Filtration of Nanoparticle Agglomerates in Aqueous Colloidal Suspensions Exposed to an External Radio-Frequency Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.3. Measurement Characterization
- Transmission Electron Microscopy. Conventional TEM images were obtained using the Fei Tecnai G2 F20 S Twin transmission electron microscope (Hillsboro, OR, USA).
- inViaTM Qontor® confocal Raman microscope using inVia Reflex Renishaw system equipped with Leica DM2700 and Leica N Plan EPI 50x/0.75na BD Objective. In this study, a laser with a maximum power of 50 mW was used. The following operating parameters were applied: 532 nm wavelength, 0.5% laser power, exposure time 10 s, measurement results averaged over the points of a grid.
- XRD measurements using Malvern Panalytical’s Empyrean X-ray diffractometer was used. The operating parameters of the X-ray diffractometer were as follows: voltage 40 kV, current 40 mA, the wavelength of the CuK-radiation 1.5406 Å.
- Dynamic Light Scattering, where particle hydrodynamic size measurements were carried out using Zetasizer Nano ZS analyzer (Malvern Instruments Ltd., Worcestershire, UK), operated in the 173 backscattered mode on diluted aqueous solutions of nanoclusters.
- Radio-frequency magnetic field generator for heating a magnetic colloid suspension based on a copper coil with 6 turns. The inside of the coil was cooled by flowing water. The following parameters were used in the experiments: the frequency of the magnetic field kHz and the amplitude of the magnetic field mT.
3. Results
4. Discussion
- -
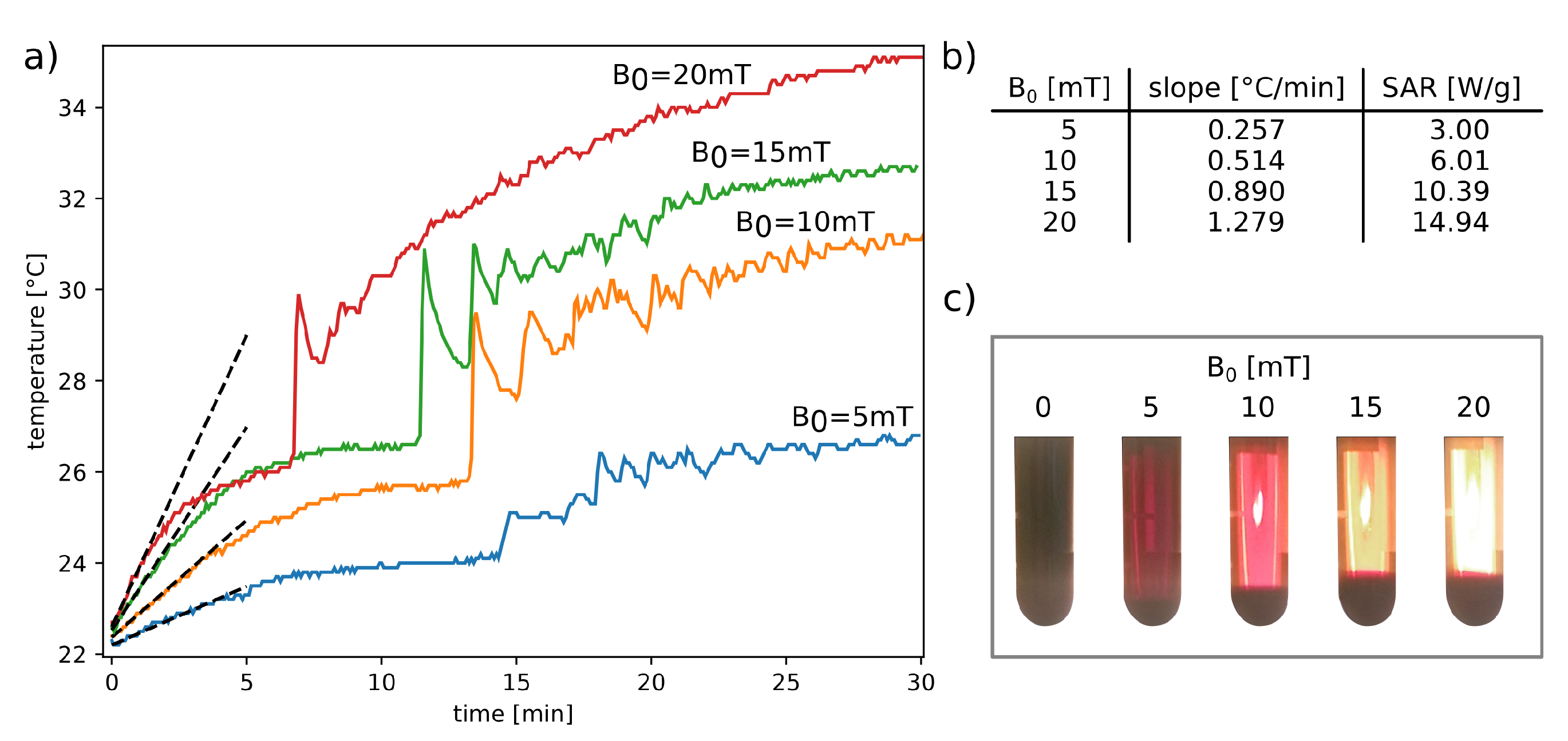
- An initially monotonic temperature increase, analogous to the temperature course in Figure 6a, corresponding to the heat transfer from nanoparticles to a fluid environment. Larger aggregates of nanoparticles begin to form and sink by gravity to the bottom of the tube (even the 50 s exposure time to an RF magnetic field is sufficient for noticeable particle segregation). Falling aggregates increase the temperature of the lower part of the magnetic suspension, activating a thermally driven convection circulation that carries finer aggregates.
