Morphology and Mechanical Properties of Fossil Diatom Frustules from Genera of Ellerbeckia and Melosira
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphology and Chemical Composition Study
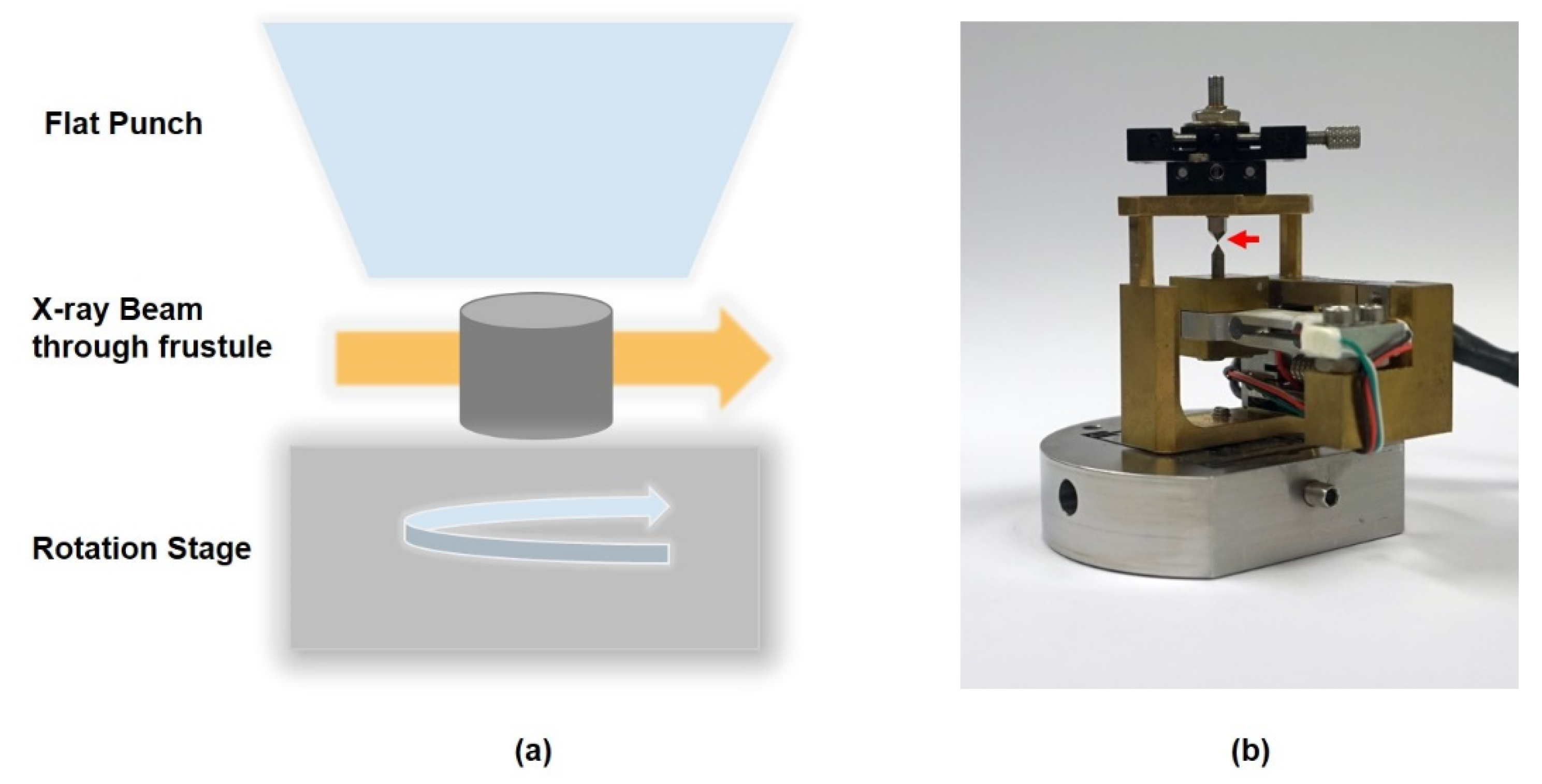
2.2. Micromechanical Behavior Study
3. Results and Discussion
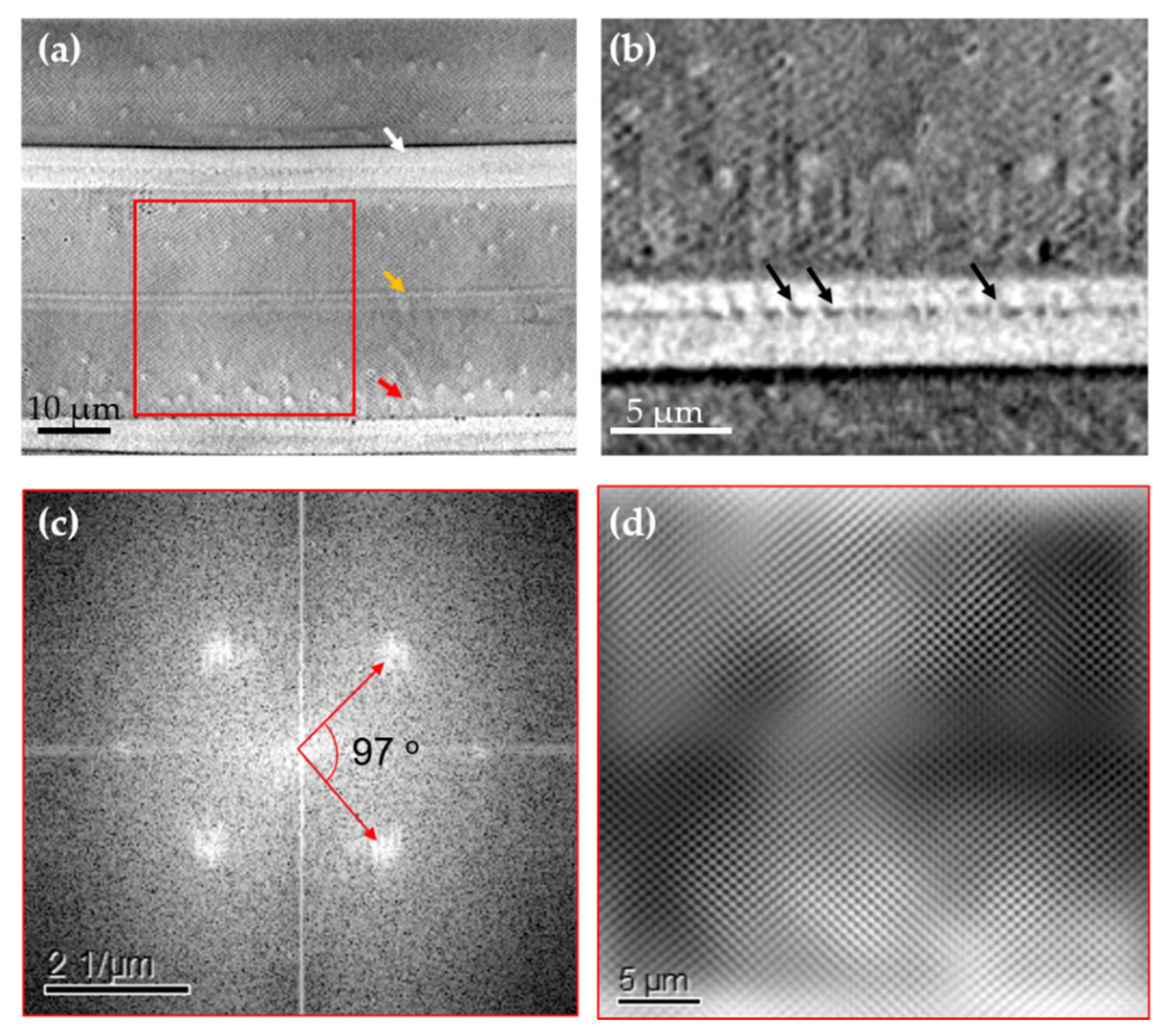
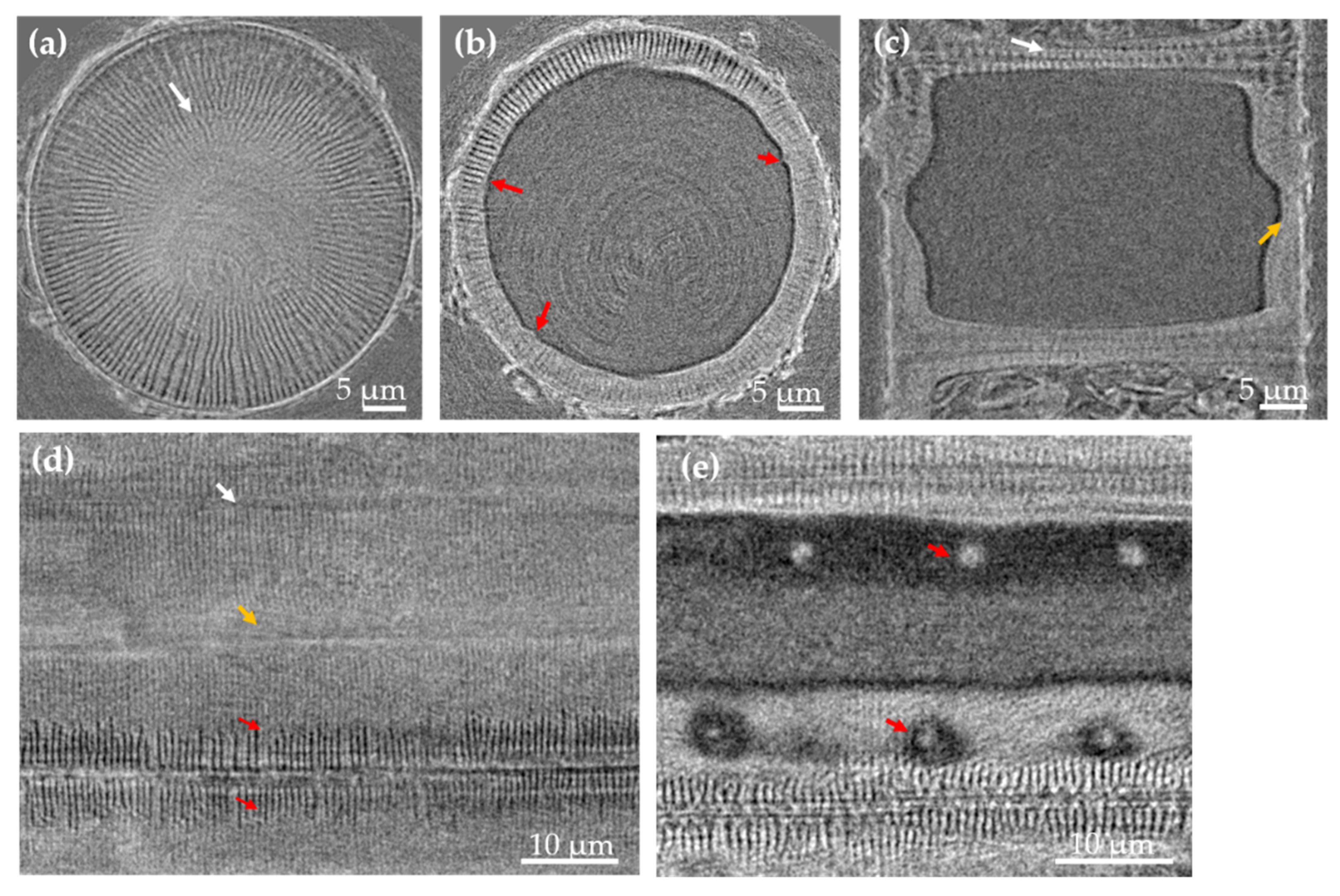
3.1. Morphology and Chemical Composition Study
3.2. Uniaxial Static Compression Test of Fossil Frustules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamat, S.; Su, X.; Ballarini, R.; Heuer, A.H. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature 2000, 405, 1036. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, T.; Zhang, D. Biotemplated materials for sustainable energy and environment: Current status and challenges. ChemSusChem 2011, 4, 1344–1378. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Nann, T.; Voelcker, N. Silicon nanowire photocathodes for photoelectrochemical hydrogen production. Nanomaterials 2016, 6, 144. [Google Scholar] [CrossRef]
