Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers
Abstract
1. Introduction
2. Materials and Methods
2.1. Acrylic Samples Preparation
2.2. Acrylic Samples Investigations
2.2.1. Cross Polarized Light Microscopy (CPOM)
2.2.2. FT-IR Spectroscopy
2.2.3. Scanning Electron Microscopy
2.3. In Vitro Testing of the Acrylic Samples’ Toxicity
2.4. Antibacterial Assays
2.5. Scanning Electron Microscopy (SEM) Investigations of the Bacterial Cells
2.6. Statistical Analysis
3. Results
3.1. Acrylic Samples Investigations
3.1.1. Cross Polarized Light Microscopy
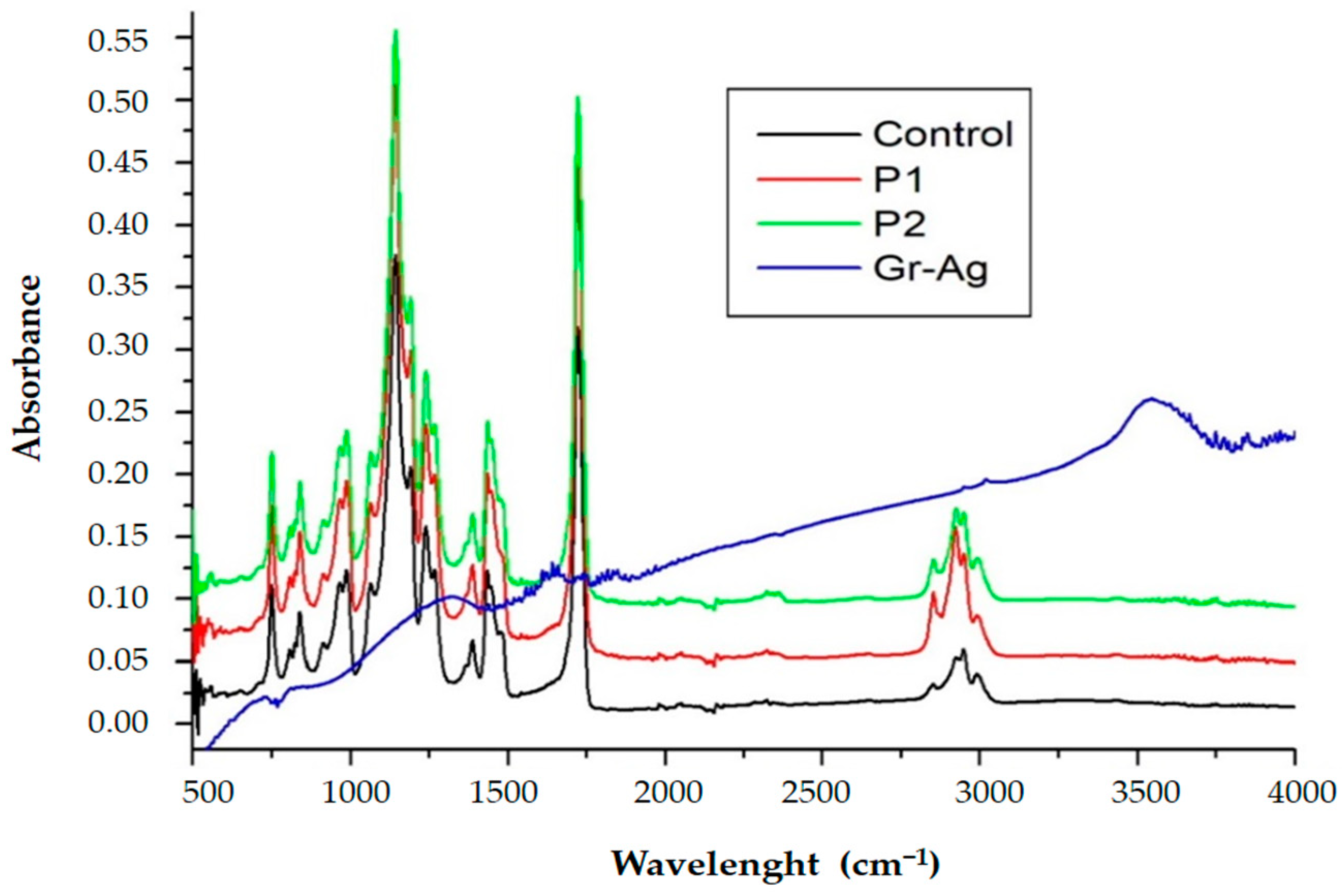
3.1.2. FTIR Spectroscopy
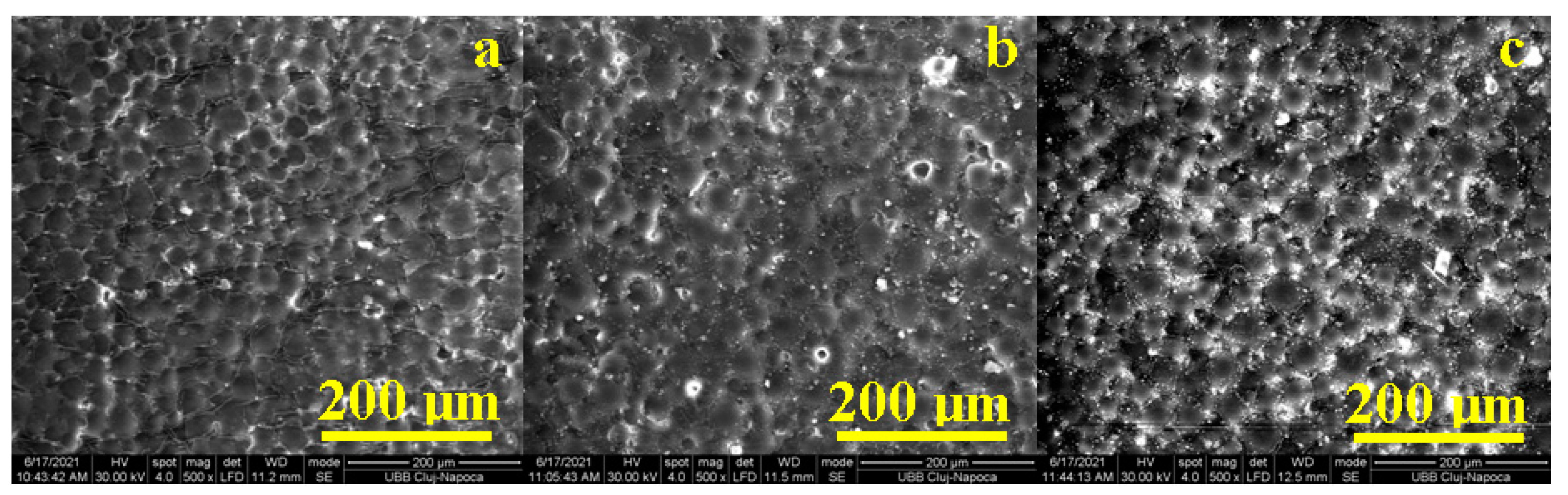
3.1.3. Scanning Electron Microscopy Investigations of the Acrylic Samples
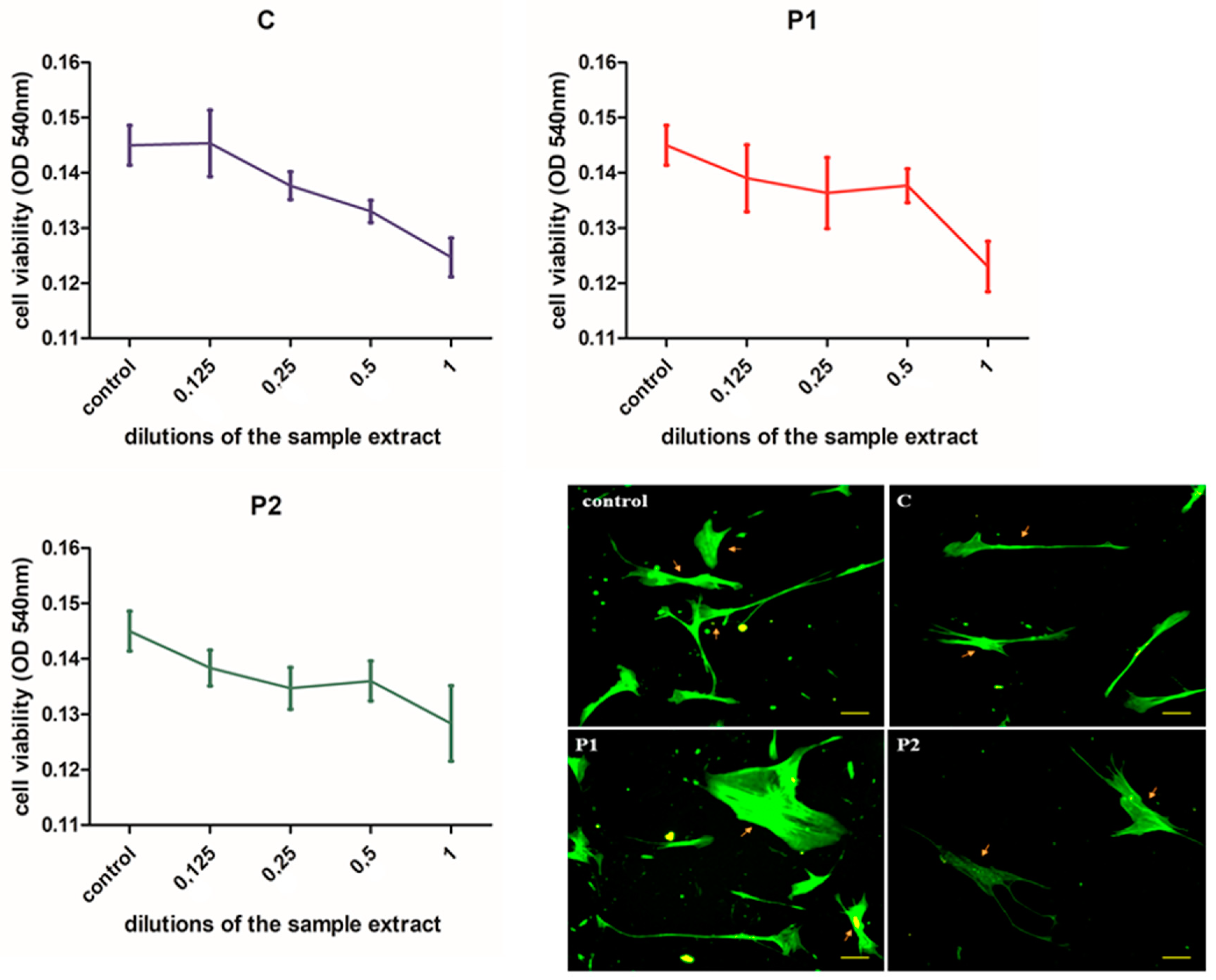
3.2. Cell Viability
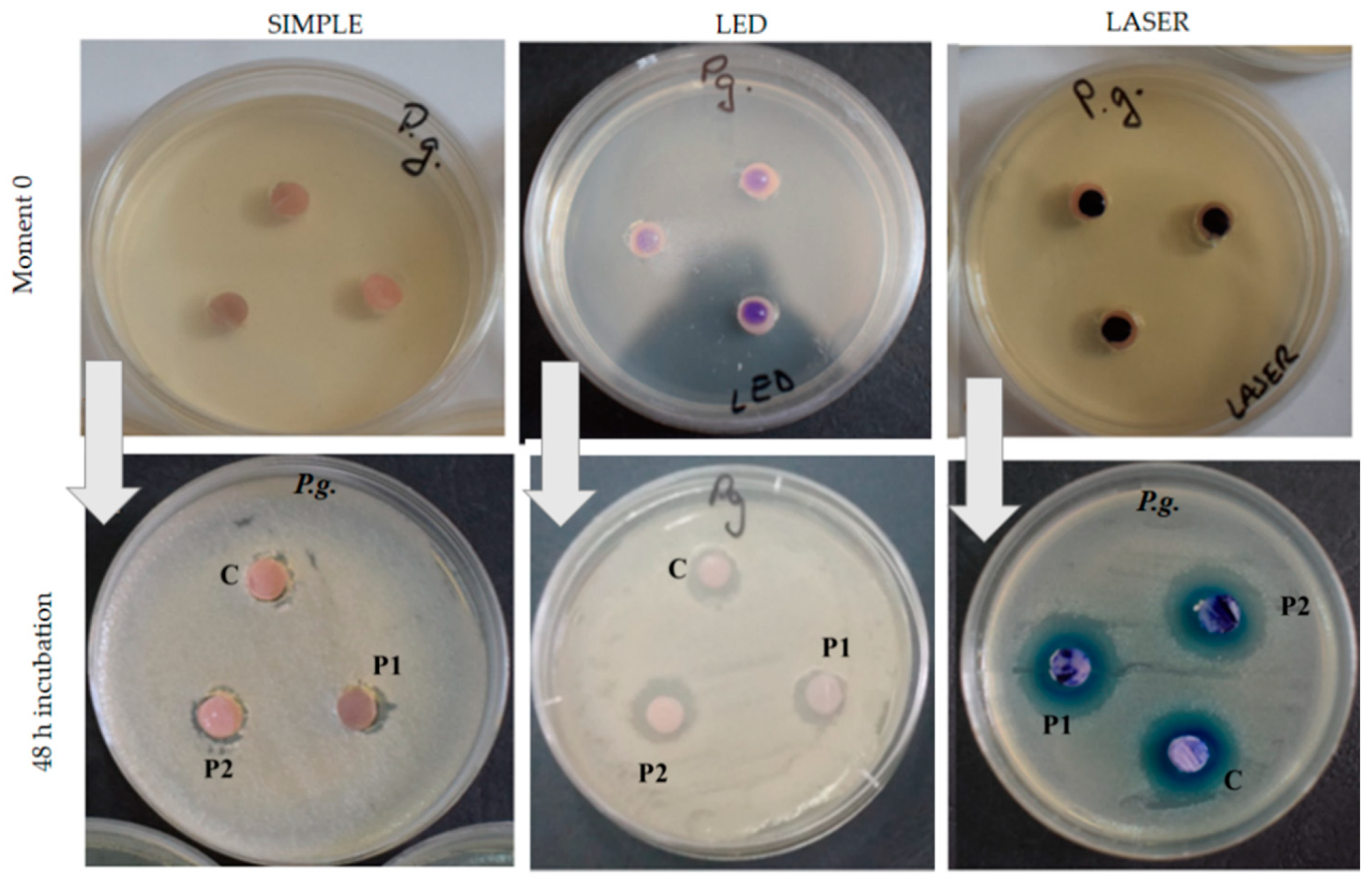
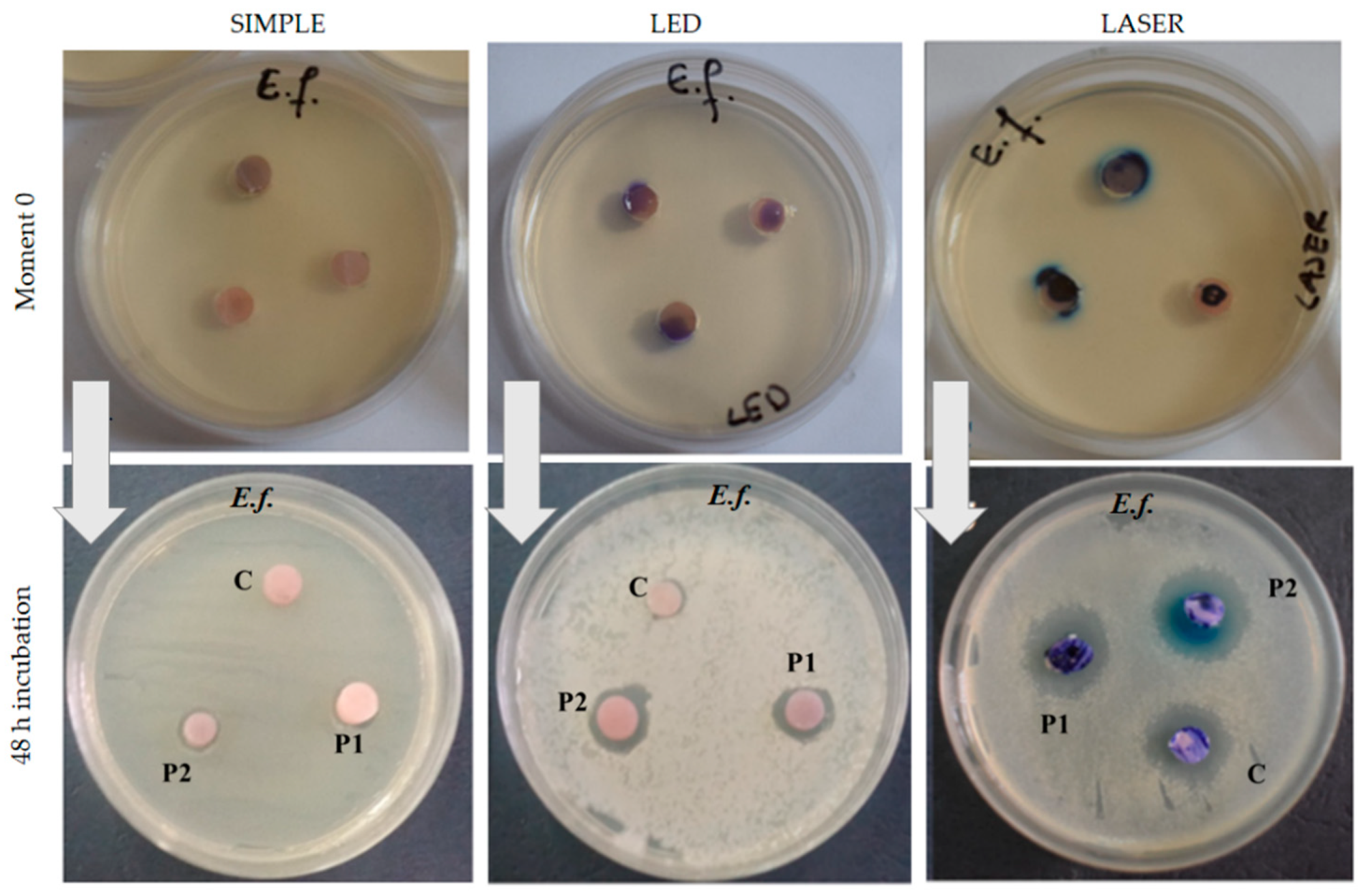
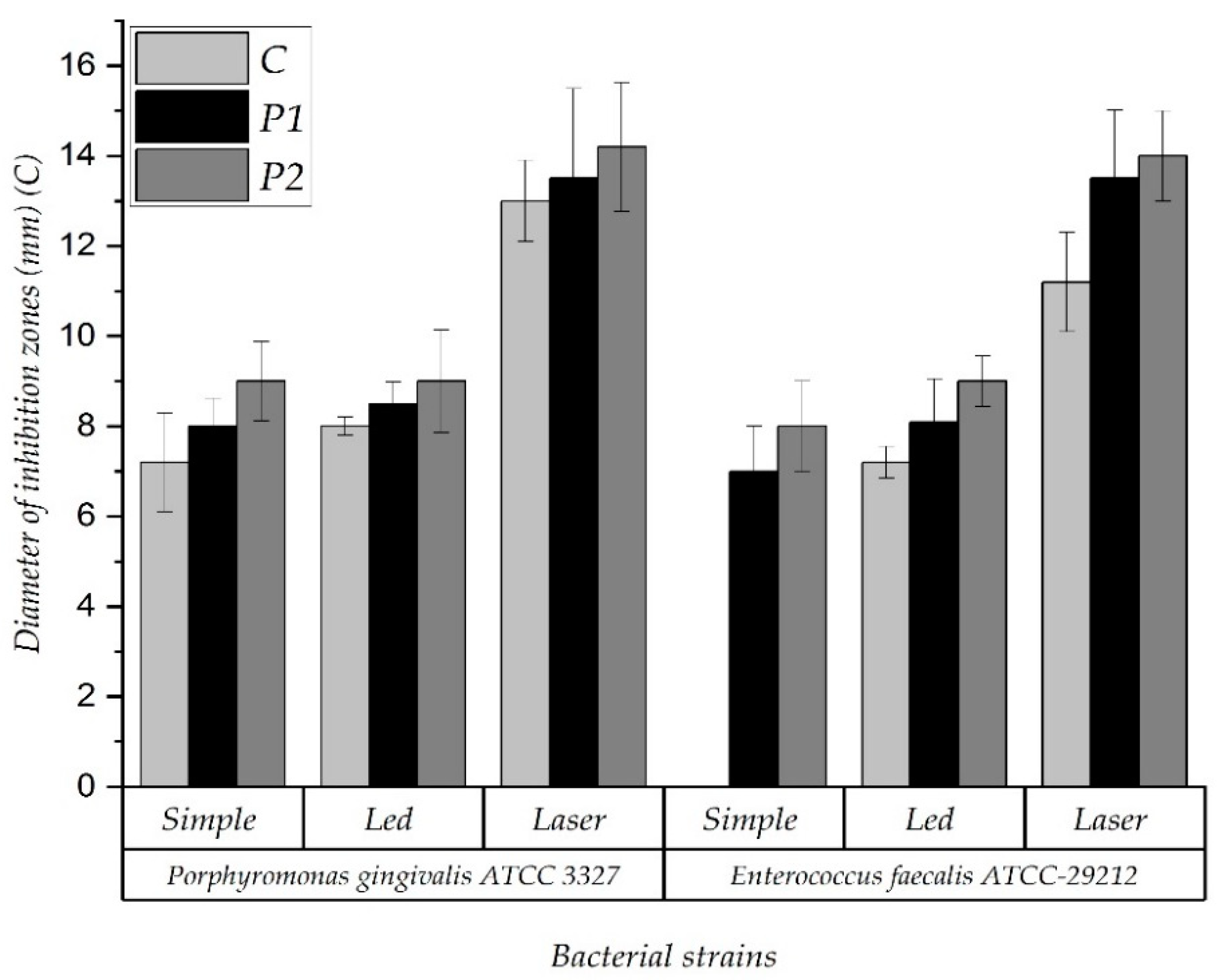
3.3. Antimicrobial Effect of the Acrylic Samples
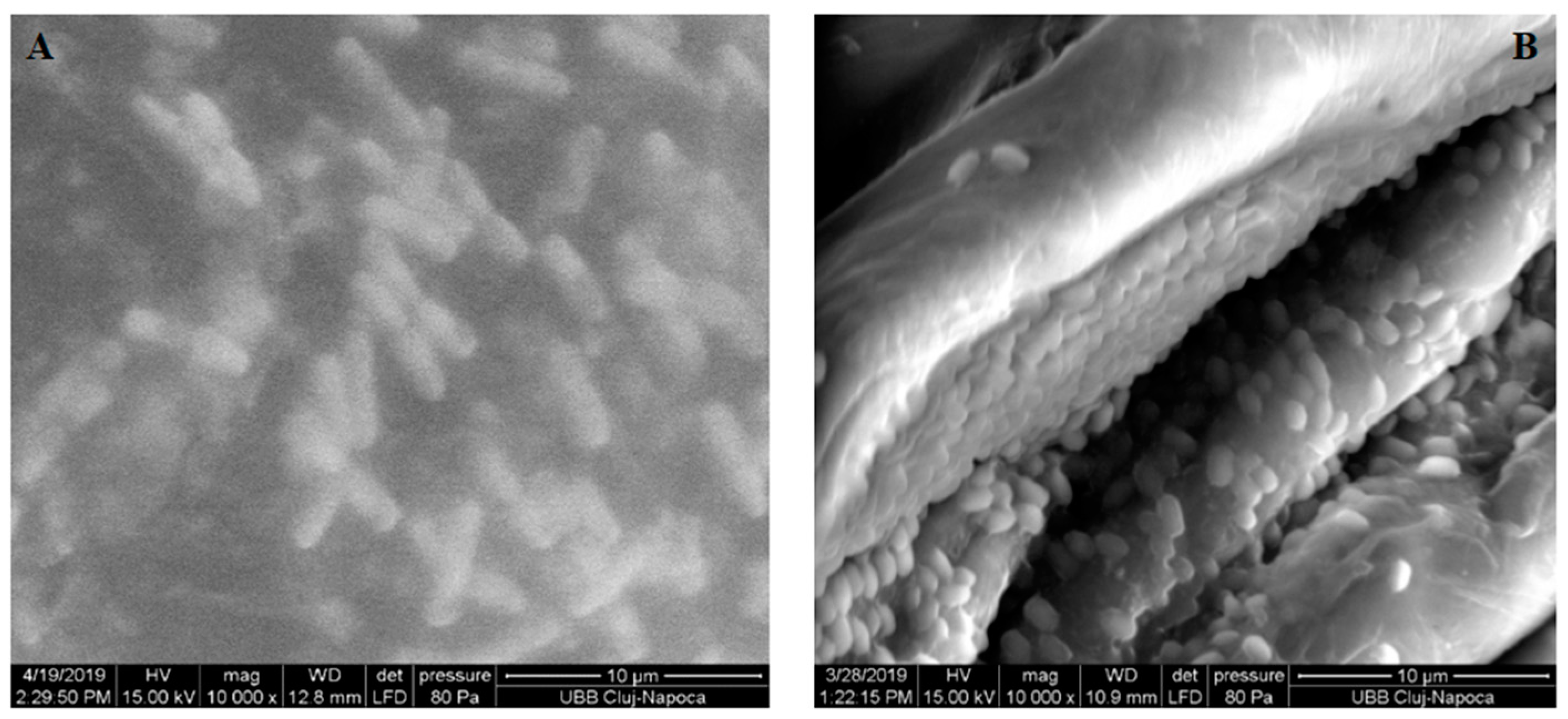
3.4. Scanning Electron Microscopy Investigations of the Bacterial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Coulthwaite, L.; Verrain, J. Potential pathogenic aspects of denture plaque. Br. J. Biom. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef]
- Sumi, Y.; Kagami, H.; Ohtsuka, Y.; Kakinoki, Y.; Haruguchi, Y.; Miyamoto, H. High correlation between the bacterial species in denture and pharyngeal microflora. Gerodontology 2003, 20, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Samnieng, P.; Palasuk, J.; Zaitsu, T.; Ueno, M.; Yoko, K. Oral malodor and its related factors in edentate elderly with and without dentures. Int. J. Clin. Prev. Dent. 2015, 11, 217–224. [Google Scholar] [CrossRef]
