Structural Transitions and Stability of FAPbI3 and MAPbI3: The Role of Interstitial Water
Abstract
1. Introduction
2. Materials and Methods
3. Results
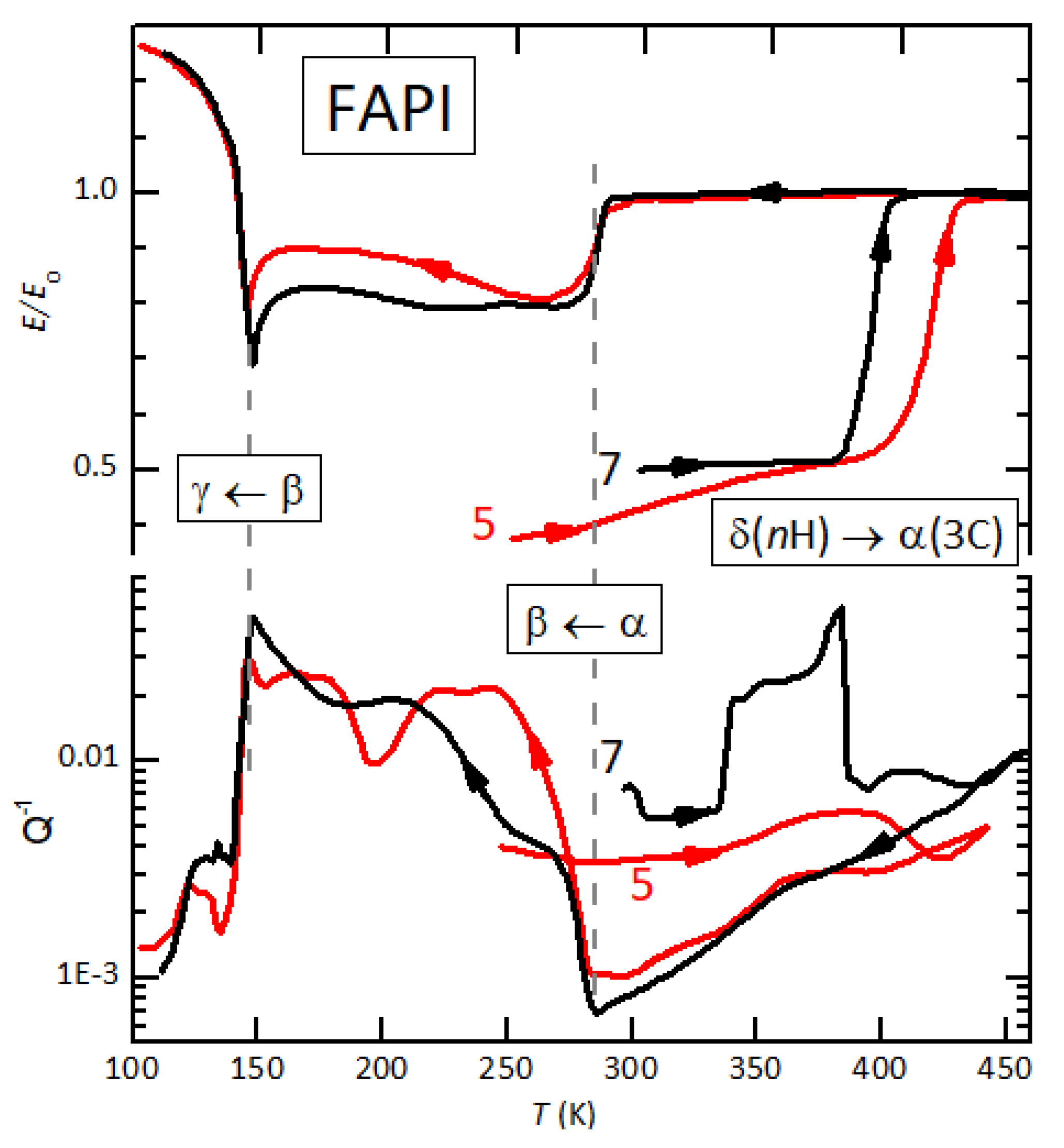
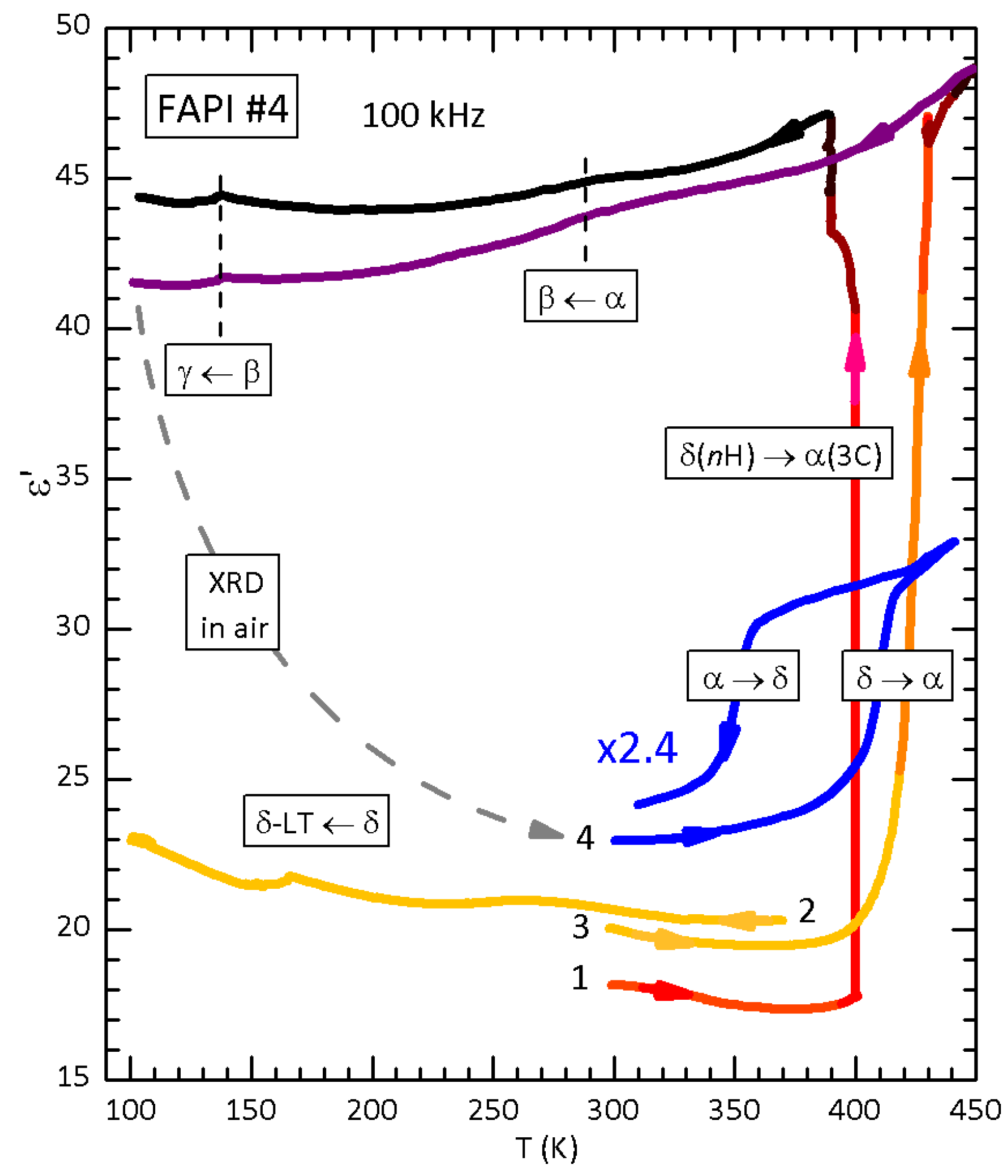
3.1. FAPI
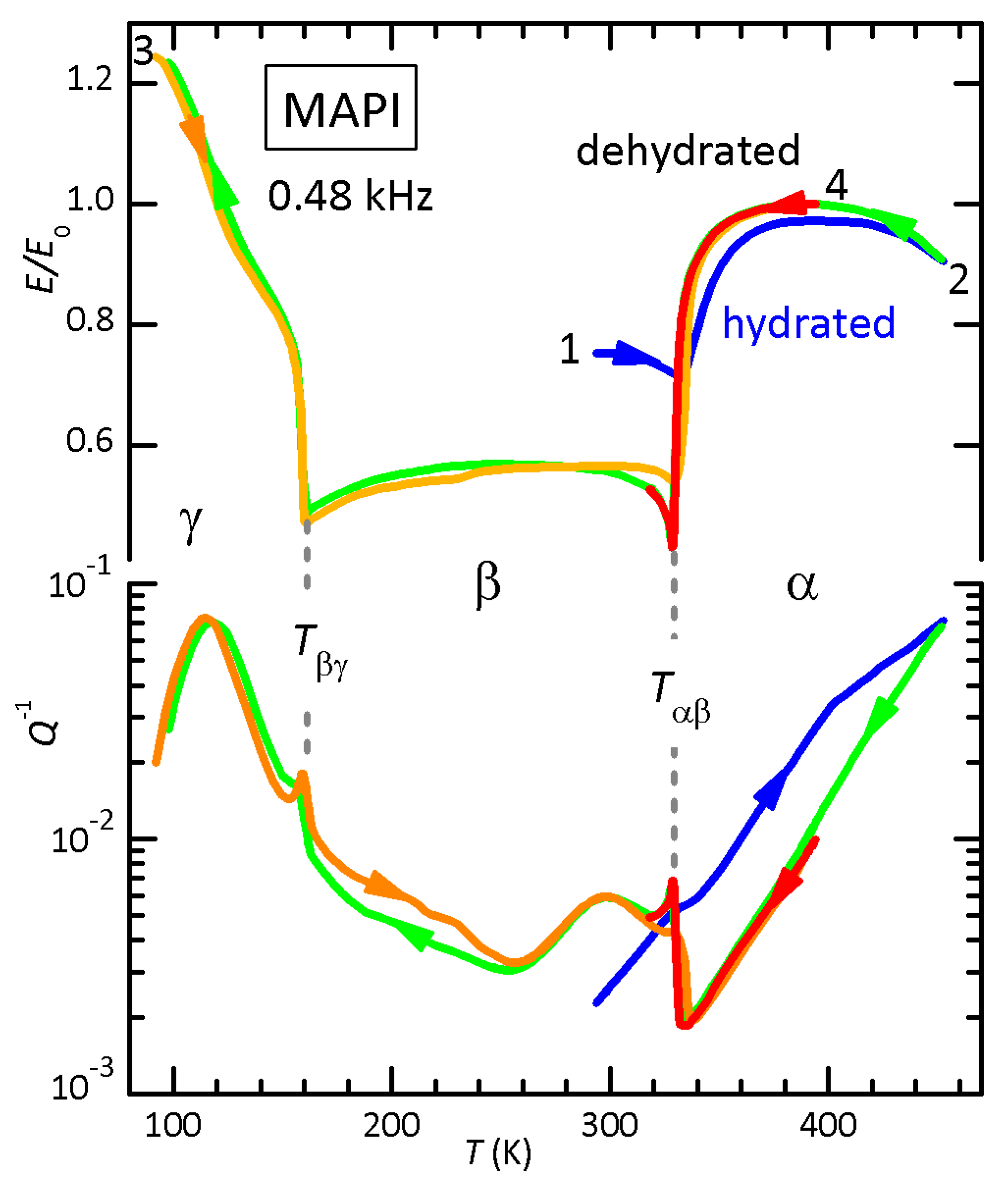
3.2. MAPI
4. Discussion
4.1. Ionic Sizes and Differences between MAPI and FAPI
4.2. Hydrated Phases and Interstitial H2O
4.3. The Reduction of the Elastic Softening at the α/β-MAPI Transition and Interstitial H2O
4.4. The Kinetics of the α/δ-FAPI Transition and Interstitial H2O
4.5. Interstitial Water, Tolerance Factor and Relative Stability of the α and δ Phases
4.6. Re-Entrant Behaviour of the δ → α Transition in FAPI and Anelastic Relaxation from the Action of H2O
4.7. Reproducibility of the Experiments with Hydrated Samples
4.8. Beneficial Role of Water during Preparation
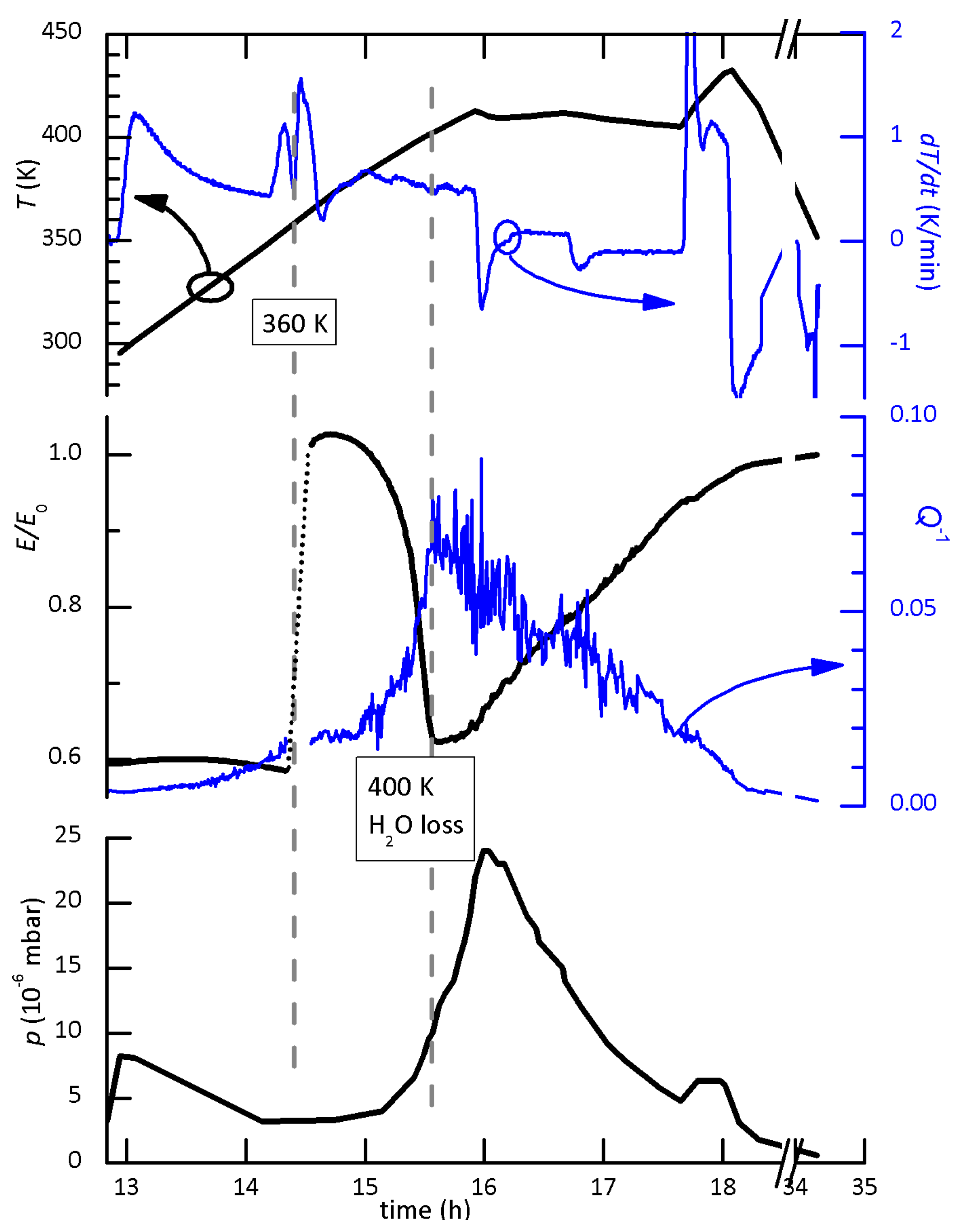
4.9. Removal of Water
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Weber, D. CH3NH3PbX3, a Pb(II)-System with Cubic Perovskite Structure. Z. Naturforsch. 1978, 33b, 1443–1445. [Google Scholar] [CrossRef]
- Poglitsch, A.; Weber, D. Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Mater. 2015, 27, 1248–1254. [Google Scholar] [CrossRef]