- Gordon, R.; Losic, D.; Tiffany, M.; Nagy, S.; Sterrenburg, F. The glass menagerie: Diatoms for novel applications in nanotechnology. Trends Biotechnol. 2009, 27, 116–127. [Google Scholar] [CrossRef]
- Rea, I.; Dardano, P.; Ferrara, A.; Stefano, L. Micro- and nano-optical devices from diatom nanostructure: Light control by mother nature. In Diatom Nanotechnology Progress and Emerging Applications, 1st ed.; Losic, D., Ed.; The Royal Society of Chemistry: London, UK, 2018; pp. 111–123. [Google Scholar]
- Pattnayak, A.; Madhu, N.; Panda, A.S.; Sahoo, M.K.; Mohanta, K. A comparative study on mechanical properties of Al-SiO2 composites fabricated using rice husk silica in crystalline and amorphous form as reinforcement. Mater. Today Proc. 2018, 5, 8184–8192. [Google Scholar] [CrossRef]
- Meindlhumer, M.; Zalesak, J.; Pitonak, R.; Todt, J.; Sartory, B.; Burghammer, M.; Stark, A.; Schell, N.; Daniel, R.; Keckes, J.F.; et al. Biomimetic hard and tough nanoceramic Ti-Al-N film with self-assembled six-level hierarchy. Nanoscale 2019, 11, 7986. [Google Scholar] [CrossRef]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dabek, P.; Witkowski, A.; Buszewski, B. “Outsourcing” diatoms in fabrication of metal-doped 3D biosilica. Materials 2020, 13, 2576. [Google Scholar] [CrossRef]
- Xing, Y.; Yu, L.; Wang, X.; Jia, J.; Liu, Y.; He, J.; Jia, Z. Characterization and analysis of Coscinodiscus genus frustule based on FIB-SEM. Prog. Nat. Sci. Mater. Int. 2017, 27, 391–395. [Google Scholar] [CrossRef]
- Zglobicka, I.; Chmielewska, A.; Topal, E.; Kutukova, K.; Gluch, J.; Krüger, P.; Kilroy, C.; Swieszkowski, W.; Kurzydlowski, K.J.; Zschech, E. 3D diatom–designed and selective laser melting (SLM) manufactured metallic structures. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gurel, A.; Yildiz, A. Diatom communities, lithofacies characteristics and paleo environmental interpretation of Pliocene diatomite deposits in the lhlara–Selime plain (Aksaray, Central Anatolia, Turkey). J. Asian Earth Sci. 2007, 30, 170–180. [Google Scholar] [CrossRef]
- Owen, R.B.; Potts, R.; Behrensmeyer, A.K.; Ditchfield, P. Diatomaceous sediments and environmental change in the Pleistocene Olorgesailie Formation, Southern Kenya Rift Valley. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 269, 17–37. [Google Scholar] [CrossRef]
- Crangle, R.D. Diatomite: U.S. Geological Survey Mineral Commodity Summaries; U.S. Geological Survey: Washington, DC, USA, 2010; pp. 52–53.
- Bunch, T.R.; Bond, C.; Buhl, K.; Stone, D. Diatomaceous Earth General Fact Sheet. 2013. Available online: http://npic.orst.edu/factsheets/degen.html (accessed on 25 October 2020).
- Lutynski, M.; Sakiewicz, P.; Lutynska, S. Characterization of diatomaceous earth and halloysite resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Antonides, L.E. Diatomite. USGS. Available online: https://www.usgs.gov/centers/nmic/diatomite-statistics-and-information (accessed on 25 October 2020).