- Koshimune, S.; Awano, S.; Gohara, K.; Kurihara, E.; Ansai, T.; Takehara, T. Low salivary flow and volatile sulfur compounds in mouth air. Oral surgery, oral medicine, oral pathology. Oral Radiol. Endod. 2003, 96, 38–41. [Google Scholar] [CrossRef]
- Kleinberg, I.; Wolff, M.; Codipilly, D. Role of saliva in oral dryness, oral feel and oral malodour. Int. Dent. J. 2002, 52, 236–240. [Google Scholar] [CrossRef]
- Krespi, Y.; Shrime, M.; Kacker, A. The relationship between oral malodor and volatile sulfur compound-producing bacteria. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2006, 135, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Devji, T. Professional tooth cleaning and scaling and root planing with oral hygiene instructions may reduce volatile sulfur compounds in patients with periodontal disease. J. Am. Dent. Assoc. 2018, 149, e26. [Google Scholar] [CrossRef]
- Shagana, J.A. Evaluation of bacterial loads in removable dentures: A review. J. Pharm. Sci. Res. 2016, 8, 1117–1118. [Google Scholar]
- Rostoka, D.; Kroiča, J.; Iriste, V.; Reinis, A.; Kuznetsova, V.; Teibe, U. Treatment of halitosis with mouth rinsing agents containing essential oils. Stomatologia 2012, 91, 27–34. [Google Scholar]
- Köroğlu, A.; Şahin, O.; Kürkçüoğlu, I.; Dede, D.Ö.; Özdemir, T.; Hazer, B. Silver nanoparticle incorporation effect on mechanical and thermal properties of denture base acrylic resins. J. Appl. Oral Sci. 2016, 24, 590–596. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Fang, J.; Wang, C.; Li, Y.; Zhao, Z.; Mei, L. Comparison of bacterial adhesion to dental materials of polyethyleneterephthalate (PET) and polymethyl methacrylate (PMMA) using atomic force microscopy and scanning electron microscopy. Scanning 2016, 38, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.; Enoki, C.; Ito, I.Y.; Faria, G.; Matsumoto, M.A.; Nelson-Filho, P. In-vivo evaluation of the bacterial contamination and disinfection of acrylic baseplates of removable orthodontic appliances. Am. J. Orthod. Dentofacial. Orthop. 2007, 131, 705.e11–705.e17. [Google Scholar] [CrossRef]
- Paolone, G.; Moratti, E.; Goracci, C.; Gherlone, E.; Vichi, A. Effect of finishing systems on surface roughness and gloss of full-body bulk resin -fill composites. Materials 2020, 13, 5657. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Ferracane, J.L.; Paolone, G.; Brambilla, E. In vitro biofilm formation on resin-based composites under different surface conditions. J. Dent. 2018, 77, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Juan Carlos, F.A.; Rene, G.C.; Germán, V.S.; Laura Susana, A.T. Antimicrobial Poly (methyl methacrylate) with Silver Nanoparticles for Dentistry: A Systematic Review. Appl. Sci. 2020, 10, 4007. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Lee, M.C.; Lin, Z.I.; Lee, C.A.; Tung, Y.C.; Lou, C.W.; Law, W.C.; Chen, N.T.; Lin, K.A.; Lin, J.H. Intensifying the Antimicrobial Activity of Poly[2-(tert-butylamino)ethyl Methacrylate]/Polylactide Composites by Tailoring Their Chemical and Physical Structures. Mol. Pharm. 2019, 16, 709–723. [Google Scholar] [CrossRef]
- Hoque, J.; Adhikary, U.; Yadav, V.; Samaddar, S.; Konai, M.M.; Prakas RGParamanandham, K.; Shome, B.R.; Sanyal, K.; Haldar, J. Chitosan derivatives active against multidrug-resistant bacteria and pathogenic fungi. In vivo evaluation as topical antimicrobials. Mol. Pharm. 2016, 13, 3578–3589. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Lombardo, G.; Marinucci, L.; Kenny, J.M.; Torre, L.; Cianetti, S. Effect of nanohydroxyapatite, antibiotic, and mucosal defensive agent on the mechanical and thermal properties of glass ionomer cements for special needs patients. J. Mater. Res. 2018, 33, 638–649. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Massey, V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 2000, 28, 283–296. [Google Scholar] [CrossRef]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and late onset side effects of photodynamic therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef]
- Sterer, N.; Feuerstein, O. Effect of visible light on malodour production by mixed oral microflora. J. Med. Microbiol. 2005, 54 Pt 12, 1225–1229. [Google Scholar] [CrossRef]
- Soukos, N.S.; Som, S.; Abernethy, A.D.; Ruggiero, K.; Dunham, J.; Lee, C.; Doukas, A.; Goodson, J.M. Phototargeting Oral Black-Pigmented Bacteria. Antimicrob. Agents Chemother. 2005, 49, 1391–1396. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef] [PubMed]
- Biris, A.R.; Dervishi, E.; Ardelean, S.; Lazar, M.D.; Watanabe, F.; Biris, G.L.; Misan, I.; Biris, A.S. Synthesis of Ag-decorated, few-layer graphene structures over a novel Ag/MgO catalytic system by radio-frequency chemical vapor deposition. Mater. Chem. Phys. 2013, 138, 454–461. [Google Scholar] [CrossRef]
- ISO 10993-12; 2012 Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2012.
- Carpa, R.; Dragan-Bularda, M.; Muntean, V. General Microbiology-Practical Guide; Presa Universitara Clujeana: Cluj-Napoca, Romania, 2014; pp. 58–61. (In Romanian) [Google Scholar]
- Rodríguez-León, E.; Íñiguez-Palomares, R.; Urrutia-Bañuelos, E.; Herrera-Urbina, R.; Tánori JMaldonado, A. Self-alignment of silver nanoparticles in highly ordered 2D arrays. Nanoscale Res. Lett. 2015, 10, 1–7. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef]
