Quaternary Alloy Quantum Dots as Fluorescence Probes for Total Acidity Detection of Paper-Based Relics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CdZnSeS-pATP QDs
2.3. Characterization of CdZnSeS-pATP QDs
2.4. Preparation of Aqueous Solutions with Standardized pH Values
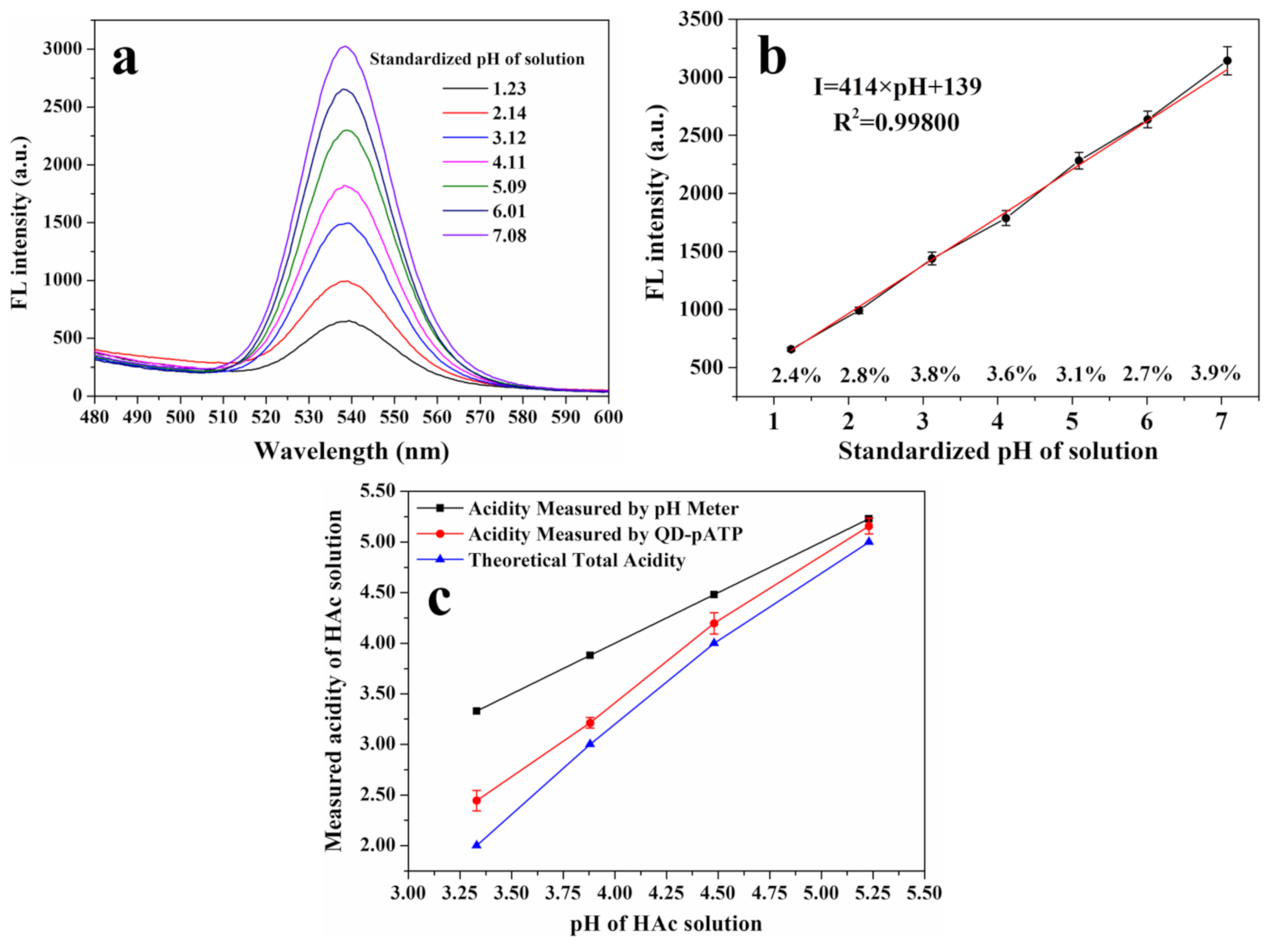
2.5. Establishment of Standard Curves for the Acidity Measurement of Aqueous Solutions
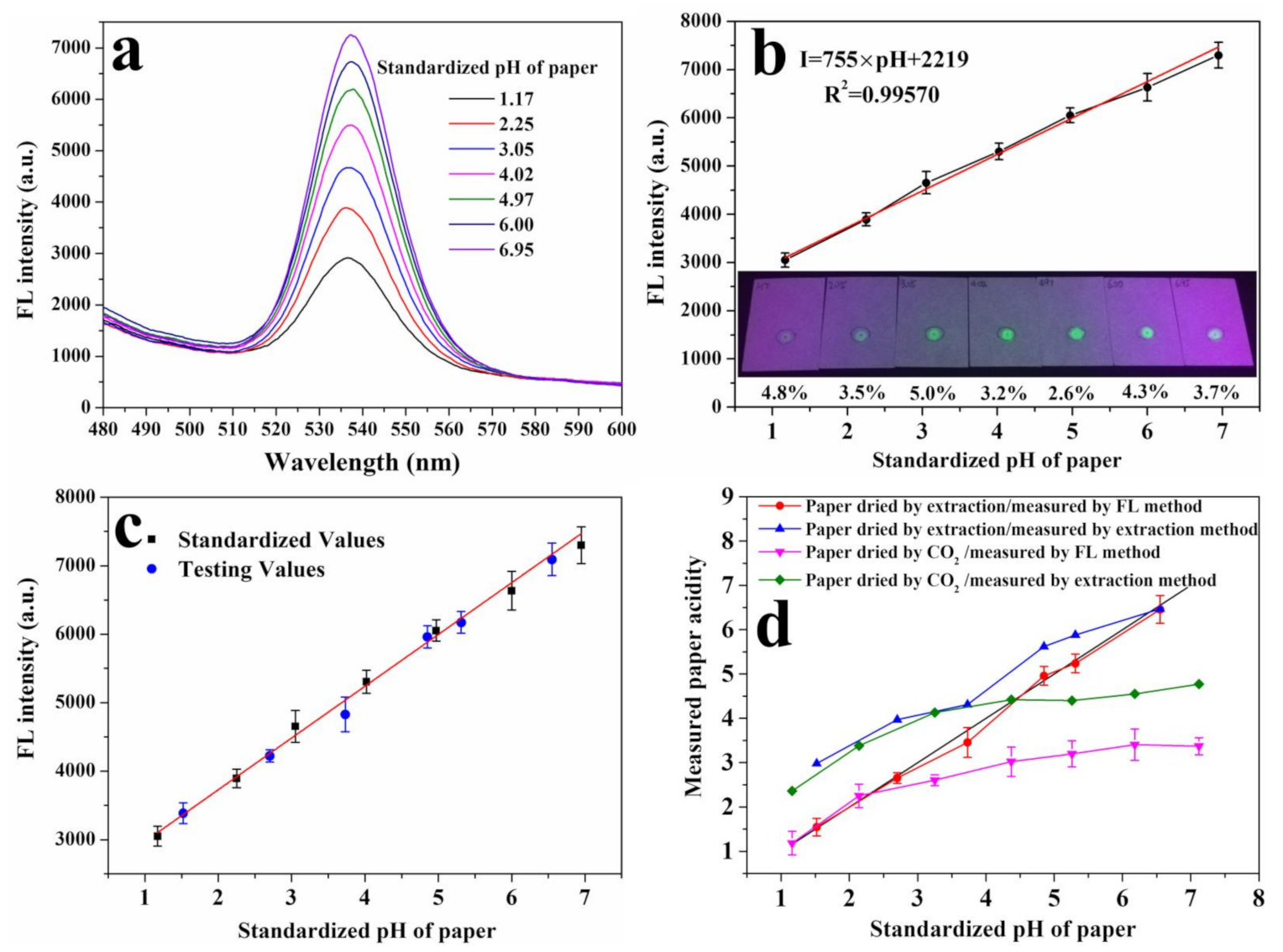
2.6. Establishment of a Standard Curve for the Acidity Measurement of Paper
2.7. Paper Acidity Measured by Using Fluorescence Spectroscopy and a pH Meter
3. Results and Discussion
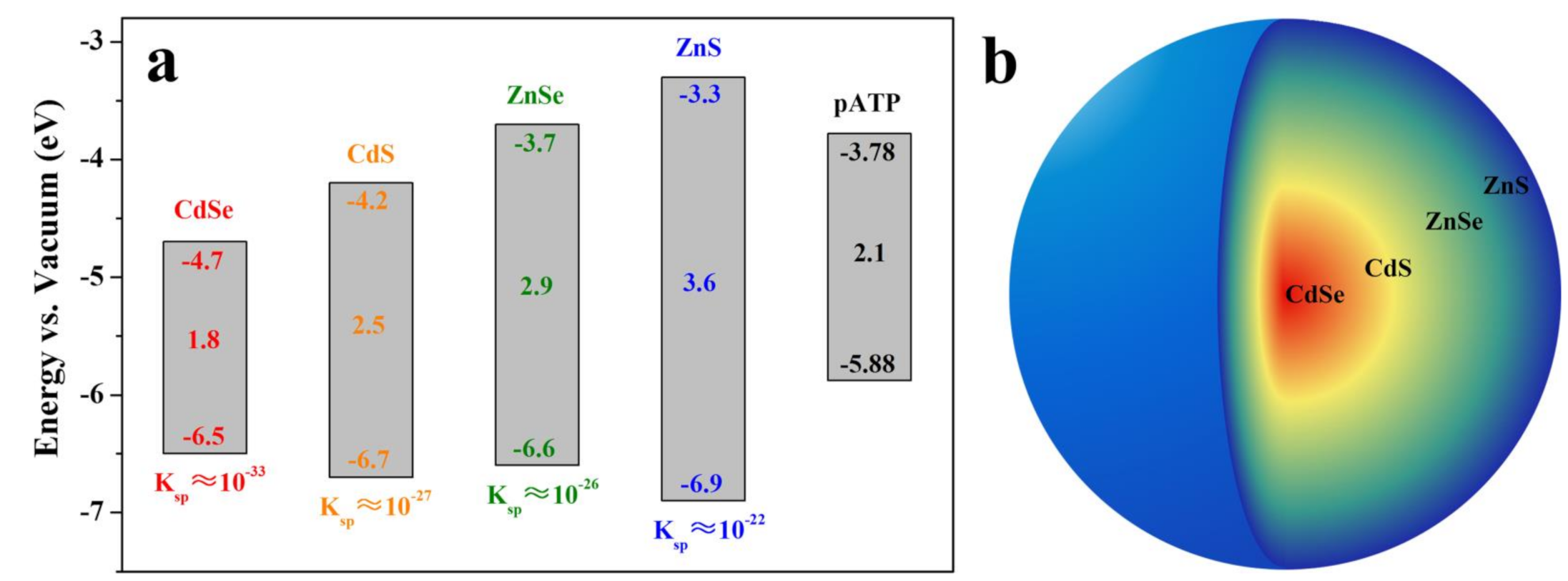
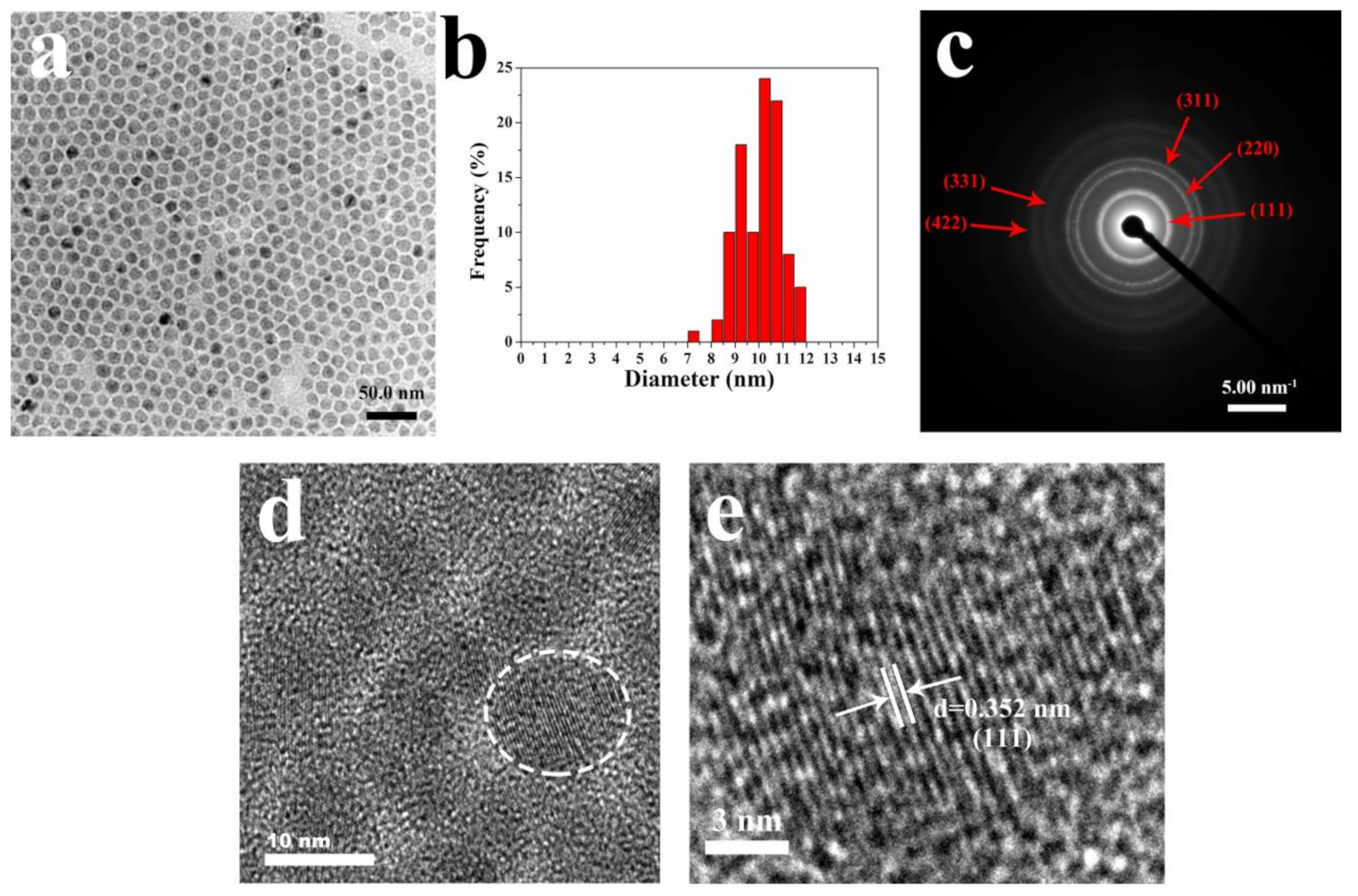
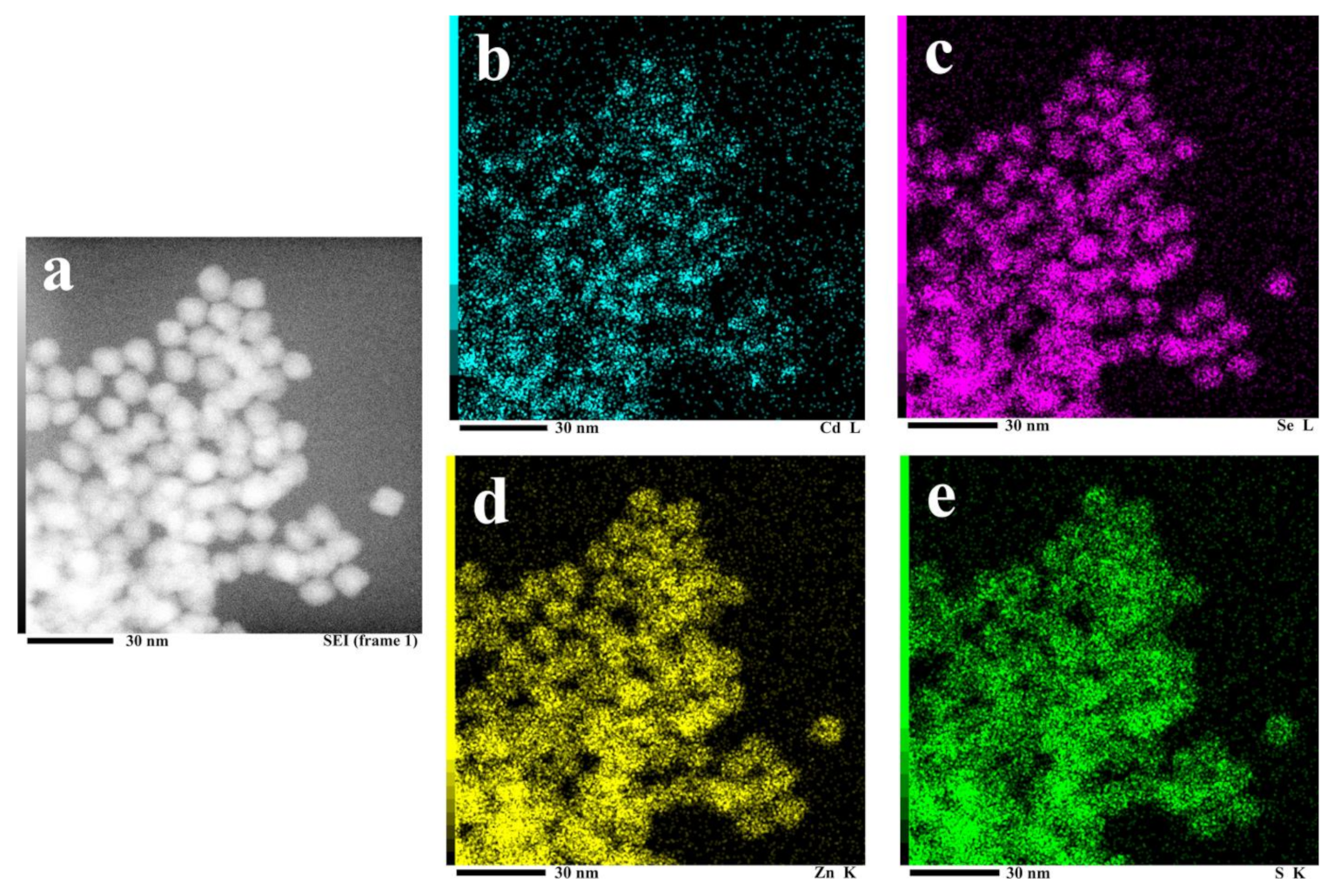
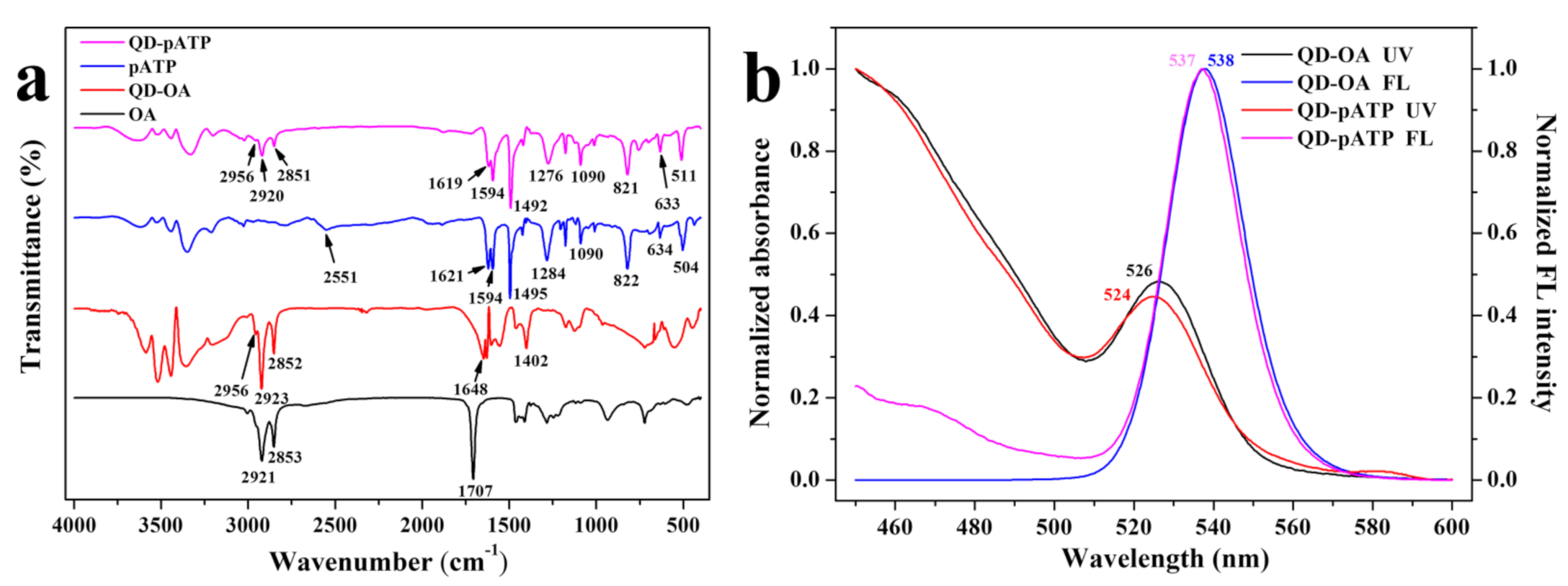
3.1. Composition and Spectral Characteristics of CdZnSeS-pATP QDs
3.2. Fluorescence Response of CdZnSeS-pATP QDs to Acidity
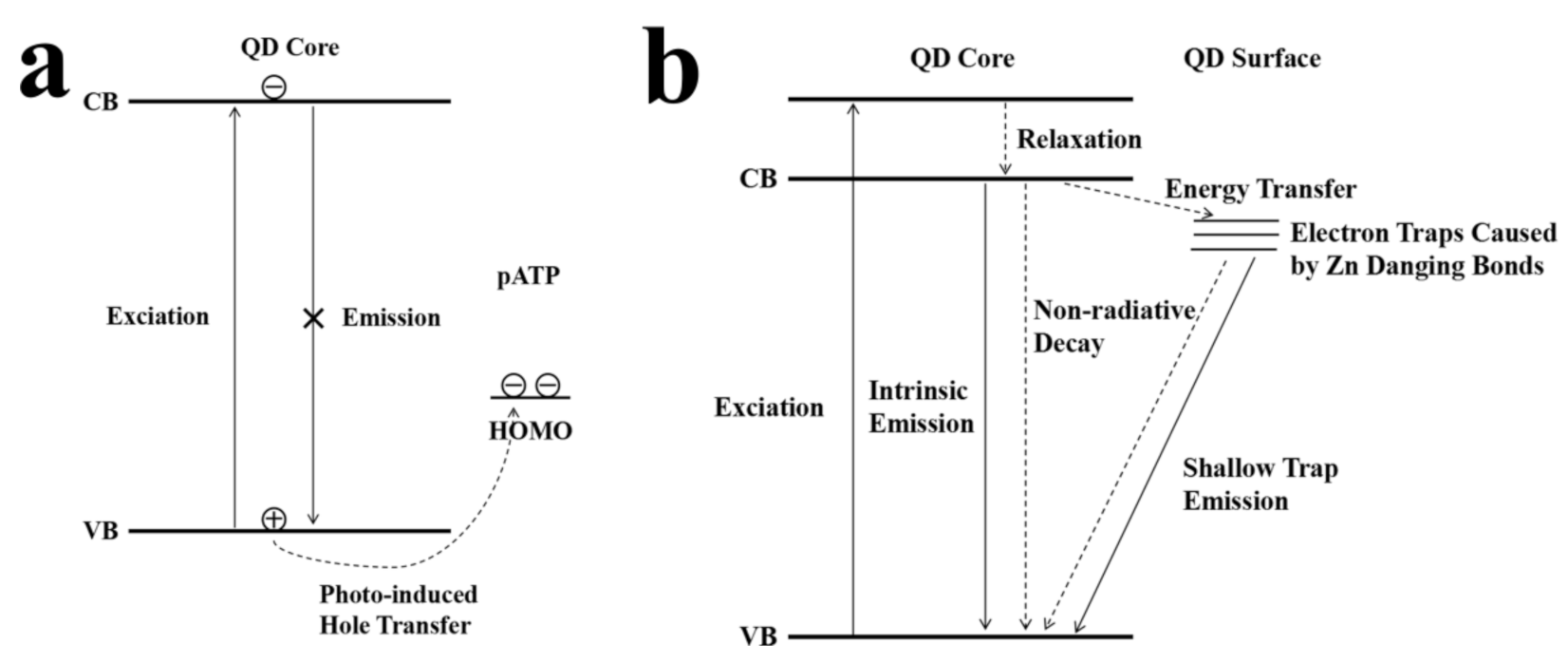
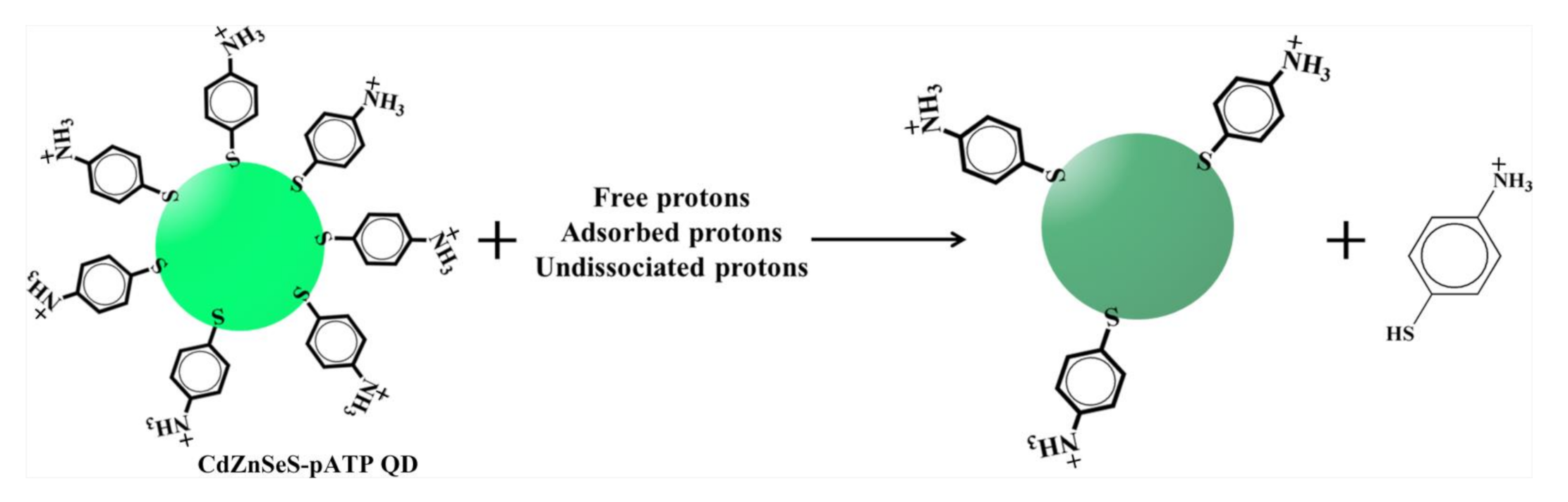
3.3. Mechanism of the Fluorescence Response of CdZnSeS-pATP QDs to Acidity
