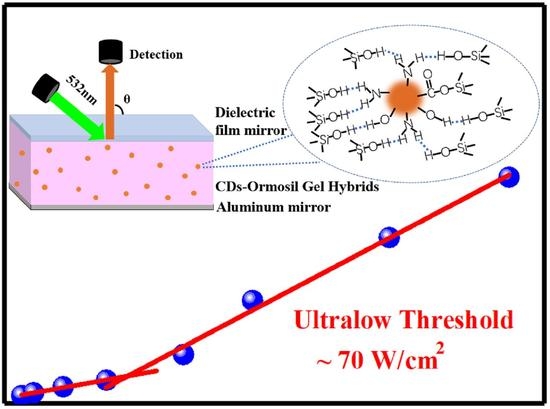
Ultralow Threshold Lasing from Carbon Dot–Ormosil Gel Hybrid-Based Planar Microcavity
Abstract
:1. Introduction
2. Results and Discussion
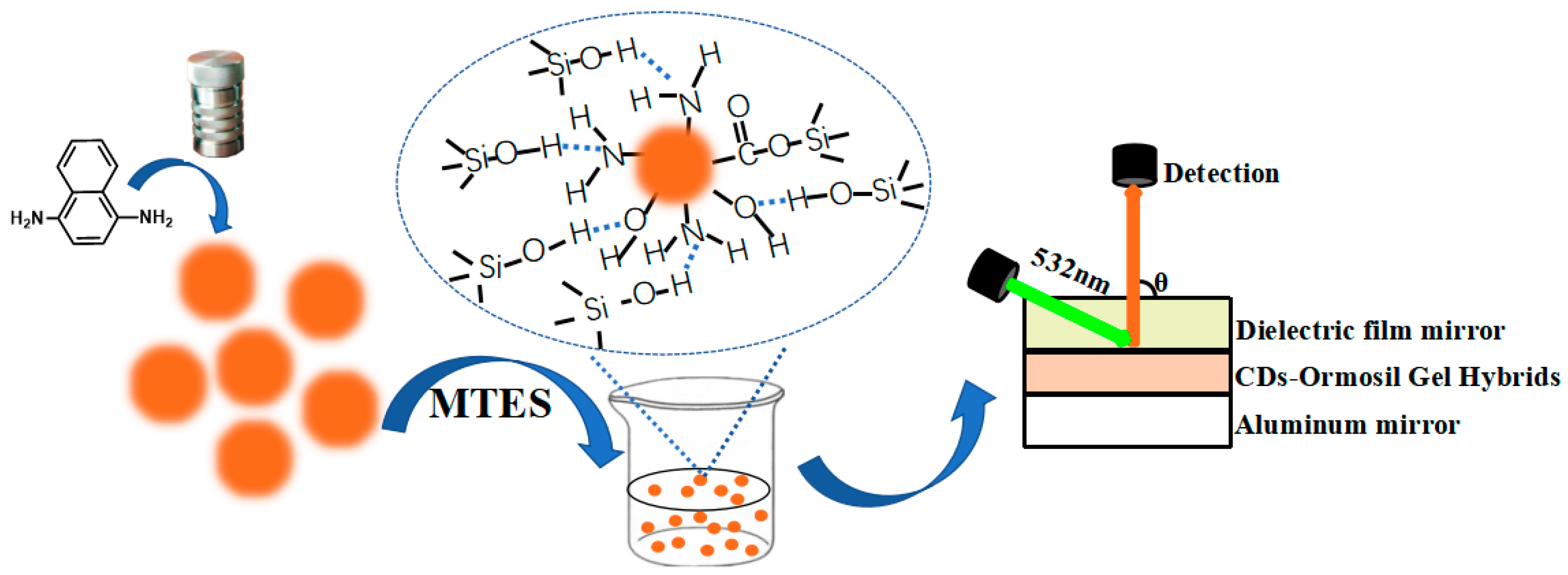
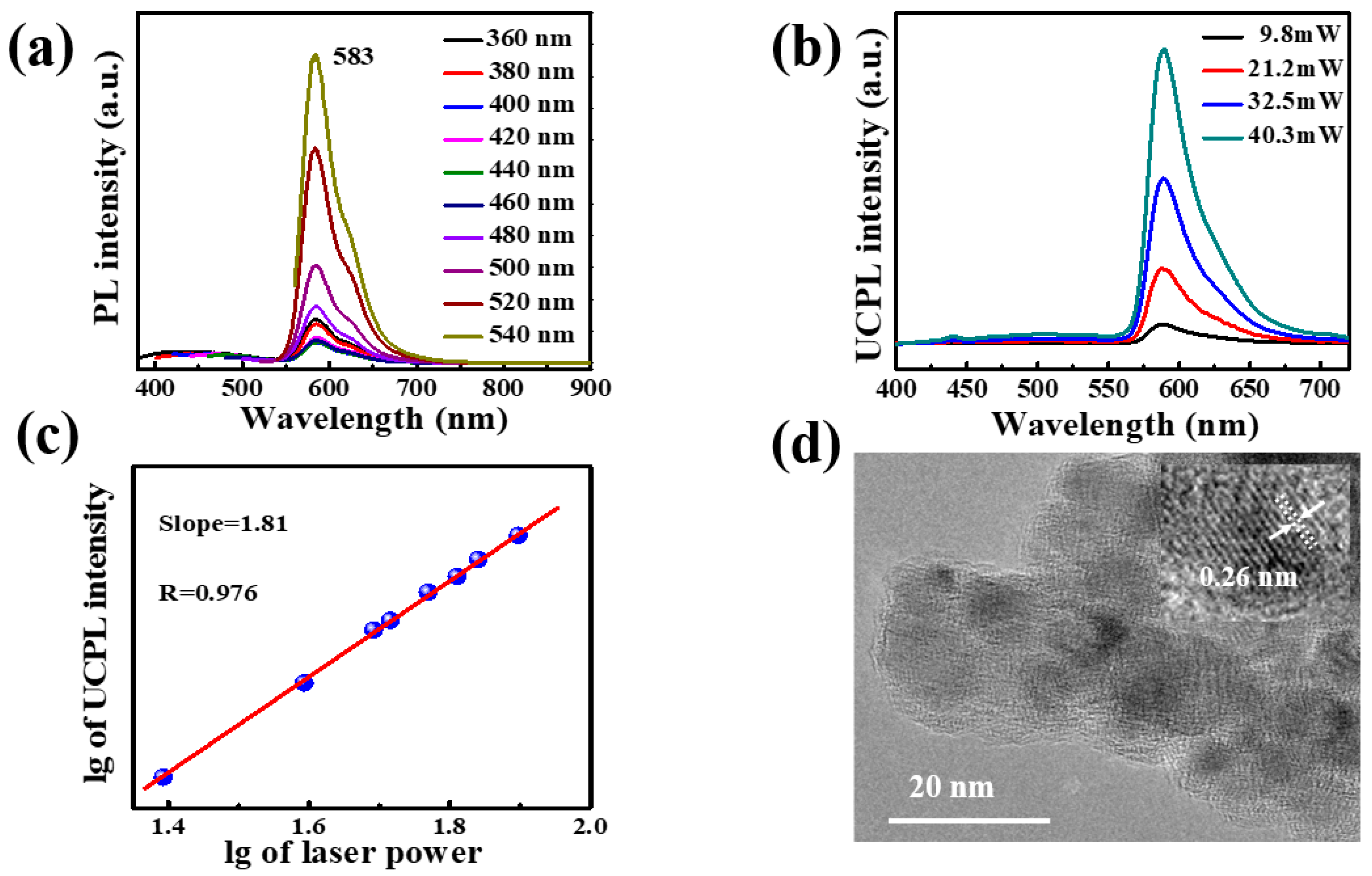
2.1. Optical Properties of CD-gel
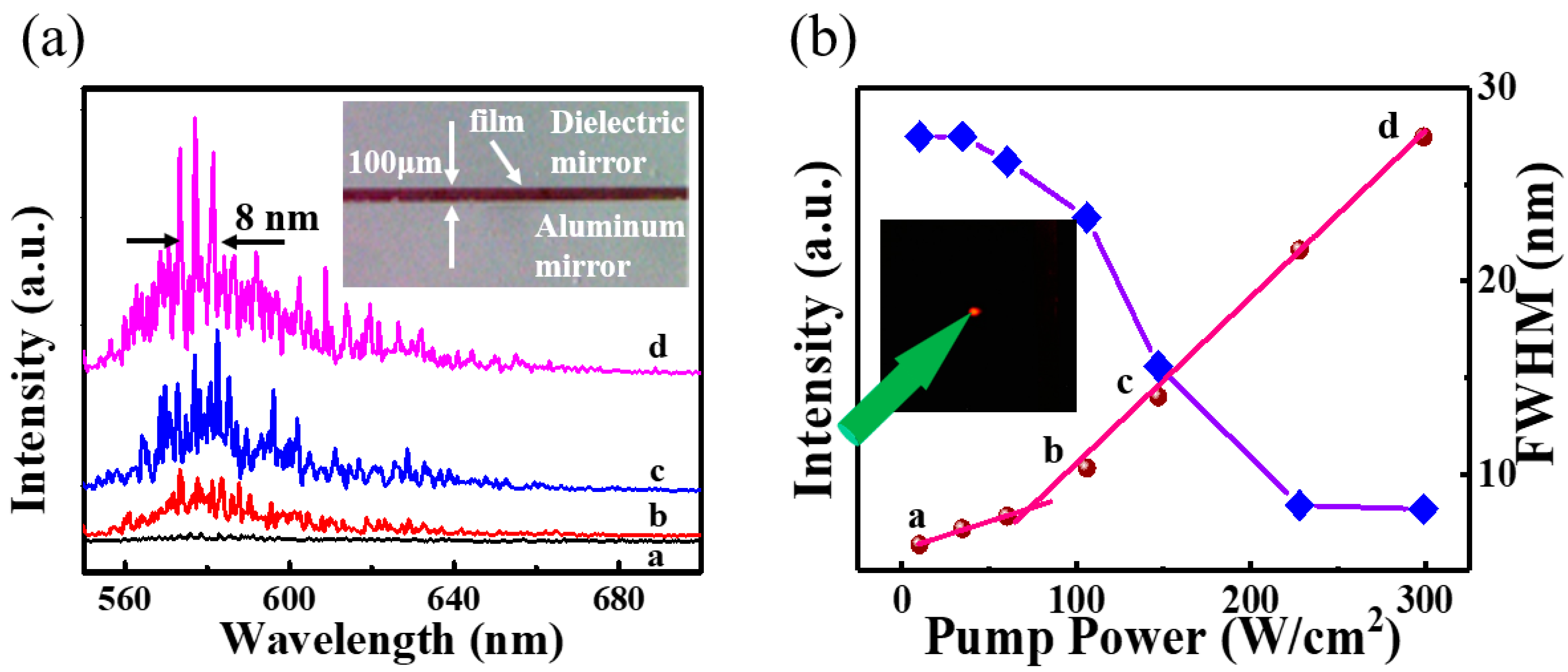
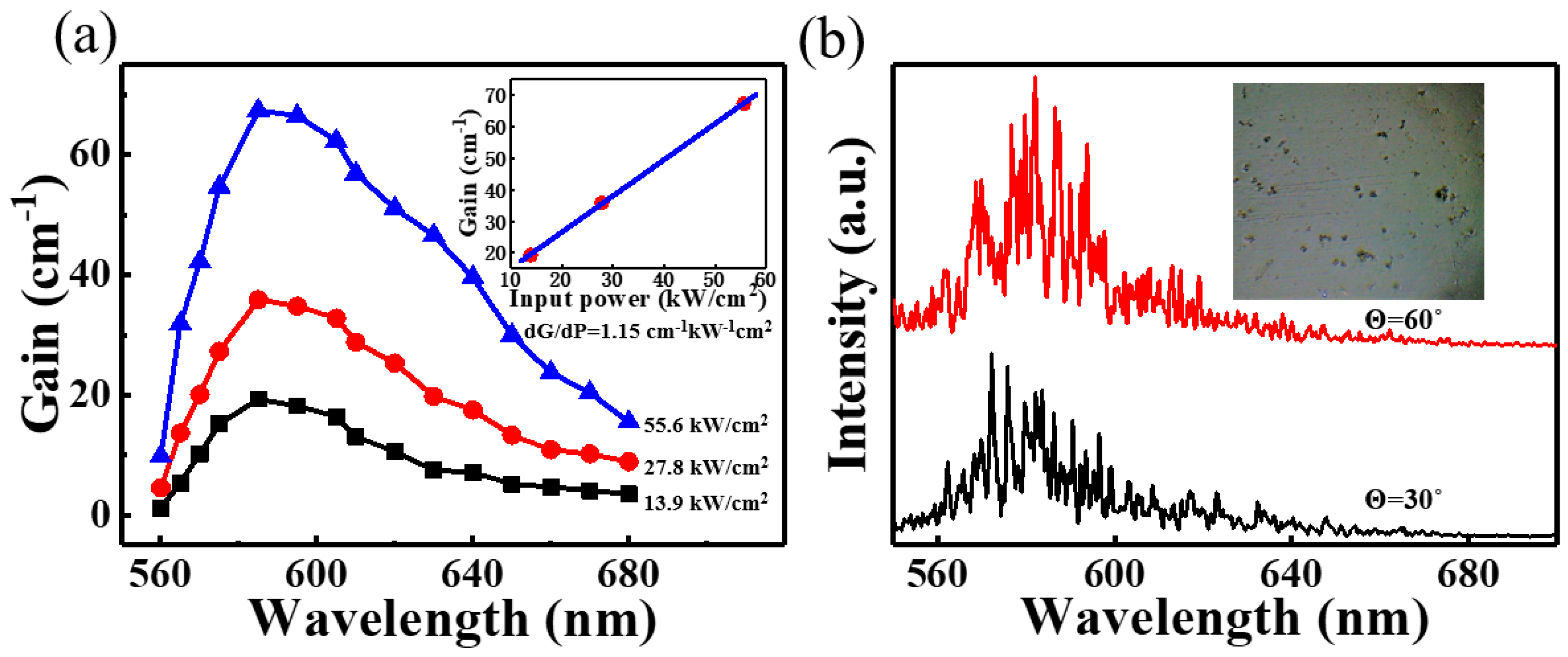
2.2. Ultralow Threshold Laser
3. Conclusions
4. Experimental Section
4.1. Synthesis of CD-gel
4.2. Fabrication of the Laser Cavity
4.3. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging, Angewandte Chemie-International Edition. Angew. Chem. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Zhang, W.; Mei, S.; He, L.; Zhu, J.; Chen, G.; Guo, R. Microwave-Assisted Aqueous Synthesis of Transition Metal Ions Doped ZnSe/ZnS Core/Shell Quantum Dots with Tunable White-Light Emission. Appl. Surf. Sci. 2015, 351, 655–661. [Google Scholar] [CrossRef]
- Fan, F.; Turkdogan, S.; Liu, Z.; Shelhammer, D.; Ning, C.Z. A Monolithic White Laser. Nat. Nanotechnol. 2015, 10, 796–803. [Google Scholar] [CrossRef]
- Li, G.; Zhai, T.; Jiang, Y.; Bando, Y.; Golberg, D. Enhanced Field-Emission and Red Lasing of Ordered CdSe Nanowire Branched Arrays. J. Phys. Chem. C 2011, 115, 9740–9745. [Google