- Yu, X.; Tsao, H.N.; Zhang, Z.; Gao, P. Miscellaneous and Perspicacious: Hybrid Halide Perovskite Materials Based Photodetectors and Sensors. Adv. Opt. Mater. 2020, 8, 2001095. [Google Scholar] [CrossRef]
- Yakunin, S.; Dirin, D.N.; Shynkarenko, Y.; Morad, V.; Cherniukh, I.; Nazarenko, O.; Kreil, D.; Nauser, T.; Kovalenko, M.V. Detection of gamma photons using solution-grown single crystals of hybrid lead halide perovskites. Nat. Photonics 2016, 10, 585–589. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Kanatzidis, M.G. The Renaissance of Halide Perovskites and Their Evolution as Emerging Semiconductors. Acc. Chem. Res. 2015, 48, 2791. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Zhu, X.; Zuo, S.; Yang, Z.; Feng, J.; Wang, Z.; Zhang, X.; Priya, S.; Liu, S.F.; Yang, D. In Situ Grain Boundary Modification via Two-Dimensional Nanoplates to Remarkably Improve Stability and Efficiency of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 39802. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, D.; Kam, M.; Zhang, Q.; Poddar, S.; Fu, Y.; Mo, X.; Fan, Z. Significantly Improved Black Phase Stability of FAPbI3 Nanowires via Spatially Confined Vapor Phase Growth in Nanoporous Templates. Nanoscale 2018, 10, 15164–15172. [Google Scholar] [CrossRef]