- Gyanaranjan, S.; Niladri, S.; Sahua, D.; Swain, S.K. Nano gold decorated reduced graphene oxide wrapped polymethylmethacrylate for supercapacitor applications. RSC Adv. 2017, 7, 2137–2150. [Google Scholar]
- Richter, J.L. Diagnostic and treatment of halitosis. Compend. Contin. Educ. Dent. 1996, 17, 370–372, 374–376. [Google Scholar]
- Kossioni, A.E.; Hajto-Bryk, J.; Janssens, B.; Maggi, S.; Marchini, L.; MCkenna, G.; Müller, F.; Petrovic, M.; Roller-Wirnsberger, R.E.; Schimmel, M.; et al. Practical guidelines for physicians in promoting oral health in frail older adults. J. Am. Med. Dir. Assoc. 2018, 19, 1039–1046. [Google Scholar] [CrossRef]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef]
- Liu, J.; Ling, J.Q.; Wu, C.D. Cetylpyridinium chloride suppresses gene expression associated with halitosis. Arch. Oral Biol. 2013, 58, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gu, J.-Y.; Zhang, Y.-Y.; Gong, D.-J.; Zhu, Y.-M.; Sun, Y. Tolerance induced by Porphyromonas gingivalis may occur independently of TLR2 and TLR4. PLoS ONE 2018, 13, e0200946. [Google Scholar] [CrossRef]
- Colaco, A.S. Extreme resistance of Enterococcus faecalis and its role in endodontic treatment failure. Prog. Med. Sci. 2018, 2, 9–13. [Google Scholar] [CrossRef]
- Kumari, S.V.; Taruna, M.; Chittaranjan, B.; Reddy, S.M.; Reddy, K.K.; Kulkarni, G. A qualitative analysis to compare the effects of surface machining of conventional denture base resin and two soft liners: A scanning electron microscopic study. J. Clin. Diagn. Res. 2015, 9, ZC30–ZC34. [Google Scholar] [CrossRef]