Scholar] [CrossRef]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Nayak, T.; Goel, S.; Bean, J.; Theuer, C.P.; et al. In Vivo Targeting and Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Geng, Y.; Li, D.; Yao, H.; Huo, Z.; Li, Y.; Zhang, K.; Zhu, S.; Wei, H.; Xu, W.; et al. Deep Red Emissive Carbonized Polymer Dots with Unprecedented Narrow Full Width at Half Maximum. Adv. Mater. 2020, 32, e1906641. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ni, Y.; Ren, J.; Zhang, W.; Wang, Y.; Xie, Z.; Zhou, S.; Yu, S.F. Highly Efficient and Ultra-Narrow Bandwidth Orange Emissive Carbon Dots for Microcavity Lasers. Nanoscale 2019, 11, 11577–11583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ni, Y.; Xu, X.; Lu, W.; Ren, P.; Yan, P.; Siu, C.K.; Ruan, S.; Yu, S.F. Realization of Multiphoton Lasing from Carbon Nanodot Microcavities. Nanoscale 2017, 9, 5957–5963. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Li, X.; Zhang, T.; Zhang, W.; Chen, M.; Zhou, X. Facile Synthesis of Yellow Emissive Carbon Dots with High Quantum Yield and Their Application in Construction of Fluorescence-Labeled Shape Memory Nanocomposite. J. Alloy. Compd. 2020, 834, 154399. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright Multicolor Bandgap Fluorescent Carbon Quantum Dots for Electroluminescent Light-Emitting Diodes. Adv. Mater. 2017, 29, 6. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.-A. Carbon Dots with Continuously Tunable Full-Color Emission and Their Application in Ratiometric pH Sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Kwon, W.; Do, S.; Lee, J.; Hwang, S.; Kim, J.K.; Rhee, S.-W. Freestanding Luminescent Films of Nitrogen-Rich Carbon Nanodots toward Large-Scale Phosphor-Based White-Light-Emitting Devices. Chem. Mater. 2013, 25, 1893–1899. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef]
- Sun, M.; Qu, S.; Hao, Z.; Ji, W.; Jing, P.; Zhang, H.; Zhang, L.; Zhao, J.; Shen, D. Towards Efficient Solid-State Photoluminescence Based on Carbon-Nanodots and Starch Composites. Nanoscale 2014, 6, 13076–13081. [Google Scholar] [CrossRef] [Green Version]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated sp2-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Zhang, W.F.; Jin, L.M.; Yu, S.F.; Zhu, H.; Pan, S.S.; Zhao, Y.H.; Yang, H.Y. Wide-Bandwidth Lasing from C-Dot/Epoxy Nanocomposite Fabry–Perot Cavities with Ultralow Threshold. J. Mater. Chem. C 2014, 2, 1525. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.; Chen, Y.; Xu, Y.; Song, Q. Polymer-Assisted Self-Assembly of Multicolor Carbon Dots as Solid-State Phosphors for Fabrication of Warm, High-Quality, and Temperature-Responsive