- -
- After about 10–12 min, rapid changes in the temperature of the surface colloid layer can be observed. During this time, the temperature difference between the bottom part and the upper part of the colloidal suspension reaches its maximum value, triggering the upward convective flow of liquid. For silica-stabilized magnetic nanoparticles, the effect is weaker.
- -
- becomes saturated, which is accompanied by the disappearing circulation and smaller and smaller upward ejection of warm liquid. At this stage of the RFMH process, the distribution of nanoparticles in the colloid is practically unchanged.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sangaiya, P.; Jayaprakash, R.A. Review on iron oxide nanoparticles and their biomedical applications. J. Supercond. Nov. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic acid-coated iron oxide magnetic nanoparticles: The polymer molecular weight influence. Colloids Surf. 2018, 543, 28–37. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.V.; Amme, T.F. Synthesis, Principles, and Properties of Magnetite Nanoparticles for In Vivo Imaging Applications—A Review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Review Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Perez, J.M.; O’Loughin, T.; Simeone, F.J.; Weissleder, R.; Josephson, L. DNA-Based Magnetic Nanoparticle Assembly Acts as a Magnetic Relaxation Nanoswitch Allowing Screening of DNA-Cleaving Agents. J. Am. Chem. Soc. 2002, 124, 2856–2857. [Google Scholar] [CrossRef]
- Cantillon-Murphy, P.; Wald, L.L.; Zahn, M.; Adalsteinsson, E. Proposing Magnetic Nanoparticle Hyperthermia in Low-Field MRI. Concepts Magn. Reson. Part A 2010, 36A, 36–47. [Google Scholar] [CrossRef]
- Hurley, K.R.; Ring, H.L.; Etheridge, M.; Zhang, J.; Gao, Z.; Shao, Q.; Klein, N.D.; Szlag, V.M.; Chung, C.; Reineke, T.M.; et al. Predictable Heating and Positive MRI Contrast from a Mesoporous Silica-Coated Iron Oxide Nanoparticle. Mol. Pharm. 2016, 13, 2172–2183. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Schirra, H.; Schiestel, T.; Schmidt, H.; Felix, R. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J. Magn. Magn. Mater. 1999, 194, 185–196. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticle in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Soleymani, M.; Edrissi, M. Heating ability and biocompatibility study of silica-coated magnetic nanoparticles as heating mediators for magnetic hyperthermia and magnetically triggered drug delivery systems. Bull. Mater. Sci. 2015, 38, 1633–1638. [Google Scholar] [CrossRef]
- Dríždal, T.; Togni, P.; Vxixšek, L.; Vrba, J. Comparison of Constant and Temperature Dependent Blood Perfusion in Temperature Prediction for Superficial Hyperthermia. Radioengineering 2010, 19, 281–289. [Google Scholar]
- Soleymani, M.; Velashjerdi, M.; Shaterabadi, Z.; Barati, A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020, 237, 116130. [Google Scholar] [CrossRef]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samiaa, A.C.S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Prog. Nat. Sci. 2016, 26, 440–448. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, Á.V.; Iglesias, G.R. Modulation of the Magnetic Hyperthermia Response Using Different Superparamagnetic Iron Oxide Nanoparticle Morphologies. Nanomaterials 2021, 11, 627. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Schillinger, U.; Brill, T.; Rudolph, C.; Huth, S.; Gersting, S.; Krötz, F.; Hirschberger, J.; Bergemann, C.; Plank, C. Advances in magnetofection—Magnetically guided nucleic acid delivery. J. Magn. Magn. Mater. 2005, 293, 501–508. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S.-Y. Stimuli-Responsive Controlled-Release Delivery System Based on Mesoporous Silica Nanorods Capped with Magnetic Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 5038–5044. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Guskos, N.; Kozioł, J.J.; Dudek, M.R. Preparation of the narrow size distribution USPIO in mesoporous silica for magnetic field guided drug delivery and release. J. Magn. Magn. Mater. 2015, 374, 96–102. [Google Scholar] [CrossRef]