- An, Y.; Hidalgo, J.; Perini, A.R.; Castro-Méndez, A.F.; Vagott, J.N.; Bairley, K.; Wang, S.; Li, X.; Correa-Baena, J.P. Structural Stability of Formamidinium- and Cesium-Based Halide Perovskites. ACS Energy Lett. 2021, 6, 1942–1969. [Google Scholar] [CrossRef]
- Knight, A.J.; Herz, L.M. Preventing phase segregation in mixed-halide perovskites: A perspective. Energy Environ. Sci. 2020, 13, 2024–2046. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Ustinova, M.I.; Gutsev, L.; Tsarev, S.A.; Dremova, N.N.; Zhidkov, I.; Luchkin, S.Y.; Ramachandran, B.R.; Frolova, L.; Kurmaev, E.Z.; et al. When iodide meets bromide: Halide mixing facilitates the light-induced decomposition of perovskite absorber films. Nano Energy 2021, 86, 106082. [Google Scholar] [CrossRef]
- Tenuta, E.; Zheng, C.; Rubel, O. Thermodynamic origin of instability in hybrid halide perovskites. Sci. Rep. 2016, 6, 37654. [Google Scholar] [CrossRef]
- Zheng, C.; Rubel, O. Unraveling the Water Degradation Mechanism of CH3NH3PbI3. J. Phys. Chem. C 2019, 123, 19385–19394. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, R.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-Induced Degradation via Grain Boundaries of HC(NH2)2PbI3 Planar Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1705363. [Google Scholar] [CrossRef]