White-Light-Emitting Devices. ACS Appl. Mater. Interfaces 2019, 11, 22332–22338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, J.; Hu, C.; Zhang, H.; Lei, B.; Liu, Y. Room Temperature Phosphorescence from Moisture-Resistant and Oxygen-Barred Carbon Dot Aggregates. J. Mater. Chem. C 2017, 5, 6243–6250. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, F.; Liu, C.-Y. Organic-Inorganic Hybrid Functional Carbon Dot Gel Glasses. Adv. Mater. 2012, 24, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sun, X.; Wang, Y.; Song, R.; Xie, Z.; Zhou, S.; Chen, P. Controllable Photoluminescent and Nonlinear Optical Properties of Polymerizable Carbon Dots and Their Arbitrary Copolymerized Gel Glasses. Adv. Opt. Mater. 2018, 6, 7. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhu, H.; Yu, S.F.; Yang, H.Y. Observation of Lasing Emission from Carbon Nanodots in Organic Solvents. Adv. Mater. 2012, 24, 2263–2267. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, W.; Yu, S.F. Realization of Lasing Emission from Graphene Quantum Dots Using Titanium Dioxide Nanoparticles as Light Scatterers. Nanoscale 2013, 5, 1797–1802. [Google Scholar] [CrossRef]
- Qu, S.; Liu, X.; Guo, X.; Chu, M.; Zhang, L.; Shen, D. Amplified Spontaneous Green Emission and Lasing Emission from Carbon Nanoparticles. Adv. Funct. Mater. 2013, 24, 2689–2695. [Google Scholar] [CrossRef]