- Şuhani, M.F.; Băciuţ, G.; Băciuţ, M.; Şuhani, R.; Bran, S. Current perspectives regarding the application and incorporation of silver nanoparticles into dental biomaterials. Clujul Med. 2018, 91, 274–279. [Google Scholar] [CrossRef]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agen. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef]
- Vallé, C.; Kinloch, I.A.; Young RJWilson, N.R.; Rourke, J.P. Graphene oxide and base-washed graphene oxide as reinforcements in PMMA nanocomposites. Compos. Sci. Technol. 2013, 88, 158–164. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, Z.; Yu, Y.; Shao, C.; Suo, Y.; Wei, X.; Zhou, Y. Toluidine blue O and porphyrin-mediated photodynamic therapy on three main pathogenic bacteria of periodontitis using portable LED phototherapy device. J. Innov. Opt. Health Sci. 2015, 8, 1550017. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hassan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Gambarini, G.; Plotino, G.; Grande, N.; Nocca, G.; Lupi, A.; Giardina, B.; De Luca, M. TestarellI. In vitro evaluation of the cytotoxicity of FotoSan light-activated disinfection on human fibroblasts. Med. Sci. Monit. 2011, 17, MT21–MT25. [Google Scholar] [CrossRef]
- Kato, H. History of photodynamic therapy past, present and future. Gan Kagaku Ryoho 1996, 23, 8–15. [Google Scholar]
- Javed, F.; Samaranayake, L.P.; Romanos, G.E. Treatment of oral fungal infections using antimicrobial photodynamic therapy: A systematic review of currently available evidence. Photochem. Photobiol. Sci. 2014, 13, 726–734. [Google Scholar] [CrossRef]
- Mima, E.G.; Vergani, C.E.; Machado, A.L.; Massucato, E.M.S.; Colombo, A.L.; Bagnato, V.S.; Pavarina, A.C. Comparison of photodynamic therapy versus conventional antifungal therapy for the treatment of denture stomatitis: A randomized clinical study. Clin. Microbiol. Infect. 2012, 18, E380–E388. [Google Scholar] [CrossRef]