- Mleczko, J.; Defort, A.; Kozioł, J.J.; Nguyen, T.T.; Mirończyk, A.; Zapotoczny, B.; Nowak-Jary, J.; Gronczewska, E.; Marć, M.; Dudek, M.R. Limitation of tuning the antibody-antigen reaction by changing the value of pH and its consequence for hyperthermia. J. Biochem. 2016, 159, 421–427. [Google Scholar] [CrossRef]
- Shah, A.; ADobrovolskaia, M.A. Immunological Effects of Iron Oxide Nanoparticles and Iron-based Complex Drug Formulations: Therapeutic Benefits, Toxicity, Mechanistic Insights, and Translational Considerations. Nanomedicine 2018, 14, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Doong, R.; Kim, H.; Varma, R.S.; Dionysiou, D.D. Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation; American Chemical Society: Washington, DC, USA, 2016. [Google Scholar]
- Pasandideh, E.K.; Kakav, I.B.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Esrafili, A.; Kalantary, R.R. Silica-coated magnetite nanoparticles core-shell spheres (Fe3O4@SiO2) for natural organic matter removal. J. Environ. Health Sci. Eng. 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Alp, E.; Aydogan, N. A comparative study: Synthesis of superparamagnetic iron oxide nanoparticles in air and N2 atmosphere. Colloids Surf. 2016, 510, 205–212. [Google Scholar] [CrossRef]
- Rani, S.; Varma, G. Superparamagnetism and metamagnetic transition in Fe3O4 nanoparticles synthesized via co-precipitation method at different pH. Phys. B Condens. Matter 2015, 472, 66–77. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal decomposition synthesis of iron oxide nanoparticles with diminished magnetic dead layer by controlled addition of oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Toyos-Rodríguez, C.; Calleja-García, J.; Torres-Sánchez, L.; López, A.; Abu-Dief, A.M.; Costa, A.; Elbaile, L.; Crespo, R.D.; Garitaonia, J.S.; Lastra, E.; et al. A Simple and Reliable Synthesis of Superparamagnetic Magnetite Nanoparticles by Thermal Decomposition of Fe(acac)3. J. Nanomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Kaur, G.; Dogra, V.; Kumar, R.; Kumar, S.; Singh, K. Fabrication of iron oxide nanocolloids using metallosurfactant-based microemulsions: Antioxidant activity, cellular, and genotoxicity toward Vitis vinifera. J. Biomol. Struct. Dyn. 2019, 37, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Song, X.; Liu, H. Facile hydrothermal synthesis of Fe3O4 nanoparticle and effect of crystallinity on performances for supercapacitor. Funct. Mater. Lett. 2019, 12, 1950019. [Google Scholar] [CrossRef]
- Hu, P.; Chang, T.; Chen, W.J.; Deng, J.; Li, S.L.; Zuo, Y.G.; Kang, L.; Yang, F.; Hostetter, M.; Volinsky, A.A. Temperature effects on magnetic properties of Fe3O4 nanoparticles synthesized by the sol-gel explosion-assisted method. J. Alloys Compd. 2019, 773, 605–611. [Google Scholar] [CrossRef]
- Hajnorouzi, A.; Modaresi, N. Direct sono electrochemical method for synthesizing Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2020, 166732. [Google Scholar] [CrossRef]
- Maurizi, L.; Bouyer, F.; Paris, J.; Demoisson, F.; Saviot, L.; Millot, N. One step continuous hydrothermal synthesis of very fine stabilized superparamagnetic nanoparticles of magnetite. Chem. Commun. 2011, 47, 11706–11708. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Liivat, A.; Zhu, Y. Microwave-solvothermal synthesis of Fe3O4 magnetic nanoparticles. Mater. Lett. 2013, 107, 23–26. [Google Scholar] [CrossRef]
- Ditsch, A.; Laibinis, P.E.; Wang, D.I.C.; Hatton, T.A. Controlled Clustering and Enhanced Stability of Polymer-Coated Magnetic Nanoparticles. Langmuir 2005, 21, 6006–6018. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Functionalization of Monodisperse Magnetic Nanoparticles. Langmuir 2007, 23, 2158–2168. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Szabó, Á.; Iván, B.; Turcu, R.; Vékásd, L.; Zupkó, I.; Jaics, G.; Tombácz, E. Multifunctional PEG-carboxylate copolymer coated superparamagnetic iron oxide nanoparticles for biomedical application. J. Magn. Magn. Mater. 2018, 451, 710–720. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.; Ooi, B.S.; Ahmad, A.L. Agglomeration, colloidal stability, and magnetic separation of magnetic nanoparticles: Collective influences on environmental engineering applications. J. Nanoparticle Res. 2017, 19, 368. [Google Scholar] [CrossRef]