- Yuan, F.; Xi, Z.; Shi, X.; Li, Y.; Li, X.; Wang, Z.; Fan, L.; Yang, S. Ultrastable and Low-Threshold Random Lasing from Narrow-Bandwidth-Emission Triangular Carbon Quantum Dots. Adv. Opt. Mater. 2019, 7, 1801202. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Lin, J.; Fan, Y.; Li, Y.; Lv, Y.; Liu, X. Excitation Wavelength Independence: Toward Low-Threshold Amplified Spontaneous Emission from Carbon Nanodots. ACS Appl. Mater. Interfaces 2016, 8, 25454–25460. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, S.; Guan, S.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Confined Synthesis of Carbon Nitride in a Layered Host Matrix with Unprecedented Solid-State Quantum Yield and Stability. Adv. Mater. 2018, 30, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Sun, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Zheng, M.; Xiao, Y.; Liang, Y.; Dong, H.; Hu, H.; et al. Enhancement of Fluorescence Emission for Tricolor Quantum Dots Assembled in Polysiloxane toward Solar Spectrum-Simulated White Light-Emitting Devices. Small 2020, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, L.; Xiao, S.; Zhou, X.; Wang, W.; Xu, L. Unidirectional High Intensity Narrow-Linewidth Lasing from a Planar Random Microcavity Laser. Phys. Rev. Lett. 2006, 96, 033902. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yu, S.; Zhang, W.; Zhu, H.; Lu, W.; Jin, L. Low Threshold Amplified Spontaneous Emission from Tin Oxide Quantum Dots: A Instantiation of Dipole Transition Silence Semiconductors. Nanoscale 2013, 5, 11561–11567. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Han, Z.; Ren, J.; Wang, Z.; Zhang, W.; Xie, Z.; Shao, Y.; Zhou, S. Ultralow Threshold Lasing from Carbon Dot–Ormosil Gel Hybrid-Based Planar Microcavity. Nanomaterials 2021, 11, 1762. https://doi.org/10.3390/nano11071762
Ni Y, Han Z, Ren J, Wang Z, Zhang W, Xie Z, Shao Y, Zhou S. Ultralow Threshold Lasing from Carbon Dot–Ormosil Gel Hybrid-Based Planar Microcavity. Nanomaterials. 2021; 11(7):1762. https://doi.org/10.3390/nano11071762
Chicago/Turabian StyleNi, Yiqun, Zhixia Han, Junkai Ren, Zhen Wang, Wenfei Zhang, Zheng Xie, Yonghong Shao, and Shuyun Zhou. 2021. "Ultralow Threshold Lasing from Carbon Dot–Ormosil Gel Hybrid-Based Planar Microcavity" Nanomaterials 11, no. 7: 1762. https://doi.org/10.3390/nano11071762
APA StyleNi, Y., Han, Z., Ren, J., Wang, Z., Zhang, W., Xie, Z., Shao, Y., & Zhou, S. (2021). Ultralow Threshold Lasing from Carbon Dot–Ormosil Gel Hybrid-Based Planar Microcavity. Nanomaterials, 11(7), 1762. https://doi.org/10.3390/nano11071762