- De Namor, A.D.; Elgamouz, A.; Frangie, S.; Martinez, V. Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J. Hazard. Mater. 2012, 241–242, 14–31. [Google Scholar] [CrossRef]
- Hamm, C.E. The evolution of advanced mechanical defenses and potential technological applications of diatom shells. J. Nanosci. Nanotechnol. 2005, 5, 108–119. [Google Scholar] [CrossRef]
- Aitken, Z.H.; Luo, S.; Reynolds, S.N.; Thaulow, C.; Greer, J.R. Microstructure provides insights into evolutionary design and resilience of Coscinodiscus sp. frustule. Proc. Natl. Acad. Sci. USA 2016, 113, 2017–2022. [Google Scholar] [CrossRef]
- Mavhungu, S.T.; Akinlabi, E.T.; Onitiri, M.A.; Varachia, F.M. Aluminum matrix composites for industrial use: Advances and trends. Procedia Manuf. 2017, 7, 178–182. [Google Scholar] [CrossRef]
- Rizkalla, H.L.; Abdulwahed, A. Some mechanical properties of metal-nonmetal Al-SiO2 particulate composites. J. Mater. Process. Technol. 1996, 56, 398–403. [Google Scholar] [CrossRef]
- Sulaiman, S.; Sayuti, M.; Samin, R. Mechanical properties of the as-cast quartz particulate reinforced LM6 alloy matrix composites. J. Mater. Process. Technol. 2008, 201, 731–735. [Google Scholar] [CrossRef]
- Hashemi, M.; Jamaati, R.; Toroghinejad, M.R. Microstructure and mechanical properties of Al/SiO2 composite produced by CAR process. Mater. Sci. Eng. A 2012, 532, 275–281. [Google Scholar] [CrossRef]
- Zglobicka, I. Frustules of Didymosphenia geminata as a modifier of resin. Inzynieria Mater. 2018, 1, 10–16. [Google Scholar] [CrossRef]
- Svensson, F.; Norberg, J.; Snoeijs, P. Diatom cell size, coloniality and motility: Trade-offs between temperature, salinity and nutrient supply with climate change. PLoS ONE 2014, 9, e109993. [Google Scholar] [CrossRef]
- Almqvist, N.; Delamo, Y.; Smith, B.L.; Thomson, N.H.; Bartholdson, A.; Lal, R.; Brzezinski, M.; Hansma, P.K. Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy. J. Microsc. 2001, 202, 518–532. [Google Scholar] [CrossRef]
- Losic, D.; Short, K.; Mitchell, J.G.; Lal, R.; Voelcker, N.H. AFM nanoindentations of diatom biosilica surfaces. Langmuir 2007, 23, 5014–5021. [Google Scholar] [CrossRef]
- Topal, E.; Rajendran, H.; Zglobicka, I.; Gluch, J.; Liao, Z.; Clausner, A.; Kurzydlowski, J.K.; Zschech, E. Numerical and experimental study of the mechanical response of diatom frustules. Nanomaterials 2020, 10, 959. [Google Scholar] [CrossRef]
- Hamm, C.E.; Merkel, R.; Springe, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Weng, D.; Du, C.; Wang, J.; Cao, S. Contribution of frustules and mucilage trails to the mobility of diatom Navicula sp. Sci. Rep. 2019, 9, 7342. [Google Scholar] [CrossRef] [PubMed]
- Zgłobicka, I.; Li, Q.; Gluch, J.; Płocinska, M.; Noga, T.; Dobosz, R.; Szoszkiewicz, R.; Witkowski, A.; Zschech, E.; Kurzydłowski, K.J. Visualization of the internal structure of Didymosphenia geminata frustules using nano X-ray tomography. Sci. Rep. 2017, 7, 9086. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, A.; Duewer, F.; Cui, H.; Feser, M.; Wang, S.; Yun, W. X-ray computed tomography in Zernike phase contrast mode at 8 keV with 50 nm resolution using Cu rotating anode X-ray source. Z. Kristallogr. Cryst. Mater. 2007, 222, 650–655. [Google Scholar] [CrossRef]
- Li, Q.; Gluch, J.; Krüger, P.; Gall, M.; Neinhuis, C.; Zschech, E. Pollen structure visualization using high-resolution laboratory-based hard X-ray tomography. Biochem. Biophys. Res. Commun. 2016, 479, 272–276. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zschech, E.; Löffler, M.; Krüger, P.; Gluch, J.; Kutukova, K.; Zgłobicka, I.; Topal, E. Laboratory computed X-ray tomography–A nondestructive technique for 3D microstructure analyis of materials. Pract. Metallogr. 2018, 55, 539–555. [Google Scholar] [CrossRef]
- Crawford, R.M.; Sims, P.A. Ellerbeckia baileyi (H. L. Smith) Crawford &Sims Comb. Nov.: Typification and frustule morphology of a rare freshwater fossil diatom. Diatom Res. 2007, 22, 17–26. [Google Scholar]
- Wolle, F. Diatomaceae of North America; The Commenius Press: Bethlehem, PA, USA, 1890; pp. 15–47. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2020. Available online: http://www.algaebase.org (accessed on 25 October 2020).