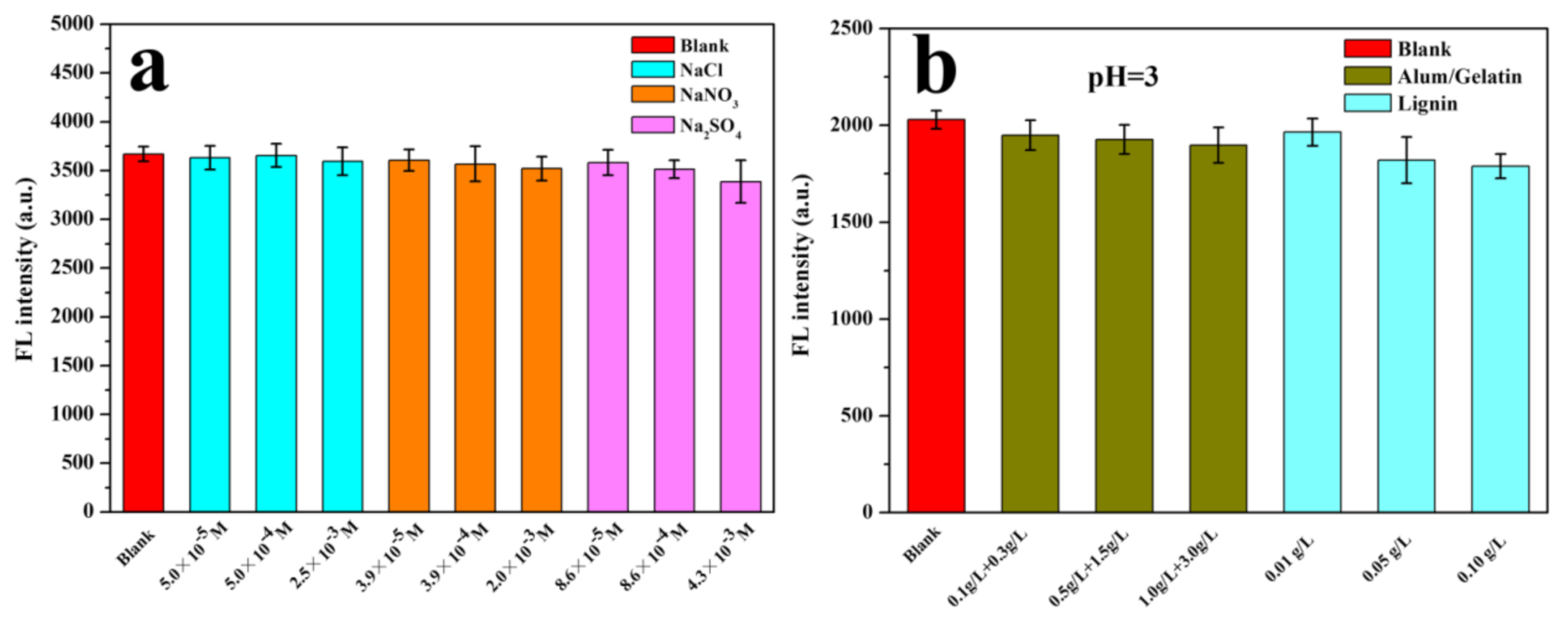
3.4. The Influences of Impurities in Paper on the Fluorescence of CdZnSeS-pATP QDs
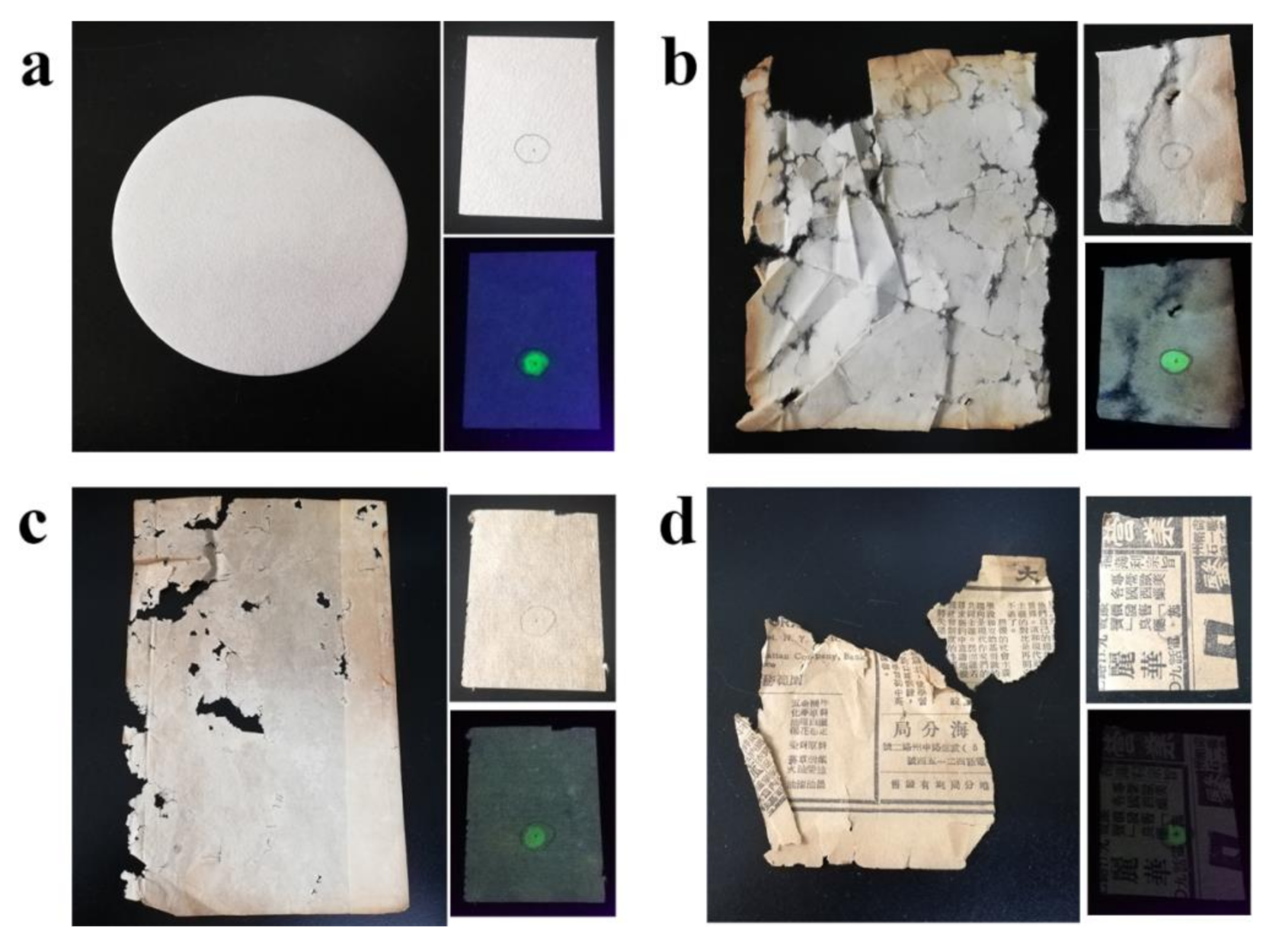
3.5. Detection of the Total Acidity of Paper-based Relics
4. Conclusions
- The prepared CdZnSeS-pATP QDs have a CdSe and CdS core, ZnSe middle shell, and ZnS outer shell. The alloy structure of CdZnSeS QDs could eliminate lattice mismatch to acquire high fluorescence quantum efficiency (88.9% for CdZnSeS-OA).
- OA ligand could be exchanged by pATP at the surface of CdZnSeS QDs to obtain specific optical properties concerning a response to acidity because of the dissociation of the pATP from the QDs under acidic conditions. The fluorescence response of CdZnSeS-pATP QDs to the acidity of paper samples has a wide acidity sensing range (1–7), high sensitivity (ca. 2.5-fold fluorescence intensity enhancement with pH values increasing from 1 to 7), good linearity (R2 = 0.99570) and excellent reproducibility (RSD of four measurements less than 5% at the same pH).