- Yang, B.; Cai, H.; Qin, W.; Zhang, B.; Zhai, C.; Jiang, B.; Wu, Y. Bcl-2-functionalized ultrasmall superparamagnetic iron oxide nanoparticles coated with amphiphilic polymer enhance the labeling efficiency of islets for detection by magnetic resonance imaging. Int. J. Nanomed. 2013, 8, 3977–3990. [Google Scholar] [CrossRef][Green Version]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VIII. Update. J. Colloid Interface Sci. 2020, 275, 102064. [Google Scholar] [CrossRef] [PubMed]
- Kazeminezhad, I.; Mosiv, S. Size dependence of electrooxidized Fe3O4 nanoparticles on surfactant concentration. World Acad. Sci. Eng. Technol. 2011, 74, 338–341. [Google Scholar]
- Barale, M.; Mansour, C.; Carrette, F.; Pavageau, E.M.; Catalette, H.; Lefèvre, G.; Fedoroff, M.; Cote, G. Characterization of the surface charge of oxide particles of PWR primary water circuits from 5 to 320 °C. J. Nucl. Mater. 2008, 381, 302–308. [Google Scholar] [CrossRef]
- Tombàcz, E.; Majzik, A.; Horvxaxt, Z.S.; Illés, E. Magnetite in aqueous medium: Coating its surface and surface coated with it. Romanian Rep. Phys. 2006, 58, 281–286. [Google Scholar]
- Namdeo, M.; Bajpai, S.K. Investigation of hexavalent chromium uptake by synthetic magnetite nanoparticles. EJEAFChe 2009, 8, 367–381. [Google Scholar]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Barrera, G.; Allia, P.; Tiberto, P. Temperature-dependent heating efficiency of magnetic nanoparticles for applications in precision nanomedicine. Nanoscale 2020, 12, 6360–6377. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub1, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Marć, M.; Dudek, M.; Kozioł, J.J.; Zapotoczny, B. Adsorption of Methylene Blue on Titanate Nanotubes Synthesized with Ultra-Small Fe3O4 Nanoparticles. Nano Brief Rep. Rev. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Bakhteeva, I.A.; Medvedeva, I.V.; Uimin, M.A.; Byzov, I.V.; Zhakov, S.V.; Yermakov, A.E.; Shchegoleva, N.N. Magnetic sedimentation and aggregation of Fe3O4@SiO2 nanoparticles in water medium. Sep. Purif. Technol. 2016, 159, 35–42. [Google Scholar] [CrossRef]
- Wolak, W.; Kolomeisky, A.B.; Dudek, M.R.; Marć, M.; Najder-Kozdrowska, L. Enhancing silica surface deprotonation by using magnetic nanoparticles as heating agents. J. Phys. D Appl. Phys. 2019, 52, 465001. [Google Scholar] [CrossRef]
- Suwa, M.; Uotani, A.; Tsukahara, S. Alignment and small oscillation of superparamagnetic iron oxide nanoparticle in liquid under alternating magnetic field. J. Appl. Phys. 2019, 125, 123901. [Google Scholar] [CrossRef]
- Shasha, C.; Krishnan, K.M. Nonequilibrium Dynamics of Magnetic Nanoparticles with Applications in Biomedicine. Adv. Mater. 2020, 1904131. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J. Sol.-Gel. Sci. Technol. 2016, 77, 753–758. [Google Scholar] [CrossRef]
- Bhowmik, R.N.; Ranganathan, R. Mossbauer Spectroscopy: An Essential Tool for Nanoparticle Magnetism in Co0.2Zn0.8Fe2O4 Ferrite. Indian J. Pure Appl. Phys. 2007, 45, 810. [Google Scholar]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J.; Foner, S. Surface Spin Disorder in NiFe2O4 Nanoparticles. Phys. Rev. Lett. 1996, 77, 394. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A new method for the identification and quantification of magnetite-maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Goettingen Math. Phys. Kl. 1918, 1918, 98–100. [Google Scholar]
- The Database Containing Information on Minerals. Available online: https://www.mindat.org/min-2538.html or https://www.mindat.org/min-2533.html (accessed on 26 June 2021).
