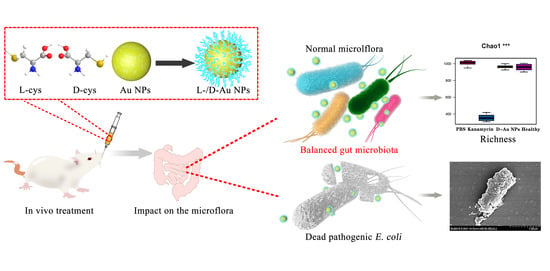
Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chiral Cysteine Functionalized Gold Nanoparticles
2.3. Bacterial Culture
2.4. Antibacterial Activity Assays
2.5. Live/Dead Bacterial Assay
2.6. In Vivo Antibacterial Activity in Infected Mice
2.7. Changes of Intestinal Microflora in Mice
3. Results and Discussion
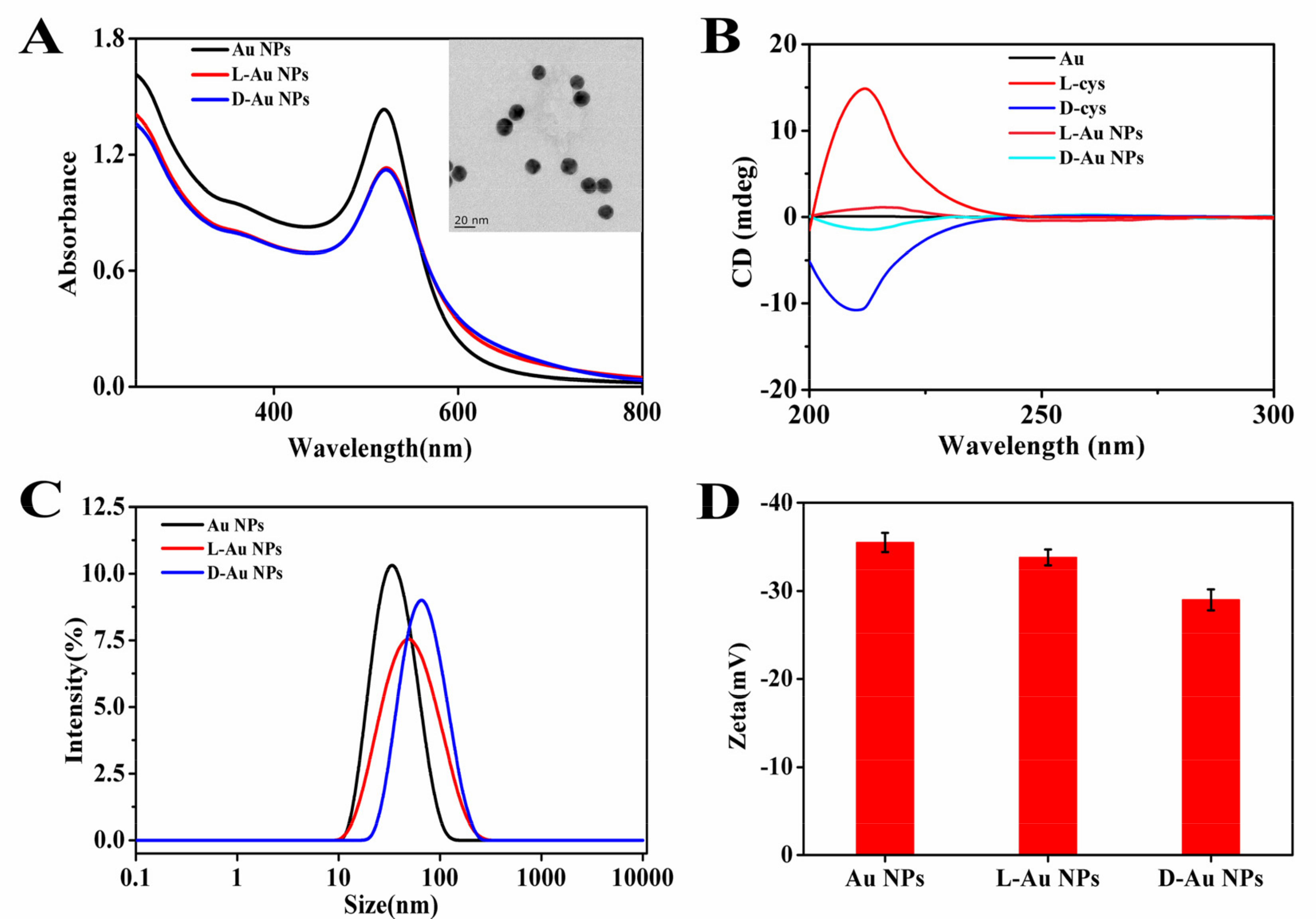
3.1. Characterization
3.2. In Vitro Antibacterial Activities of Chiral Au NPs
3.3. Antibacterial Mechanism of d-/l-Au NPs
3.4. In Vivo Therapy of Mice Suffering from E. coli Infection
3.5. Effects on the Structure of Intestinal Microflora in Mice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Penha, C.B.; Bonin, E.; da Silva, A.F.; Hioka, N.; Zanqueta, É.B.; Nakamura, T.U.; Filho, B.A.D.A.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Photodynamic Inactivation of Foodborne and Food Spoilage Bacteria by Curcumin. LWT 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics Interaction with Foodborne Pathogens: A Potential Alternative to Anti-biotics and Future Challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Laserna, A.K.C.; He, Y.; Feng, X.; Yang, H. Synergistic Action of Electrolyzed Water and Mild Heat for Enhanced Microbial Inactivation of Escherichia coli O157:H7 Revealed by Metabolomics Analysis. Food Control. 2020, 110, 107026. [Google Scholar] [CrossRef]
- Hameed, A.S.H.; Louis, G.; Karthikeyan, C.; Thajuddin, N.; Ravi, G. Impact of l-Arginine and l-Histidine on the Structural, Optical and Antibacterial Properties of Mg Doped ZnO Nanoparticles Tested Against Extended-spectrum Beta-lactamases (ESBLs) Producing Escherichia coli. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 211, 373–382. [Google Scholar] [CrossRef]
- Klebba, P.E.; Newton, S.M.C.; Six, D.A.; Kumar, A.; Yang, T.; Nairn, B.L.; Munger, C.; Chakravorty, S. Iron Acquisition Systems of Gram-Negative Bacterial Pathogens Define Tonb-Dependent Pathways to Novel Antibiotics. Chem. Rev. 2021. [Google Scholar] [CrossRef]
- Zheng, W.; Peña, A.; Ilangovan, A.; Clark, J.N.-B.; Frankel, G.; Egelman, E.H.; Costa, T.R.D. Cryoelectron-microscopy Structure of the Enteropathogenic Escherichia coli type III Secretion System EspA filament. Proc. Natl. Acad. Sci. USA 2021, 118, 2022826118. [Google Scholar] [CrossRef] [PubMed]
- Godinez, W.J.; Chan, H.; Hossain, I.; Li, C.; Ranjitkar, S.; Rasper, D.; Simmons, R.L.; Zhang, X.; Feng, B.Y. Morphological Decon-volution of Beta-Lactam Polyspecificity in E. coli. ACS Chem. Biol. 2019, 14, 1217–1226. [Google Scholar] [CrossRef]
- Levy, N.; Bruneau, J.-M.; Le Rouzic, E.; Bonnard, D.; Le Strat, F.; Caravano, A.; Chevreuil, F.; Barbion, J.; Chasset, S.; Ledoussal, B.; et al. Structural Basis for E. coli Penicillin Binding Protein (PBP) 2 Inhibition, a Platform for Drug Design. J. Med. Chem. 2019, 62, 4742–4754. [Google Scholar] [CrossRef]
- Wang, W.; Hao, C.; Sun, M.; Xu, L.; Wu, X.; Xu, C.; Kuang, H. Peptide Mediated Chiral Inorganic Nanomaterials for Combating Gram-Negative Bacteria. Adv. Funct. Mater. 2018, 28, 1805112. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive Resistome Analysis Reveals the Prevalence of NDM and MCR-1 in Chinese Poultry Production. Nat. Microbiol. 2017, 2, 16260. [Google Scholar] [CrossRef]