- Diatoms of North America. Available online: https://diatoms.org/genera/ellerbeckia (accessed on 25 October 2020).
- Diatoms of North America. Available online: https://diatoms.org/genera/melosira (accessed on 25 October 2020).
- Kutukova, K. Combination of X-Ray Tomography and In-situ Micro Mechanical Testing of Composites. Master’s Thesis, DIU Dresden International University, Dresden, Germany, 9 December 2015. submission. [Google Scholar]
- Park, J.S.; Jung, S.W.; Lee, S.D.; Yun, S.M.; Lee, J.H. Species diversity of the genus Thalassiosira (Thalassiosirales, Bacillariophyta) in South Korea and its biogeographical distribution in the world. Phycologia 2016, 55, 403–423. [Google Scholar] [CrossRef]
- Li, Q.; Gluch, J.; Zschech, E. Laboratory-based high-resolution X-ray tomography for three-dimensional visualizing the natural diatoms. Biotechnol. Biotechnol. Equip. 2021, 35, S80. [Google Scholar]


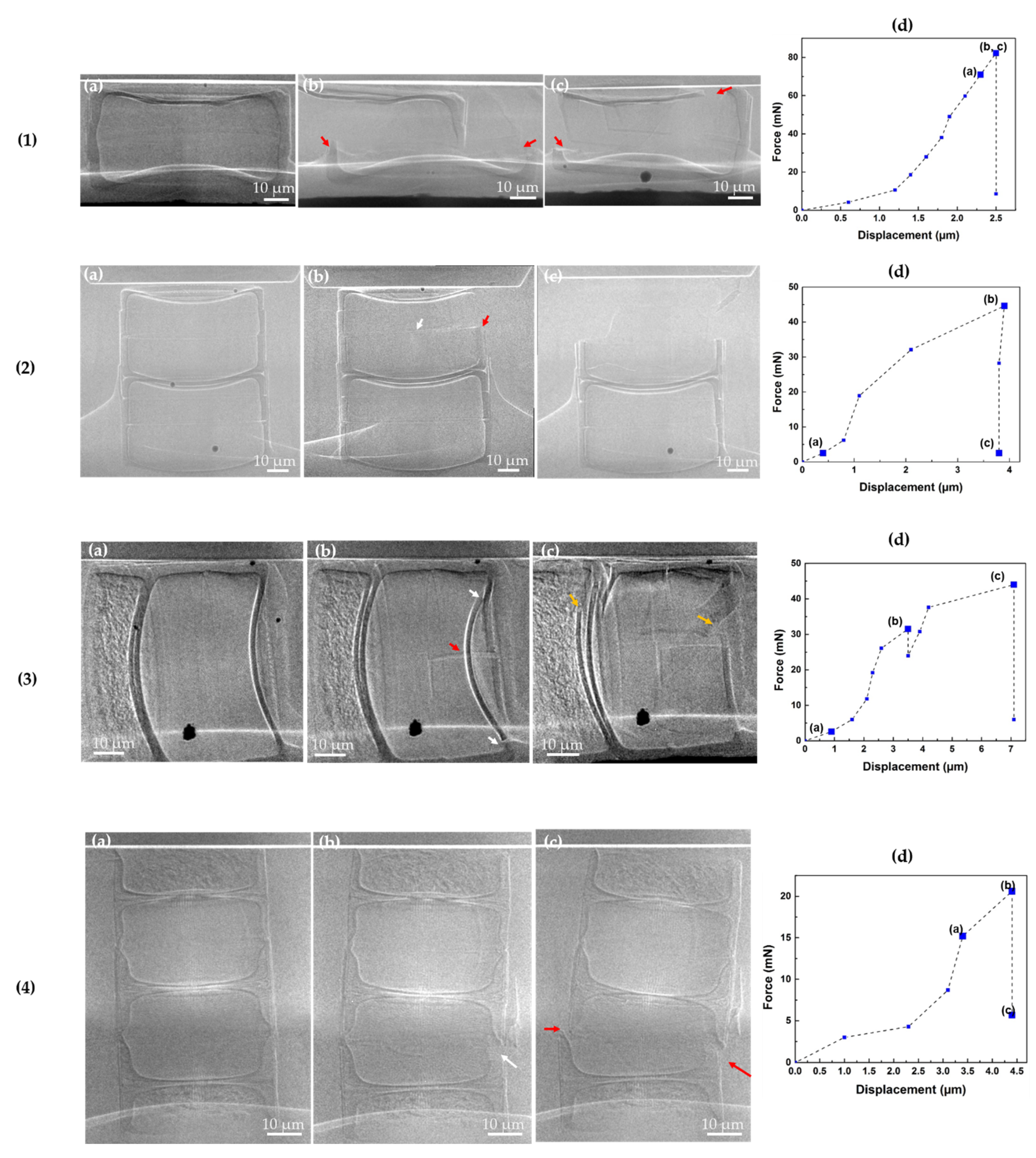

| Sample | Fmax | Cylindrical Diameter (CD) | Valve Thickness (VFT) | Mantle Wall Thickness (MT) | Copulae Thickness (CT) |
|---|---|---|---|---|---|
| mN | µm | µm | µm | µm | |
| Frustule 1 (Ellerbeckia) | 82.2 ± 0.5 | 80.0 ± 0.9 | 2.43 ± 0.1 | 3.5 ± 0.2 | 0.81 ± 0.07 |
| Frustule 2 (Ellerbeckia) | 45.2 ± 0.3 | 69.5 ± 0.7 | 1.99 ± 0.07 | 2.9 ± 0.3 | 0.75 ± 0.03 |
| Frustule 3 (Ellerbeckia) | 44.1 ± 0.2 | 62.5 ± 0.7 | 1.71 ± 0.08 | 2.6 ± 0.2 | 0.67 ± 0.02 |
| Frustule 4 (Melosira) | 20.6 ± 0.5 | 46.0 ± 0.4 | 1.6 ± 0.3 | 1.16 ± 0.09 * | 0.78 ± 0.08 |