- Amoian, B.; Mirzaee, A.; Hosseini, S.M. Assessment of the effect of photodynamic therapy on treatment of moderate-to-severe periodontitis; a randomized clinical trial study. Glob. J. Health Sci. 2016, 9, 83. [Google Scholar] [CrossRef]
- Feuerstein, O.; Persman, N.; Weiss, E.I. Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: An in vitro study. Photochem. Photobiol. 2004, 80, 412–415. [Google Scholar] [CrossRef]
- Fukui, M.; Yoshioka, M.; Satomura, K.; Nakanishi, H.; Nagayama, M. Specific-wavelength visible light irradiation inhibits bacterial growth of Porphyromonas gingivalis. J. Periodontal. Res. 2008, 43, 174–178. [Google Scholar] [CrossRef]
- Chan, Y.; Lai, C.H. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med. Sci. 2003, 18, 51–55. [Google Scholar] [CrossRef]
- Tennert, C.; Feldmann, K.; Haamann, E.; AL-Ahmad, A.; Follo, M.; Wrbas, K.T.; Hellwig, E.; Altenburger, M.J. Effect of photodynamic therapy (PDT) on Enterococcus faecalis biofilm in experimental primary and secondary endodontic infections. BMC Oral Health 2014, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, L.; Fuste, E.; Martinez-Garriga, B.; Arnabat-Dominguez, J.; Vinuesa, T.; Vinas, M. Effects of photodynamic therapy on Enterococcus faecalis biofilms. Lasers Med. Sci. 2015, 30, 1519–1526. [Google Scholar] [CrossRef]
- Jurič, I.B.; Anić, I. The use of lasers in disinfection and cleanliness of root canals: A review. Acta Stomatol. Croat. 2014, 48, 6–15. [Google Scholar] [CrossRef]