- Cordero, F.; Craciun, F.; Trequattrini, F.; Generosi, A.; Paci, B.; Paoletti, A.M.; Zanotti, G. Influence of Temperature, Pressure, and Humidity on the Stabilities and Transition Kinetics of the Various Polymorphs of FAPbI3. J. Phys. Chem. C 2020, 124, 22972–22980. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F.; Duan, C.; Yuan, L.; Zhu, H.; Li, J.; Wen, Q.; Waterhouse, G.N.; Yang, X.; Yan, K. Polymerization stabilized black-phase FAPbI3 perovskite solar cells retain 100% of initial efficiency over 100 days. Chem. Eng. J. 2021, 419, 129482. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Stoumpos, C.C.; Liu, Z.; Chang, R.H.; Kanatzidis, M.G. Controllable Perovskite Crystallization at a Gas-Solid Interface for Hole Conductor-Free Solar Cells with Steady Power Conversion Efficiency over 10%. J. Am. Chem. Soc. 2014, 136, 16411–16419. [Google Scholar] [CrossRef] [PubMed]
- Leguy, A.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397. [Google Scholar] [CrossRef]
- Raga, S.R.; Jung, M.C.; Lee, M.V.; Leyden, M.R.; Kato, Y.; Qi, Y. Influence of Air Annealing on High Efficiency Planar Structure Perovskite Solar Cells. Chem. Mater. 2015, 27, 1597–1603. [Google Scholar] [CrossRef]
