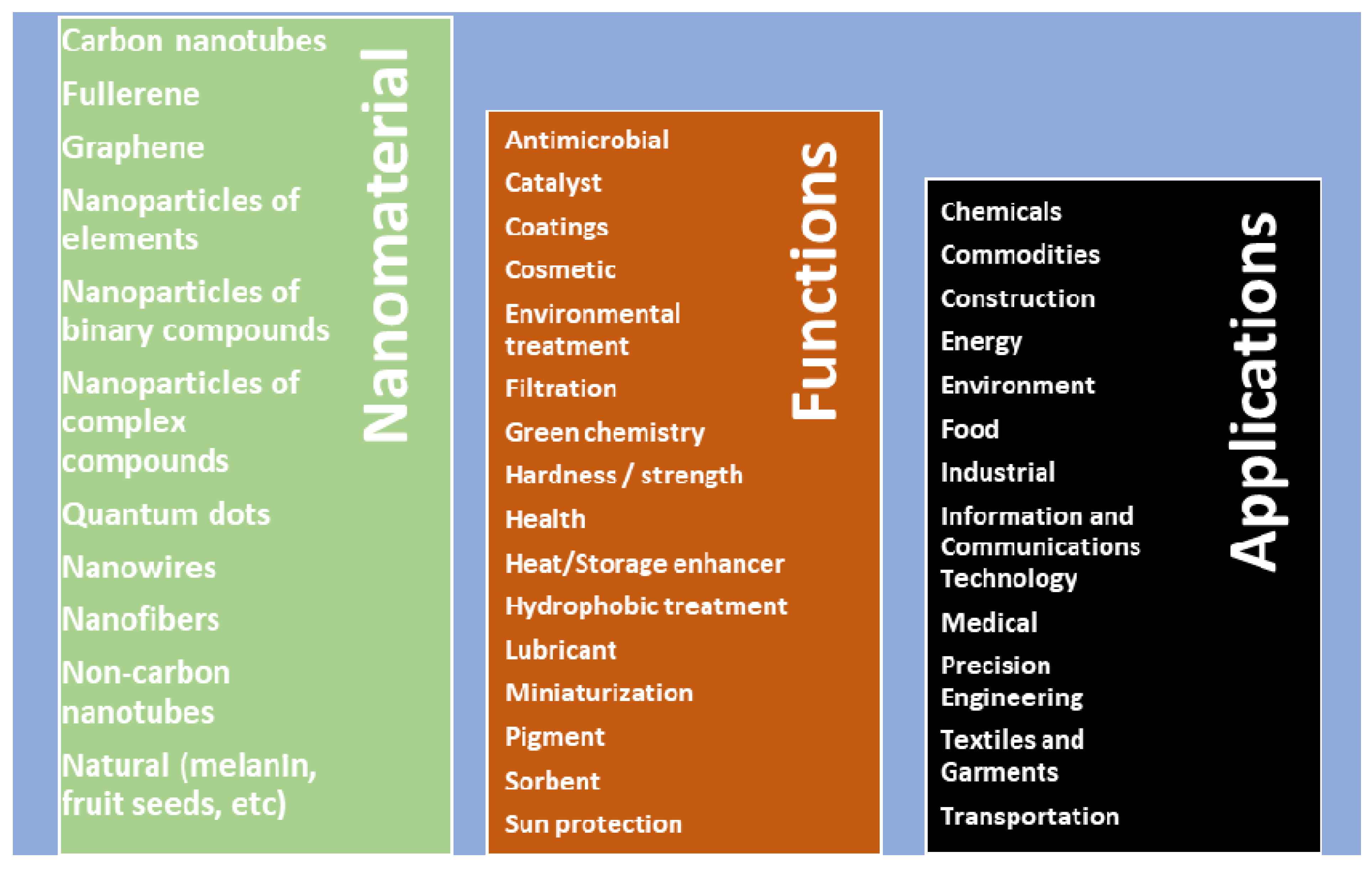
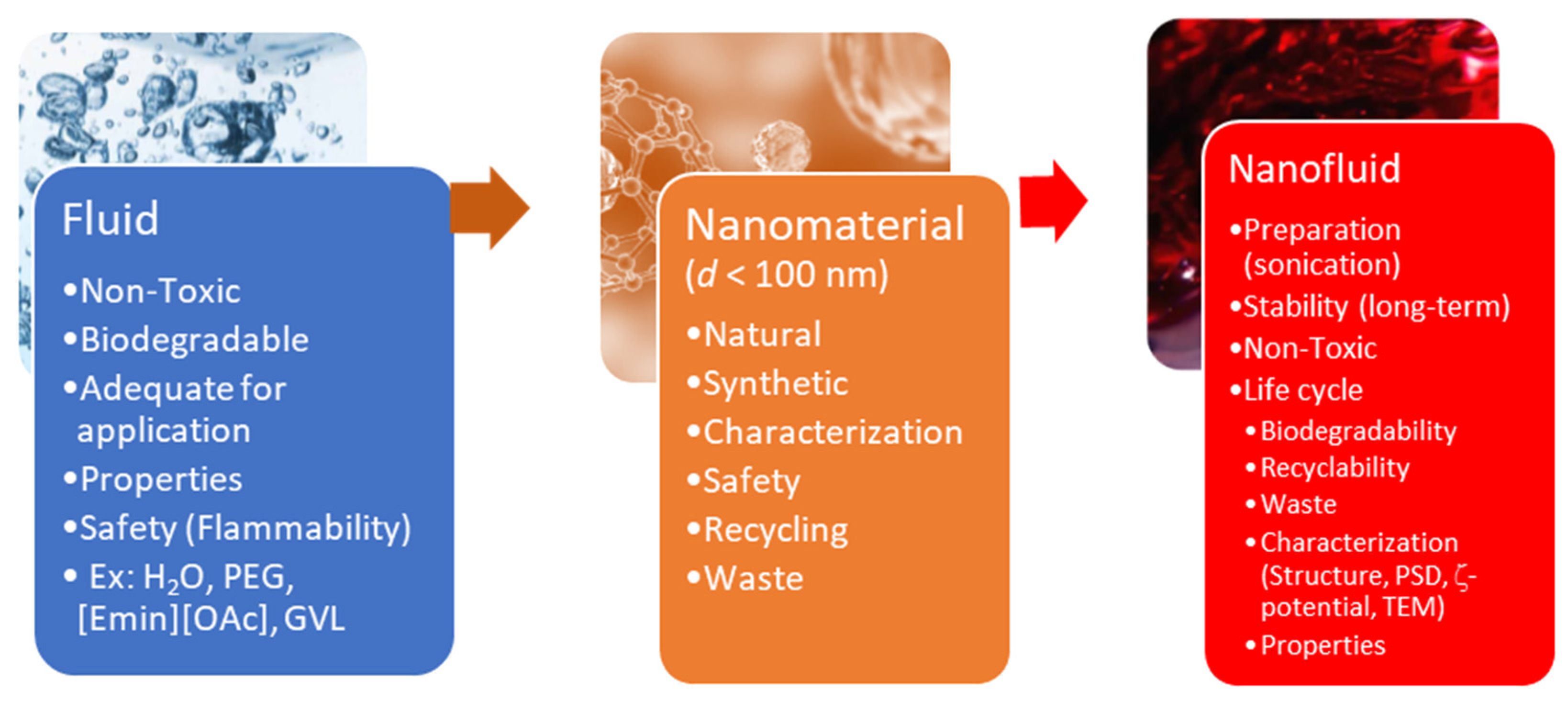

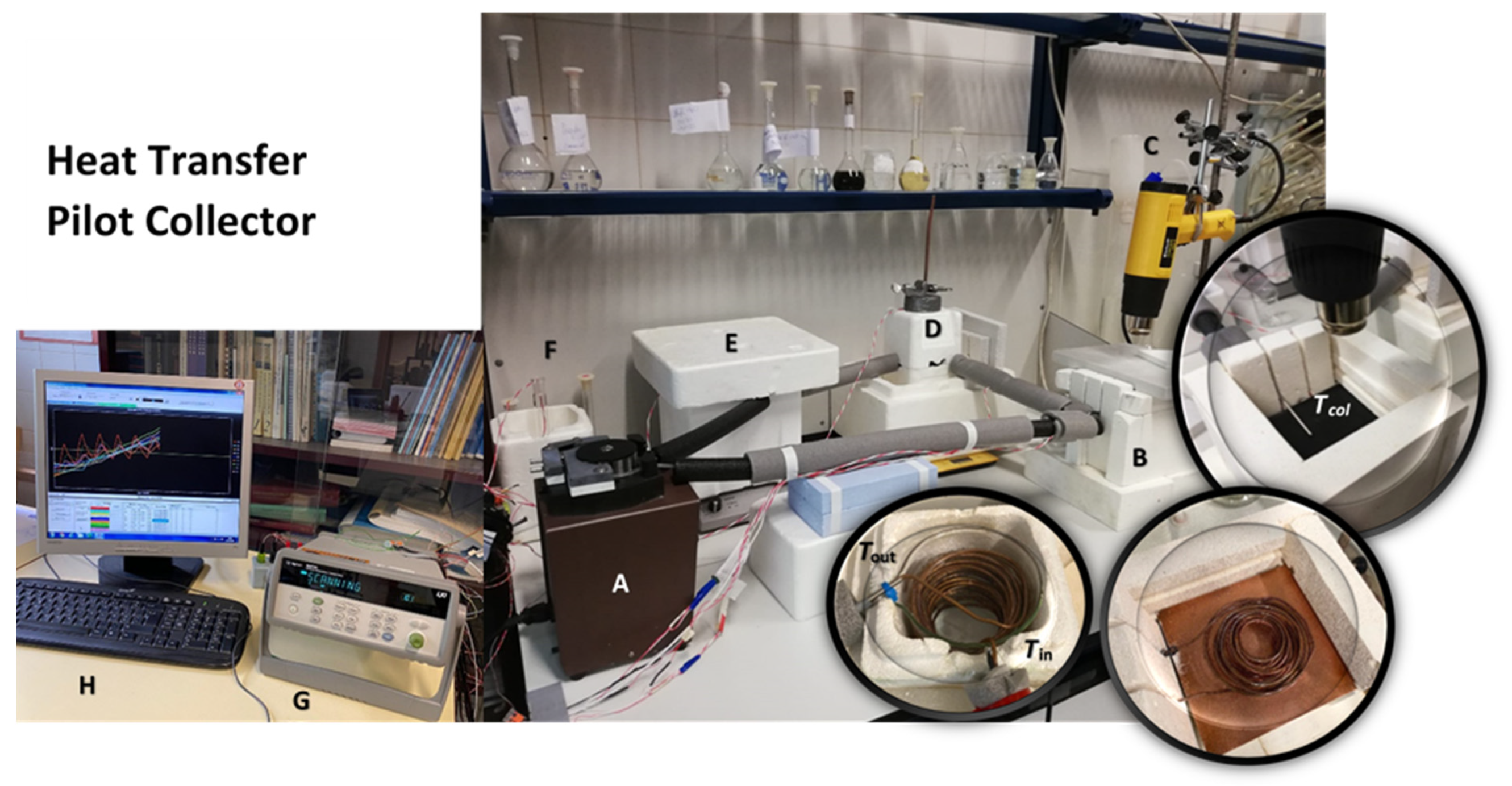
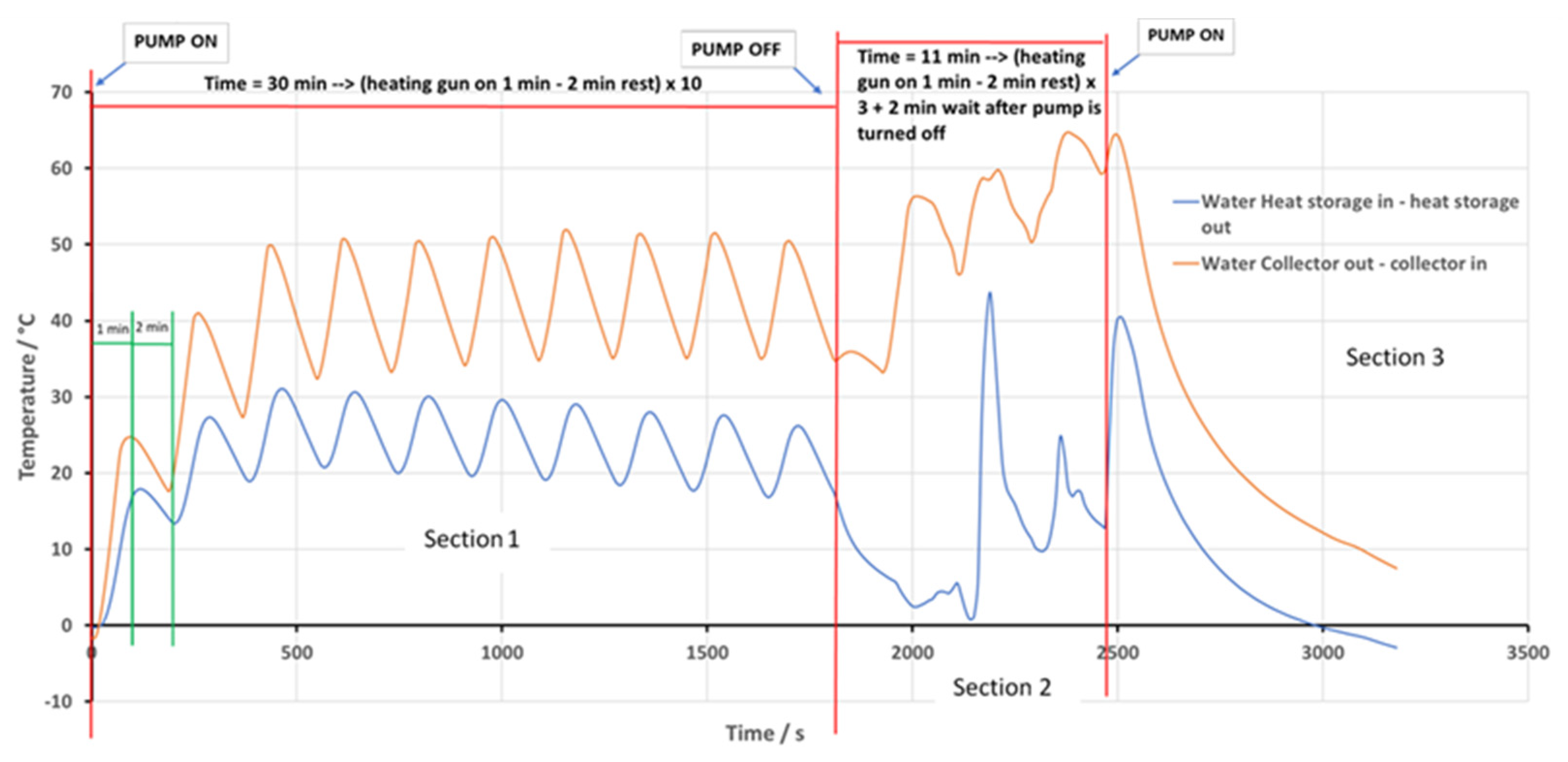
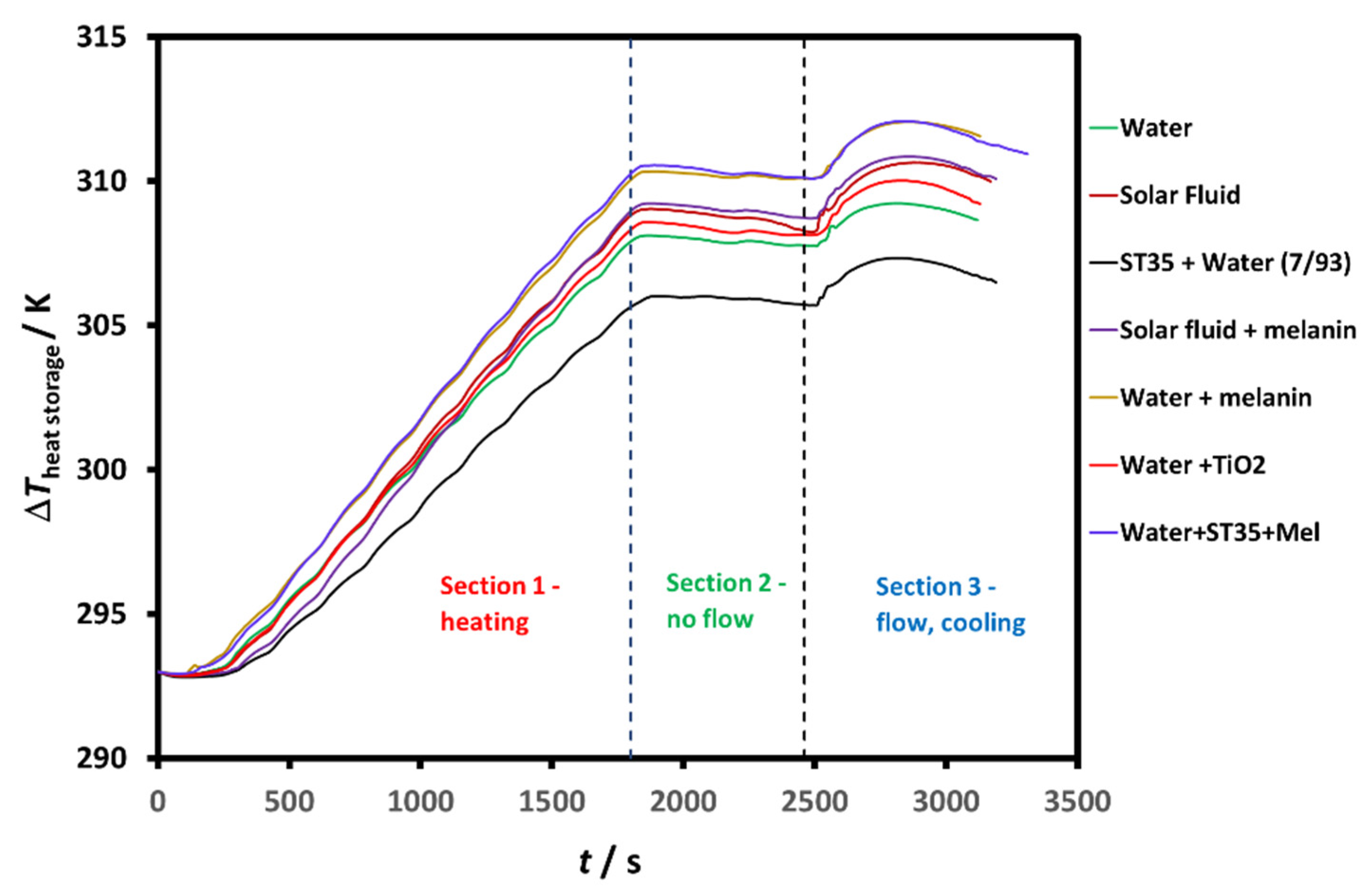
| Dispersed Phase | Dispersed Medium | Type of Colloid | Examples |
|---|---|---|---|
| Solid | Solid | Solid Sol | Glass, Coloured Glasses, and Gems |
| Solid | Liquid | Sol | Paints, Cell fluids, Blood, Mud, Ink, Nanofluids, IoNanofluids, and IoBiofluids |
| Solid | Gas | Aerosol | Smoke, Smog, Dust, and Volcanic ash |