- Card, K.J.; Thomas, M.D.; Jr, J.L.G.; Barrick, J.E.; Lenski, R.E. Genomic Evolution of Antibiotic Resistance is Contingent on Genetic Background Following a Long-term Experiment with Escherichia coli. Proc. Natl. Acad. Sci. USA 2021, 118, 2016886118. [Google Scholar] [CrossRef]
- Dik, D.A.; Fisher, J.F.; Mobashery, S. Cell-Wall Recycling of the Gram-Negative Bacteria and the Nexus to Antibiotic Resistance. Chem. Rev. 2018, 118, 5952–5984. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.W.; Lipsitch, M.; Grad, Y.H. The Role of “Spillover” in Antibiotic Resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 29063–29068. [Google Scholar] [CrossRef]
- Zhu, G.; Sun, Z.; Hui, P.; Chen, W.; Jiang, X. Composite Film with Antibacterial Gold Nanoparticles and Silk Fibroin for Treating Multidrug-Resistant E. coli-Infected Wounds. ACS Biomater. Sci. Eng. 2021, 7, 1827–1835. [Google Scholar] [CrossRef]
- Rolim, W.; Pelegrino, M.T.; Lima, B.D.A.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea Extract Mediated Biogenic Synthesis of Silver Nanoparticles: Characterization, Cytotoxicity Evaluation and Antibacterial Activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Fang, W.-X.; Ma, S.-H.; Dong, H.; Jin, X.-W.; Zou, Y.-C.; Xu, K.-X.; Zhang, L.; Luo, Y.-H. Squarelike AgCl Nanoparticles Grown Using NiCl2(Pyz)2-Based Metal–Organic Framework Nanosheet Templates for Antibacterial Applications. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Polymer-Based Coatings with Integrated Antifouling and Bactericidal Properties for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, Ph-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M. Antibacterial Activities of Green Synthesized Gold Nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, H.; Shang, Y.; Zeng, S.; Qin, Z.; Yin, S.; Li, J.; Liang, S.; Lu, G.; Liu, Z. Spiky Nanohybrids of Titanium Dioxide/Gold Nanoparticles for Enhanced Photocatalytic Degradation and Anti-bacterial Property. J. Colloid Interface Sci. 2019, 535, 516–523. [Google Scholar] [CrossRef]
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.-B.; Hochepied, J.-F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the Physicochemical Features of TiO2 Nanoparticles on Their In vitro Toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Gangopadhyay, K.; Das, R.; Purkayastha, P. Development of Non-Ionic Surfactant and Protein-Coated Ul-trasmall Silver Nanoparticles: Increased Viscoelasticity Enables Potency in Biological Applications. ACS Omega 2020, 5, 8999–9006. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A.; Parashar, S.K.S.; Patro, S.; Suar, M. Mechanistic Insight into Size-Dependent Enhanced Cytotoxicity of Industrial Antibacterial Titanium Oxide Nanoparticles on Colon Cells Because of Reactive Oxygen Species Quenching and Neutral Lipid Alteration. ACS Omega 2018, 3, 1244–1262. [Google Scholar] [CrossRef]