| Sample | VFT/CD | MT/CD | CT/CD |
|---|---|---|---|
| Frustule 1 (Ellerbeckia) | 0.030 | 0.044 | 0.010 |
| Frustule 2 (Ellerbeckia) | 0.029 | 0.041 | 0.011 |
| Frustule 3 (Ellerbeckia) | 0.027 | 0.041 | 0.011 |
| Frustule 4 (Melosira) | 0.035 | 0.025 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Gluch, J.; Liao, Z.; Posseckardt, J.; Clausner, A.; Łępicka, M.; Grądzka-Dahlke, M.; Zschech, E. Morphology and Mechanical Properties of Fossil Diatom Frustules from Genera of Ellerbeckia and Melosira. Nanomaterials 2021, 11, 1615. https://doi.org/10.3390/nano11061615
Li Q, Gluch J, Liao Z, Posseckardt J, Clausner A, Łępicka M, Grądzka-Dahlke M, Zschech E. Morphology and Mechanical Properties of Fossil Diatom Frustules from Genera of Ellerbeckia and Melosira. Nanomaterials. 2021; 11(6):1615. https://doi.org/10.3390/nano11061615
Chicago/Turabian StyleLi, Qiong, Jürgen Gluch, Zhongquan Liao, Juliane Posseckardt, André Clausner, Magdalena Łępicka, Małgorzata Grądzka-Dahlke, and Ehrenfried Zschech. 2021. "Morphology and Mechanical Properties of Fossil Diatom Frustules from Genera of Ellerbeckia and Melosira" Nanomaterials 11, no. 6: 1615. https://doi.org/10.3390/nano11061615
APA StyleLi, Q., Gluch, J., Liao, Z., Posseckardt, J., Clausner, A., Łępicka, M., Grądzka-Dahlke, M., & Zschech, E. (2021). Morphology and Mechanical Properties of Fossil Diatom Frustules from Genera of Ellerbeckia and Melosira. Nanomaterials, 11(6), 1615. https://doi.org/10.3390/nano11061615