| Liquid | Solid | Gel | Cheese, Butter, Jelly, Gelatine, Toothpaste, Natural rubber |
| Liquid | Liquid | Emulsion | Milk, Hair cream, Mayonnaise, and Brewed coffee |
| Liquid | Gas | Aerosol | Fog, Mist, Cloud, Hair sprays, and Parfum |
| Gas | Solid | Solid Sol | Pumice stone, Foam rubber (sponge) |
| Gas | Liquid | Foam | Froth, Whipped cream, Soap lather, and Fire retardant |
| HTF | Water | Solar Fluid a | ST35 + H2O (7/93) | H2O + TiO2 Nanofluid b | H2O + Melanin Nanofluid c | Solar Fluid + Melanin Nanofluid c | ST35 + H2O (7/93) + Melanin Nanofluid c |
|---|---|---|---|---|---|---|---|
| ρ/kg·m−3 | 980.8 | 996.2 | 999.3 | 1007 | 980.9 | 1000 | 999.4 |
| Cp/J·kg−1·K−1 | 4183 | 3915 d | 4004 | 4176 | 4183 | 3915 | 4004 |
| /K | 23.57 | 27.40 | 22.64 | 24.53 | 25.78 | 27.99 | 27.03 |
| ηHTF/ηWater | 1.00 | 1.09 | 0.92 | 1.04 | 1.09 | 1.11 | 1.10 |
| 16.35 | 17.74 | 14.53 | 17.16 | 19.12 | 18.02 | 19.15 | |
| ηHTF/ηWater | 1.00 | 1.02 | 0.85 | 1.05 | 1.17 | 1.03 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, M.J.; Alexandre, J.; Huisman, C.; Paredes, X.; Nieto de Castro, C. The Balance between Energy, Environmental Security, and Technical Performance: The Regulatory Challenge of Nanofluids. Nanomaterials 2021, 11, 1871. https://doi.org/10.3390/nano11081871
Lourenço MJ, Alexandre J, Huisman C, Paredes X, Nieto de Castro C. The Balance between Energy, Environmental Security, and Technical Performance: The Regulatory Challenge of Nanofluids. Nanomaterials. 2021; 11(8):1871. https://doi.org/10.3390/nano11081871
Chicago/Turabian StyleLourenço, Maria José, João Alexandre, Charlotte Huisman, Xavier Paredes, and Carlos Nieto de Castro. 2021. "The Balance between Energy, Environmental Security, and Technical Performance: The Regulatory Challenge of Nanofluids" Nanomaterials 11, no. 8: 1871. https://doi.org/10.3390/nano11081871
APA StyleLourenço, M. J., Alexandre, J., Huisman, C., Paredes, X., & Nieto de Castro, C. (2021). The Balance between Energy, Environmental Security, and Technical Performance: The Regulatory Challenge of Nanofluids. Nanomaterials, 11(8), 1871. https://doi.org/10.3390/nano11081871