- Li, J.; Chen, R.; Zhang, S.; Ma, Z.; Luo, Z.; Gao, G. Chiral Effect at Nano-Bio Interface: A Model of Chiral Gold Nanoparticle on Amylin Fibrillation. Nanomaterials 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Hove, J.B.T.; Schijven, L.M.I.; Wang, J.; Velders, A.H. Size-controlled and Water-soluble Gold Nanoparticles Using UV-induced Ligand Exchange and Phase Transfer. Chem. Commun. 2018, 54, 13355–13358. [Google Scholar] [CrossRef] [Green Version]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Li, Y.; Zhang, L.; Jiang, H.; Tao, J.; Zhu, J. Gold Nanoparticle-guarded Large-Pore Mesoporous Silica Nanocomposites for Delivery and Controlled Release of Cytochrome c. J. Colloid Interface Sci. 2021, 589, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Z.; Wang, C.; Zhang, S.; Cui, W.; Xu, Y.; Zhao, J.; Xue, H.; Li, J. Assembled Cationic Dipeptide-Gold Nanoparticle Hybrid Microspheres for Electrochemical Biosensors with Enhanced Sensitivity. J. Colloid Interface Sci. 2019, 557, 628–634. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yin, J.; Yang, J.; Li, Q.; Zheng, W.; Liu, S.; Jiang, X. The Density of Surface Coating Can Contribute to Different Antibacterial Activities of Gold Nanoparticles. Nano Lett. 2020, 20, 5036–5042. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant Extract Mediated Synthesis of Silver and Gold Nano-particles and Its Antibacterial Activity against Clinically Isolated Pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Bose, M.; Das, T.K.; Mondal, S.; Das, A.K.; Das, N.C. Sonochemical Green Reduction to Prepare Ag Nano-particles Decorated Graphene Sheets for Catalytic Performance and Antibacterial Application. Ultrasonics Sonochem. 2017, 39, 577–588. [Google Scholar] [CrossRef]
- Zheng, W.; Jia, Y.; Chen, W.; Wang, G.; Guo, X.; Jiang, X. Universal Coating from Electrostatic Self-Assembly to Prevent Multi-drug-Resistant Bacterial Colonization on Medical Devices and Solid Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 21181–21189. [Google Scholar] [CrossRef] [PubMed]
- Monteil, M.; Moustaoui, H.; Picardi, G.; Aouidat, F.; Djaker, N.; de la Chapelle, M.L.; Lecouvey, M.; Spadavecchia, J. Polyphos-phonate Ligands: From Synthesis to Design of Hybrid Pegylated Nanoparticles toward Phototherapy Studies. J. Colloid Interface Sci. 2018, 513, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Ma, W.; Zhao, X.; Wang, J.; Yao, L.; Jiang, X.; Wu, Z. Interaction between Surface Charge-Modified Gold Nanoparticles and Phospholipid Membranes. Langmuir 2018, 34, 12583–12589. [Google Scholar] [CrossRef]
- Jana, S.K.; Gucchait, A.; Paul, S.; Saha, T.; Acharya, S.; Hoque, K.M.; Misra, A.K.; Chatterjee, B.K.; Chatterjee, T.; Chakrabarti, P. Virstatin-Conjugated Gold Nanoparticle with Enhanced Antimicrobial Activity against the Vibrio cholerae El Tor Biotype. ACS Appl. Bio Mater. 2021, 4, 3089–3100. [Google Scholar] [CrossRef]
- Ferro, I.; Chelysheva, I.; Ignatova, Z. Competition for Amino Acid Flux Among Translation, Growth and Detoxification in Bacteria. RNA Biol. 2017, 15, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Loddeke, M.; Schneider, B.; Oguri, T.; Mehta, I.; Xuan, Z.; Reitzer, L. Anaerobic Cysteine Degradation and Potential Metabolic Coordination in Salmonella enterica and Escherichia coli. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.-L.; Xu, Y.; Chen, Y.-Z.; Yan, X.-S.; Li, Z.; Xie, J.-W.; Jiang, Y.