- A set of standard operations was implemented to determine paper acidity by using the fluorescence method, which can ensure excellent reproducibility. Additionally, the effects of some impurities, such as inorganic salts, gelatin-alum and lignin, on the fluorescence of CdZnSeS-pATP QDs are negligible.
- The acidity measured by the fluorescence method is higher than that by the cold extraction method in paper-based relics, which proves the conjecture that the cold extraction method detects only free protons, while the fluorescence method can detect the total acidity including free protons, adsorbed protons and undissociated protons in paper-based samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, B.; Lin, Q.; Chang, M.; Liu, C.; Fan, H.; Ren, J. A new and highly efficient conservation treatment for deacidification and strengthening of aging paper by in-situ quaternization. Carbohydr. Polym. 2019, 209, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Zou, X.; Uesaka, T.; Gurnagul, N. Prediction of paper permanence by accelerated aging II. Comparison of the predictions with natural aging results. Cellulose 1996, 3, 269–279. [Google Scholar] [CrossRef]
- Zou, X.; Uesaka, T.; Gurnagul, N. Prediction of paper permanence by accelerated aging I. Kinetic analysis of the aging process. Cellulose 1996, 3, 243–267. [Google Scholar] [CrossRef]
- Website for the International Organization for Standardization (ISO). Paper, Board and Pulps-Determination of pH of Aqueous Extracts-Part 1: Cold Extraction, ISO 6588-1:2012. Available online: https://www.iso.org/standard/59783.html (accessed on 29 June 2021).
- Website for the Technical Association of the Pulp & Paper Industry Inc. (TAPPI). Surface pH Measurement of Paper, TAPPI/ANSI T 529 om-14. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T529.aspx (accessed on 29 June 2021).
- Jiang, Z.; Fan, J.; Budarin, V.L.; Macquarrie, D.J.; Gao, Y.; Li, T.; Hu, C.; Clark, J.H. Mechanistic understanding of salt-assisted autocatalytic hydrolysis of cellulose. Sustain. Energy Fuels 2018, 2, 936–940. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, J.N.; Shevchenko, T.; Magon, A.; Bohne, C. Paper acidity estimation: Application of pH-dependent fluorescence probes. J. Photochem. Photobiol. A 1998, 113, 189–195. [Google Scholar] [CrossRef]
- Qu, Y.; Han, H.; Zheng, X.; Guo, Z.; Li, Y. Detection of surface pH of paper using a chitosan-modified silica fluorescent nanosensor. Sens. Actuators B 2014, 195, 252–258. [Google Scholar] [CrossRef]
- Rakhlin, M.; Sorokin, S.; Kazanov, D.; Sedova, I.; Shubina, T.; Ivanov, S.; Mikhailovskii, V.; Toropov, A. Bright single-photon emitters with a CdSe quantum dot and multimode tapered nanoantenna for the visible spectral range. Nanomaterials 2021, 11, 916. [Google Scholar] [CrossRef]
- Bailey, R.E.; Smith, A.M.; Nie, S. Quantum dots in biology and medicine. Physica E 2004, 25, 1–12. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef]
- Ji, B.; Koley, S.; Slobodkin, I.; Remennik, S.; Banin, U. ZnSe/ZnS core/shell quantum dots with superior optical properties through thermodynamic shell growth. Nano Let. 2020, 20, 2387–2395. [Google Scholar] [CrossRef]
- Jing, P.; Zheng, J.; Ikezawa, M.; Liu, X.; Lv, S.; Kong, X.; Zhao, J.; Masumoto, Y. Temperature-dependent photoluminescence of CdSe-core CdS/CdZnS/ZnS-multishell quantum dots. J. Phys. Chem. C 2009, 113, 13545–13550. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.; Li, D.; Wang, Y. p-Aminothiophenol-coated CdSe/ZnS quantum dots as a turn-on fluorescent probe for pH detection in aqueous media. Talanta 2017, 166, 54–62. [Google Scholar] [CrossRef]
- Kong, P.; Wang, R.; Zhang, C.; Du, Z.; Li, H.; Zou, W. Synpaper of 4-aminothiophenol functionalized quantum dots to sensitize silver nanowires and its application for solar cells. Synth. Met. 2017, 226, 50–55. [Google Scholar] [CrossRef]
- Website for the International Organization for Standardization (ISO). Reagents for Chemical Analysis-Part 1: General Test Methods. ISO 6353-1:1982. Available online: https://www.iso.org/standard/12656.html (accessed on 29 June 2021).