- Ma, M.; Wu, Y.; Zhou, J.; Sun, Y.; Zhang, Y.; Gu, N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- White, W.; De Angelis, B. Interpretation of the vibrational spectra of spinels. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 985–995. [Google Scholar] [CrossRef]
- Iliev, M.N.; Mazumdar, D.; Ma, J.X.; Gupta, A.; Rigato, F.; Fontcuberta, J. Monitoring B-site ordering and strain relaxation in NiFe2O4 epitaxial films by polarized Raman spectroscopy. Phys. Rev. B 2011, 83, 014108. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueirixnxo, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for biorelated applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Ilg, P.; Kröger, M. Dynamics of interacting magnetic nanoparticles: Effective behavior from competition between Brownian and Néel relaxation. Phys. Chem. Chem. Phys. 2020, 22, 22244–22259. [Google Scholar] [CrossRef]
- Néel, L. Influence des fluctuations thermiques sur l’aimantation de grains ferromagnétiques très fins. CR Acad. Sci. Paris 1949, 228, 664–666. [Google Scholar]
- Pilati, V.; Gomide, G.; Gomes, R.C.; Goya, G.F.; Depeyrot, J. Colloidal Stability and Concentration Effects on Nanoparticle Heat Delivery for Magnetic Fluid Hyperthermia. Langmuir 2021, 37, 1129–1140. [Google Scholar] [CrossRef]
- Das, R.; Masa, J.A.; Kalappattil, V.; Nemati, Z.; Rodrigo, I.; Garaio, E.; García, J.Á.; Phan, M.-H.; Srikanth, H. Iron Oxide Nanorings and Nanotubes for Magnetic Hyperthermia: The Problem of Intraparticle Interactions. Nanomaterials 2021, 11, 1380. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.A.; Torres, T.E.; Andrés-Vergés, M.; Costo, R.; De La Presa, P.; Serna, C.J.; Morales, M.P.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J. Solid State Chem. 2009, 182, 2779–2784. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Usov, N.A.; Gubanova, E.M.; Wei, Z.H. Specific absorption rate of assembly of magnetic nanoparticles with uniaxial anisotropy. J. Phys. Conf. Ser. 2020, 1439, 012044. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; PSouthern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D: Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Parashar, A.; Sikarwar, S.; Jain, R. Removal of pharmaceuticals from wastewater using magnetic iron oxide nanoparticles. J. Environ. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Katz, E. Magnetic Nanoparticles. Magnetochemistry 2020, 6, 6. [Google Scholar] [CrossRef]

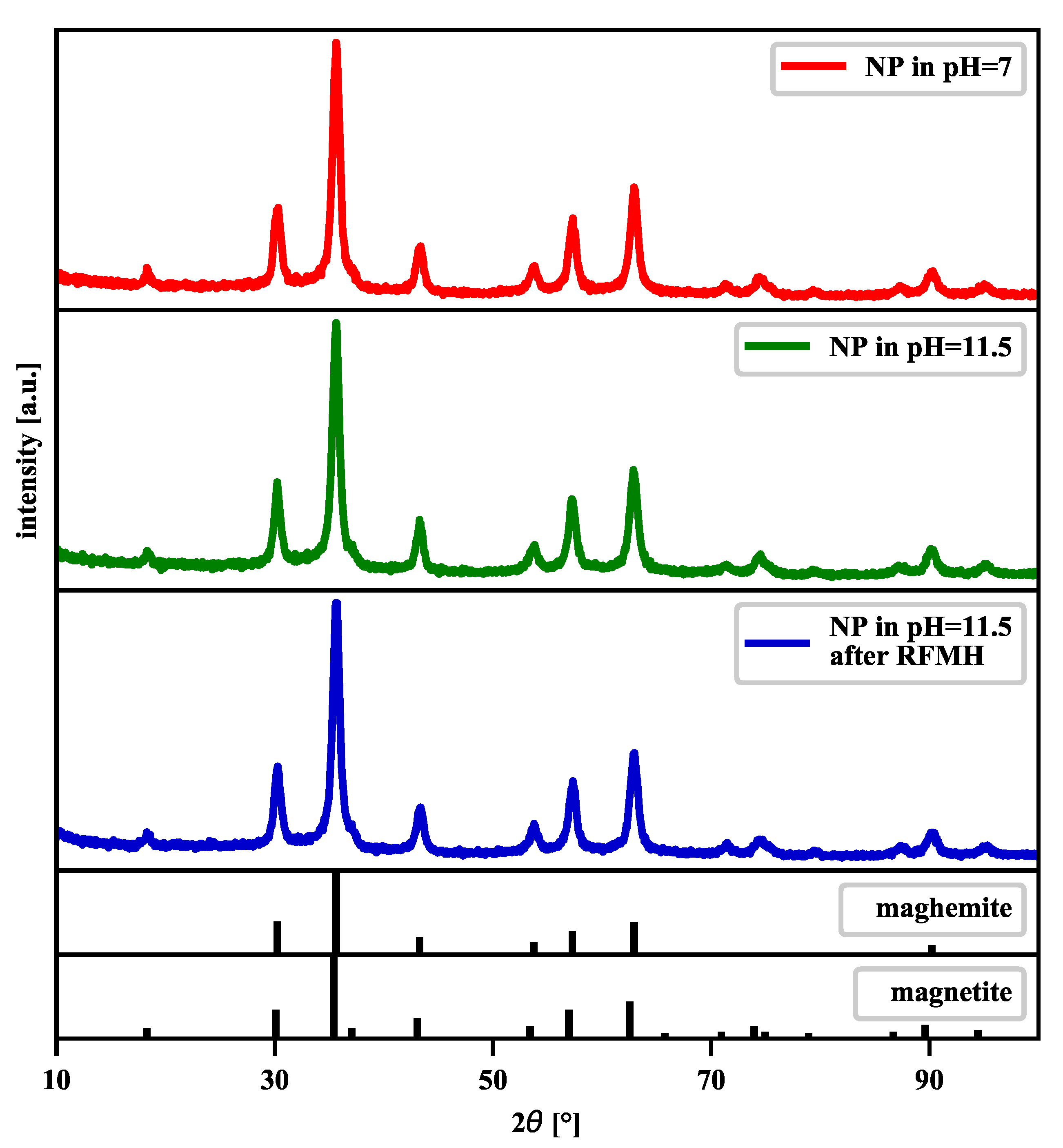
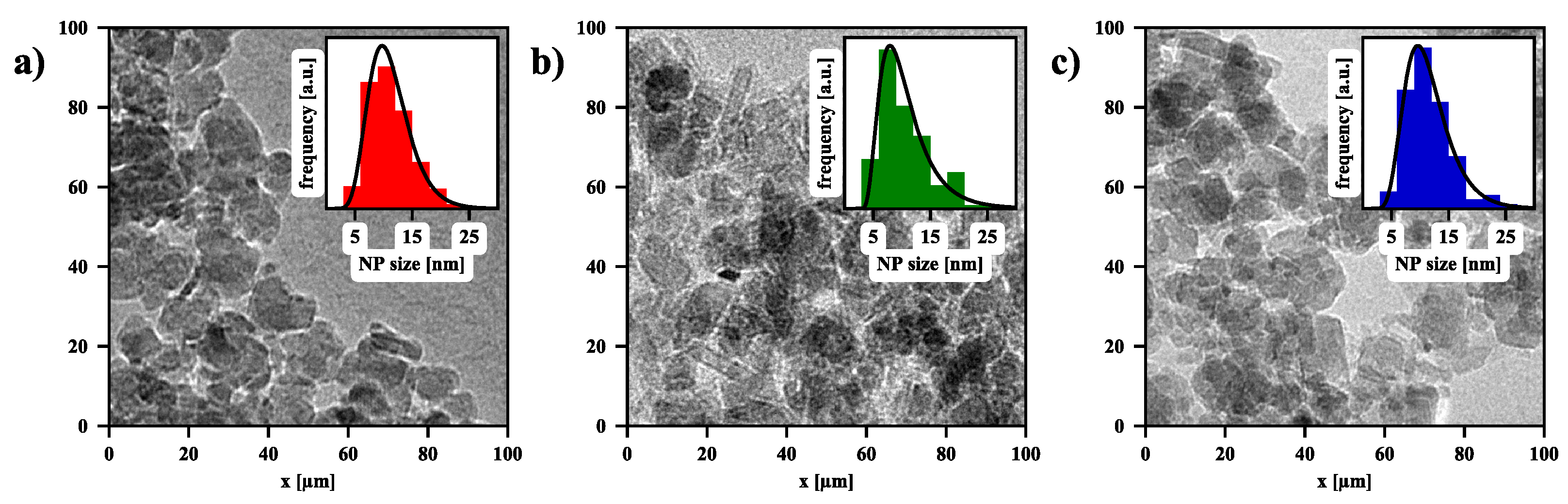
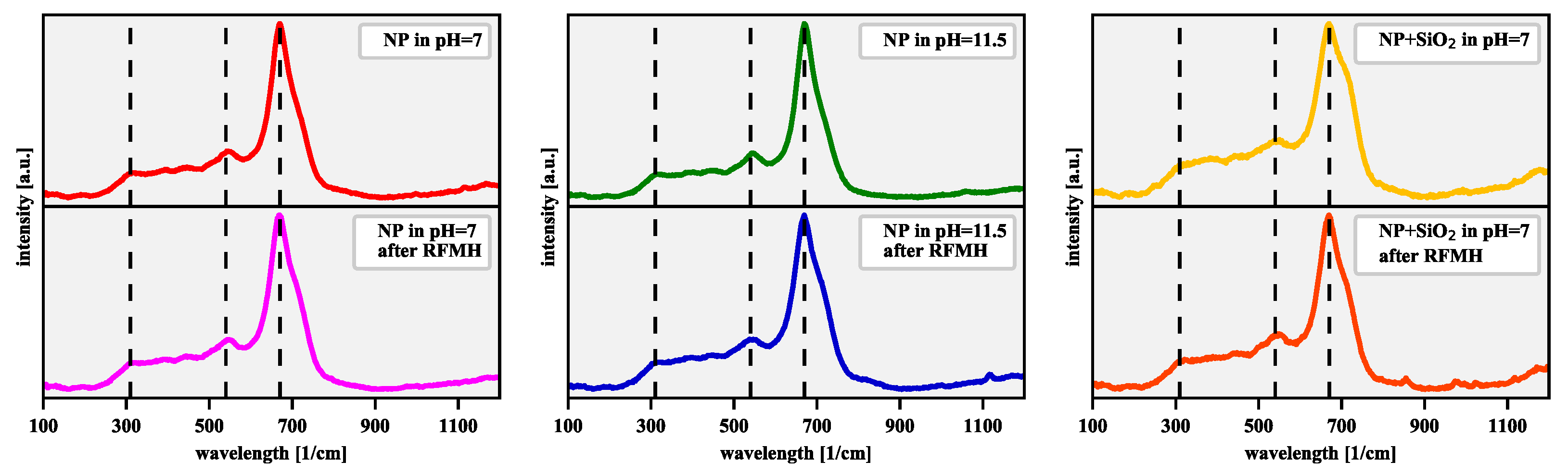
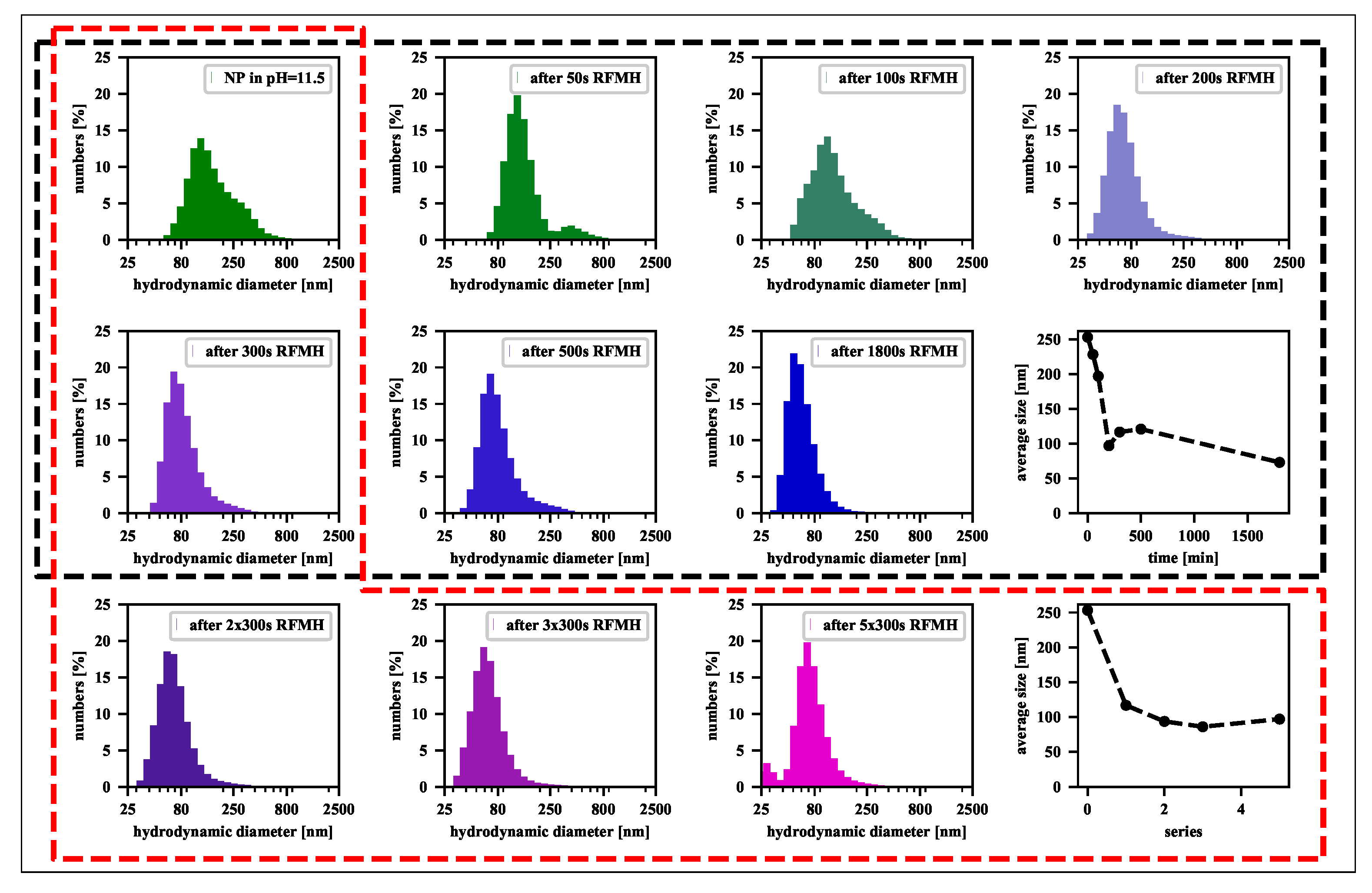

| [] | [rad] | D [nm] | |
|---|---|---|---|
| NPs in pH = 7 | 35.63 | 0.0138 | 10.54 |
| NPs in pH = 11.5 | 35.61 | 0.0137 | 10.84 |
| NPs in pH = 11.5 after RFMH | 35.63 | 0.0134 | 10.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marć, M.; Drzewiński, A.; Wolak, W.W.; Najder-Kozdrowska, L.; Dudek, M.R. Filtration of Nanoparticle Agglomerates in Aqueous Colloidal Suspensions Exposed to an External Radio-Frequency Magnetic Field. Nanomaterials 2021, 11, 1737. https://doi.org/10.3390/nano11071737
Marć M, Drzewiński A, Wolak WW, Najder-Kozdrowska L, Dudek MR. Filtration of Nanoparticle Agglomerates in Aqueous Colloidal Suspensions Exposed to an External Radio-Frequency Magnetic Field. Nanomaterials. 2021; 11(7):1737. https://doi.org/10.3390/nano11071737
Chicago/Turabian StyleMarć, Maciej, Andrzej Drzewiński, Wiktor W. Wolak, Lidia Najder-Kozdrowska, and Mirosław R. Dudek. 2021. "Filtration of Nanoparticle Agglomerates in Aqueous Colloidal Suspensions Exposed to an External Radio-Frequency Magnetic Field" Nanomaterials 11, no. 7: 1737. https://doi.org/10.3390/nano11071737
APA StyleMarć, M., Drzewiński, A., Wolak, W. W., Najder-Kozdrowska, L., & Dudek, M. R. (2021). Filtration of Nanoparticle Agglomerates in Aqueous Colloidal Suspensions Exposed to an External Radio-Frequency Magnetic Field. Nanomaterials, 11(7), 1737. https://doi.org/10.3390/nano11071737