-B. Chiral Recognition by Flexible Coordination Polymers of Ag+ with a Cysteine-Based Chiral Thiol Ligand That Bears a Binding Site. Inorg. Chem. 2021, 60, 5413–5418. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial Activity and Mechanisms of Depolymerized Fucoidans Isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, D.; Li, G.; Liu, J.; He, G.; Zhang, P.; Yang, L.; Zhu, H.; Xu, N.; Liang, S. Antibacterial Mechanism of Daptomycin Antibiotic Against Staphylococcus aureus Based on a Quantitative Bacterial Proteome Analysis. J. Proteom. 2017, 150, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Qian, J.; Gu, J.; Yan, W.; Zhang, J. Steric Configuration-enabled Selective Antimicrobial Activity of Chiral Cysteine. Biochem. Biophys. Res. Commun. 2019, 512, 505–510. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Song, C.; Rutledge, G.C. Direct Three-Dimensional Visualization of Membrane Fouling by Confocal Laser Scanning Microscopy. ACS Appl. Mater. Interfaces 2019, 11, 17001–17008. [Google Scholar] [CrossRef]
- Ledwaba, S.E.; Costa, D.V.S.; Bolick, D.T.; Giallourou, N.; Medeiros, P.H.Q.S.; Swann, J.R.; Traore, A.N.; Potgieter, N.; Nataro, J.P.; Guerrant, R.L. Enteropathogenic Escherichia coli Infection Induces Diarrhea, Intestinal Damage, Metabolic Alterations, and Increased Intestinal Permeability in a Murine Model. Front. Cell. Infect. Microbiol. 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yang, L.; Wang, H.; Zhang, J.; Shen, W. Atomic-engineered Gold@silvergold Alloy Nanoflowers for in vivo Inhibition of Bacteria. Nanoscale 2018, 10, 15661–15668. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-T.; Peng, X.; Deng, G.-H.; Sheng, H.-F.; Wang, Y.; Zhou, H.-W.; Tam, N.F.-Y. Illumina Sequencing of 16S rRNA Tag Revealed Spatial Variations of Bacterial Communities in a Mangrove Wetland. Microb. Ecol. 2013, 66, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, H.-F.; He, Y.; Wu, J.-Y.; Jiang, Y.-X.; Tam, N.F.-Y.; Zhou, H.-W. Comparison of the Levels of Bacterial Diversity in Freshwater, Intertidal Wetland, and Marine Sediments by Using Millions of Illumina Tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Trindade, G.F.; Hicks, J.M.; Potts, J.C.; Rahman, R.; Hague, R.J.M.; Amabilino, D.B.; Perez-Garcia, L.; Rawson, F.J. Mod-ulating the Biological Function of Protein by Tailoring the Adsorption Orientation on Nanoparticles. J. Colloid Interface Sci. 2021, 587, 150–161. [Google Scholar] [CrossRef]
- Yang, X.; Roling, L.T.; Vara, M.; Elnabawy, A.O.; Zhao, M. Synthesis and Characterization of Pt-Ag Alloy Nanocages with Enhanced Activity and Durability toward Oxygen Reduction. Nano Letters 2016, 16, 6644. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Yang, Y.; Wang, H.L.; Lei, W.; Liu, Y.X.; Wang, S.L. Gold Nanoparticles Modified Hollow Carbon System for Du-al-Responsive Release and Chemo-Photothermal Synergistic Therapy of Tumor. J. Colloid Interface Sci. 2019, 554, 239–249. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart Antibacterial Surfaces with Switchable Bacteria-Killing and Bacteria-Releasing Capa-bilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef] [PubMed]