- Bae, W.K.; Char, K.; Hur, H.; Lee, S. Single-step synpaper of quantum dots with chemical composition gradients. Chem. Mater. 2008, 20, 531–539. [Google Scholar] [CrossRef]
- Deng, Z.; Yan, H.; Liu, Y. Band gap engineering of quaternary-alloyed ZnCdSSe quantum dots via a facile phosphine-free colloidal method. J. Am. Chem. Soc. 2009, 131, 17744–17745. [Google Scholar] [CrossRef]
- Song, J.; Wang, O.; Shen, H.; Lin, Q.; Li, Z.; Wang, L.; Zhang, X.; Li, L. Over 30% external quantum efficiency light-emitting diodes by engineering quantum dot-assisted energy level match for hole transport layer. Adv. Funct. Mater. 2019, 29, 1808377. [Google Scholar] [CrossRef]
- Albero, J.; Martínez-Ferrero, E.; Iacopino, D.; Vidal-Ferran, A.; Palomares, E. Interfacial charge transfer dynamics in CdSe/dipole molecules coated quantum dot polymer blends. Phys. Chem. Chem. Phys. 2010, 12, 13047–13051. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Yang, H.; Sun, H. Quaternary alloy quantum dots: Toward low-threshold stimulated emission and all-solution-processed lasers in the green region. Adv. Opt. Mater. 2015, 3, 652–657. [Google Scholar] [CrossRef]
- Kim, S.; Fisher, B.; Eisler, H.J.; Bawendi, M. Type-II quantum dots: CdTe/CdSe (core/shell) and CdSe/ZnTe (core/shell) heterostructures. J. Am. Chem. Soc. 2003, 125, 11466–11467. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: Spectroscopic observation of facile metal-carboxylate displacement and binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Wang, J.; Wu, L.; Zhu, J.; He, P.; Ouyang, Q.; Chen, Q. Design of physicochemical factors for regulating the retention mechanism of 4-Aminothiophenol in surface-enhanced raman scattering toward nitrite sensing. J. Phys. Chem. C 2020, 124, 7768–7776. [Google Scholar] [CrossRef]
- Saad, H.; Rahman, M.K.A.; Yassin, I.; Muad, A.M. Characterization of ethanol concentrations at ultraviolet wavelength region. J. Fundam. Appl. Sci. 2017, 9, 384–400. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Metge, D.W.; Ray, C.; Harvey, R.W.; Chorover, J. Surface complexation of carboxylate adheres cryptosporidium parvum oocysts to the hematite-water interface. Environ. Sci. Technol. 2009, 43, 7423–7429. [Google Scholar] [CrossRef]
- Yan, L.; Qi, X. Degradation of cellulose to organic acids in its homogeneous alkaline aqueous solution. ACS Sustain. Chem. Eng. 2014, 2, 897–901. [Google Scholar] [CrossRef]
- Zhang, Z.; Tompsett, G.A.; Granados-Focil, S.; Lambert, C.R.; Timko, M.T. Rational design of solid-acid catalysts for cellulose hydrolysis using colloidal theory. Phys. Chem. Chem. Phys. 2021, 23, 10236–10243. [Google Scholar] [CrossRef]
- Gulliver, D.M.; Lowry, G.V.; Gregory, K.B. Comparative study of effects of CO2 concentration and pH on microbial communities from a saline aquifer, a depleted oil reservoir, and a freshwater aquifer. Environ. Eng. Sci. 2016, 33, 806–816. [Google Scholar] [CrossRef]
- Klinger, C.; Trinkaus, V.; Wallocha, T. Novel carbon dioxide-based method for accurate determination of pH and pCO2 in mammalian cell culture processes. Processes 2020, 8, 520. [Google Scholar] [CrossRef]
- Begin, P.; Deschatelets, S.; Grattan, D.; Guanagul, N.; Iraci, J.; Kaminska, E.; Woods, D.; Zou, X. Effect of air pollutants on paper stability. Restaurator 1999, 20, 1–21. [Google Scholar] [CrossRef]
- Munro, A.M.; Ginger, D.S. Photoluminescence quenching of single CdSe nanocrystals by ligand adsorption. Nano Lett. 2008, 8, 2585–2590. [Google Scholar] [CrossRef]
- Lin, W.; Niu, Y.; Meng, R.; Huang, L.; Cao, H.; Zhang, Z.; Qin, H.; Peng, X. Shell-thickness dependent optical properties of CdSe/CdS core/shell nanocrystals coated with thiol ligands. Nano Res. 2016, 9, 260–271. [Google Scholar] [CrossRef]
- Liu, I.S.; Lo, H.H.; Chien, C.T.; Lin, Y.Y.; Chen, C.W.; Chen, Y.F.; Su, W.F.; Liou, S.C. Enhancing photoluminescence quenching and photoelectric properties of CdSe quantum dots with hole accepting ligands. J. Mater. Chem. 2008, 18, 675–682. [Google Scholar] [CrossRef]
- Wuister, S.F.; Donega, C.M.; Meijerink, A. Influence of thiol capping on the exciton luminescence and decay kinetics of CdTe and CdSe quantum dots. J. Phys. Chem. B. 2004, 108, 17393–17397. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Jin, S.; Hamers., R.J. Influence of hole-sequestering ligands on the photostability of CdSe quantum dots. J. Phys. Chem. C 2013, 117, 313–320. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Hoy, J.; Hu, F.; Liu, H.; Gentleman, M.M.; Sfeir, M.Y.; Misewich, J.A.; Wong, S.S. Probing differential optical and coverage behavior in nanotube-nanocrystal heterostructures synthesized by covalent versus non-covalent approaches. Dalton Trans. 2014, 43, 7480–7490. [Google Scholar] [CrossRef]
- Yang, J.; Deng, D.W.; Yu, J.S. Transfer from trap emission to band-edge one in water-soluble CdS nanocrystals. J. Colloid Interface Sci. 2013, 394, 55–62. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Shallow and deep trap emission and luminescence quenching of TiO2 nanoparticles on Cu doping. Appl. Nanosci. 2014, 4, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef]
- Aldana, J.; Lavelle, N.; Wang, Y.; Peng, X. Size-dependent dissociation pH of thiolate ligands from cadmium chalcogenide nanocrystals. J. Am. Chem. Soc. 2005, 127, 2496–2504. [Google Scholar] [CrossRef]