- Komerick, N.; Curnow, A.; Macrobert, A.J.; Hopper, C.; Speight, P.M.; Wilson, M. Fluorescence biodistribution and photosensitizing activity of toluidine blue on rat buccal mucosa. Lasers Med. Sci. 2002, 17, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.M.; Riaz, I.B.; Kamal, M.U.; Paragh, G.; Zeitouni, N.C. Photodynamic therapy and pain: A systematic review. Photodiagnosis Photodyn. Ther. 2017, 19, 308–344. [Google Scholar] [CrossRef]
- Kraigsley, A.M.; Tang, K.; Lippa, K.A.; Howarter, J.; Gibson, S.L.; Lin, N.J. Effect of polymer degree of conversion on Streptococcus mutans biofilms. Macromol. Biosci 2012, 12, 1706–1713. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacali, C.; Carpa, R.; Buduru, S.; Moldovan, M.L.; Baldea, I.; Constantin, A.; Moldovan, M.; Prodan, D.; Dascalu, L.M.; Lucaciu, O.; et al. Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers. Nanomaterials 2021, 11, 1643. https://doi.org/10.3390/nano11071643
Bacali C, Carpa R, Buduru S, Moldovan ML, Baldea I, Constantin A, Moldovan M, Prodan D, Dascalu LM, Lucaciu O, et al. Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers. Nanomaterials. 2021; 11(7):1643. https://doi.org/10.3390/nano11071643
Chicago/Turabian StyleBacali, Cecilia, Rahela Carpa, Smaranda Buduru, Mirela L. Moldovan, Ioana Baldea, Annemarie Constantin, Marioara Moldovan, Doina Prodan, Laura Monica Dascalu (Rusu), Ondine Lucaciu, and et al. 2021. "Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers" Nanomaterials 11, no. 7: 1643. https://doi.org/10.3390/nano11071643
APA StyleBacali, C., Carpa, R., Buduru, S., Moldovan, M. L., Baldea, I., Constantin, A., Moldovan, M., Prodan, D., Dascalu, L. M., Lucaciu, O., Catoi, F., Constantiniuc, M., & Badea, M. (2021). Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers. Nanomaterials, 11(7), 1643. https://doi.org/10.3390/nano11071643