- Sardo, C.; Nottelet, B.; Triolo, D.; Giammona, G.; Garric, X.; Lavigne, J.-P.; Cavallaro, G.; Coudane, J. When Functionalization of PLA Surfaces Meets Thiol–Yne Photochemistry: Case Study with Antibacterial Polyaspartamide Derivatives. Biomacromolecules 2014, 15, 4351–4362. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-Amino Acids Trigger Biofilm Disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeflinger, J.L.; Davis, S.R.; Chow, J.; Miller, M. In vitro Impact of Human Milk Oligosaccharides on Enterobacteriaceae Growth. J. Agric. Food Chem. 2015, 63, 3295–3302. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, Tio2, Zno, and Fe3o4 Nps Via Protoparmeliopsis Muralis Lichen Aqueous Extract against Mul-ti-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The Molecular Mechanism of Action of Bactericidal Gold Nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zheng, W.; Zhu, G.; Lian, J.; Wang, J.; Hui, P.; He, S.; Chen, W.; Jiang, X. Albumin Broadens the Antibacterial Capabilities of Nonantibiotic Small Molecule-Capped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 45381–45389. [Google Scholar] [CrossRef] [PubMed]
- Slocik, J.M.; Dennis, P.B.; Govorov, A.O.; Bedford, N.M.; Ren, Y.; Naik, R.R. Chiral Restructuring of Peptide Enantiomers on Gold Nanomaterials. ACS Biomater. Sci. Eng. 2019, 6, 2612–2620. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Wang, H.; Wang, B.; Zhou, Y.; Liu, Y.; Shao, M.; Huang, H.; Lu, F.; Kang, Z. Chiral Control of Carbon Dots via Surface Modification for Tuning the Enzymatic Activity of Glucose Oxidase. ACS Appl. Mater. Interfaces 2021, 13, 5877–5886. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, Q.; Geng, L.; Fang, Q.; Gong, J.R. Chiral Nanoparticle as a New Efficient Antimicrobial Nanoagent. Adv. Healthc. Mater. 2016, 6, 1601011. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Zhao, X.; Guo, H.; Luo, H.; Wang, M.; Zhou, F.; Jiang, X. Gold Nanoparticles Cure Bacterial Infection with Benefit to Intestinal Microflora. ACS Nano 2019, 13, 5002–5014. [Google Scholar] [CrossRef]
- Seo, K.-H.; Kim, D.-H.; Yokoyama, W.H.; Kim, H. Synbiotic Effect of Whole Grape Seed Flour and Newly Isolated Kefir Lactic Acid Bacteria on Intestinal Microbiota of Diet-Induced Obese Mice. J. Agric. Food Chem. 2020, 68, 13131–13137. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, H.; Zhu, C.; Deng, J.; Fan, D. Hypoglycemic Effect of Ginsenoside Rg5 Mediated Partly by Modulating Gut Mi-crobiota Dysbiosis in Diabetic Db/Db Mice. J. Agric. Food Chem. 2020, 68, 5107–5117. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, H.; Zhang, M.; Zhang, J.; Yan, W. Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect. Nanomaterials 2021, 11, 1621. https://doi.org/10.3390/nano11061621
Xu Y, Wang H, Zhang M, Zhang J, Yan W. Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect. Nanomaterials. 2021; 11(6):1621. https://doi.org/10.3390/nano11061621
Chicago/Turabian StyleXu, Yuelong, Hongxia Wang, Min Zhang, Jianhao Zhang, and Wenjing Yan. 2021. "Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect" Nanomaterials 11, no. 6: 1621. https://doi.org/10.3390/nano11061621
APA StyleXu, Y., Wang, H., Zhang, M., Zhang, J., & Yan, W. (2021). Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect. Nanomaterials, 11(6), 1621. https://doi.org/10.3390/nano11061621