- Liu, Y.S.; Sun, Y.; Vernier, P.T.; Liang, C.H.; Chong, S.Y.C.; Gundersen, M.A. pH-sensitive photoluminescence of CdSe/ZnSe/ZnS quantum dots in human ovarian cancer cells. J. Phys. Chem. C 2007, 111, 2872–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lystrom, L.; Roberts, A.; Dandu, N.; Kilina, S. Surface-induced deprotonation of thiol ligands impacts the optical response of CdS quantum dots. Chem. Mater. 2021, 33, 892–901. [Google Scholar] [CrossRef]
- Bonengel, S.; Hauptstein, S.; Leonaviciute, G.; Griessinger, J.; Bernkop-Schnürch, A. Thiolated alkyl-modified carbomers: Novel excipients for mucoadhesive emulsions. Eur. J. Pharm. Sci. 2015, 75, 123–130. [Google Scholar] [CrossRef]
- Landi, B.J.; Evans, C.M.; Worman, J.J.; Castro, S.L.; Bailey, S.G.; Raffaelle, R.P. Noncovalent attachment of CdSe quantum dots to single wall carbon nanotubes. Mater. Lett. 2006, 60, 3502–3506. [Google Scholar] [CrossRef]
- Sellers, D.G.; Button, A.A.; Nasca, J.N.; Wolfe, G.E.; Chauhan, S., II; Watson, D.F. Excited-State charge transfer within covalently linked quantum dot heterostructures. J. Phys. Chem. C 2015, 119, 27737–27748. [Google Scholar] [CrossRef]
- Goswami, B.; Pal, S.; Sarkar, P. A theoretical study on the electronic structure of ZnSe/ZnS and ZnS/ZnSe core/shell nanoparticles. J. Phys. Chem. C 2008, 112, 11630–11636. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, S.; Xu, X.; Wang, Z.C.; Wang, Z.Y.; Wang, C.; Cui, Y. Co-doping of Ag into Mn:ZnSe quantum dots: Giving optical filtering effect with improved monochromaticity. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Wang, J. Discovering the effect of alum on UV photo-degradation of gelatin binder via FTIR, XPS and DFT calculation. Microchem. J. 2019, 149, 103934. [Google Scholar] [CrossRef]
- Małachowska, E.; Dubowik, M.; Boruszewski, P.; Łojewska, J.; Przybysz, P. Influence of lignin content in cellulose pulp on paper durability. Sci. Rep. 2020, 10, 19998. [Google Scholar] [CrossRef]
- Catcher, S.; Chang, G.; Zhu, Q. The problem of Chinese paper reinforcement strip repairs on a set of four hanging calligraphic scrolls. J. Inst. Conserv. 2017, 40, 49–63. [Google Scholar] [CrossRef]
- Dong, L.Y.; Zhu, Y.J. Fire-resistant inorganic analogous xuan paper with thousands of years super-durability. ACS Sustain. Chem. Eng. 2018, 6, 17239–17251. [Google Scholar] [CrossRef]
- Zhong, Z.; Peng, X. Study on the manufacturing technology of Guangdong hand-made paper. IOP Conf. Ser. Earth Environ. Sci. 2019, 358, 042064. [Google Scholar]
- Zhang, D.; Li, X.; Zhao, J. Fusarium concolor X4 pretreatment suppresses light-induced yellowing of high-yield pulp. Biomed Res. Int. 2020, 2020, 9497215. [Google Scholar] [CrossRef]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for white natural sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, J.; Cheng, Z.; Yang, R. Effective improvement of the Chinese ink diffusion properties of Xuan paper by cellulose microfibrils-precipitated calcium carbonate composite filler. Cellulose 2020, 27, 1695–1704. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, B.; Liu, S.; Tian, T.; Xv, G.; Cai, B. An optical fiber-waveguide-fiber platform for ppt level evanescent field-based sensing. Sens. Actuators B 2020, 306, 127548. [Google Scholar] [CrossRef]

| Sample | Solvent | Quantum Yield | τ1(ƒ1) | τ2(ƒ2) | τavg (ns) | R-Square |
|---|---|---|---|---|---|---|
| QD-OA | Chloroform | 0.889 | 13.92(0.971) | 46.28(0.029) | 16.84 | 0.99715 |
| QD-pATP | Chloroform | 0.340 | 5.31(0.591) | 11.70(0.409) | 9.17 | 0.99799 |
| QD-pATP(pH = 1.31) | DMSO/Water(1:1) | 0.041 | 1.73(0.359) | 6.40(0.641) | 5.79 | 0.99532 |
| QD-pATP(pH = 4.11) | DMSO/Water(1:1) | 0.278 | 3.93(0.485) | 8.19(0.515) | 6.87 | 0.99639 |
| QD-pATP(pH = 6.98) | DMSO/Water(1:1) | 0.295 | 4.20(0.554) | 8.81(0.446) | 7.10 | 0.99279 |
| Sample | Paper Acidity Measured by FL Method 1 | Paper Acidity Measured by Cold Extraction Method | Difference |
|---|---|---|---|
| Filter paper | 6.50 ± 0.19 | 6.72 | 0.22 |
| Mianlian paper | 5.33 ± 0.31 | 5.75 | 0.42 |
| Bamboo paper | 3.89 ± 0.26 | 4.74 | 0.85 |
| Newspaper | 3.14 ± 0.43 | 4.38 | 1.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Cheng, C.; Cheng, Y.; Zheng, L.; Hu, D. Quaternary Alloy Quantum Dots as Fluorescence Probes for Total Acidity Detection of Paper-Based Relics. Nanomaterials 2021, 11, 1726. https://doi.org/10.3390/nano11071726
Wang Z, Cheng C, Cheng Y, Zheng L, Hu D. Quaternary Alloy Quantum Dots as Fluorescence Probes for Total Acidity Detection of Paper-Based Relics. Nanomaterials. 2021; 11(7):1726. https://doi.org/10.3390/nano11071726
Chicago/Turabian StyleWang, Zhuorui, Cong Cheng, Yongjuan Cheng, Lizhen Zheng, and Daodao Hu. 2021. "Quaternary Alloy Quantum Dots as Fluorescence Probes for Total Acidity Detection of Paper-Based Relics" Nanomaterials 11, no. 7: 1726. https://doi.org/10.3390/nano11071726
APA StyleWang, Z., Cheng, C., Cheng, Y., Zheng, L., & Hu, D. (2021). Quaternary Alloy Quantum Dots as Fluorescence Probes for Total Acidity Detection of Paper-Based Relics. Nanomaterials, 11(7), 1726. https://doi.org/10.3390/nano11071726