- Smecca, E.; Numata, Y.; Deretzis, I.; Pellegrino, G.; Boninelli, S.; Miyasaka, T.; Magna, A.L.; Alberti, A. Stability of Solution-Processed MAPbI3 and FAPbI3 Layers. Phys. Chem. Chem. Phys. 2016, 18, 13413–13422. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; Xie, F.; Li, L.; Chen, J.; Fan, J.; Zhao, N. Organic Cation-Dependent Degradation Mechanism of Organotin Halide Perovskites. Adv. Funct. Mater. 2016, 26, 3417–3423. [Google Scholar] [CrossRef]
- Daub, M.; Ketterer, I.; Hillebrecht, H. Syntheses, Crystal Structures, and Optical Properties of the Hexagonal Perovskites Variants ABX3 (B = Ni, A = Gu, FA, MA, X = Cl, Br; B = Mn, A = MA, X = Br). Z. Anorg. Allg. Chem. 2018, 644, 280–287. [Google Scholar] [CrossRef]
- Alberti, A.; Bongiorno, C.; Smecca, E.; Deretzis, I.; Magna, A.L.; Spinella, C. Pb clustering and PbI2 nanofragmentation during methylammonium lead iodide perovskite degradation. Nat. Comm. 2019, 10, 2196. [Google Scholar] [CrossRef]
- Mannino, G.; Deretzis, I.; Smecca, E.; Giannazzo, F.; Valastro, S.; Fisicaro, G.; Magna, A.L.; Ceratti, D.; Alberti, A. CsPbBr3, MAPbBr3, and FAPbBr3 Bromide Perovskite Single Crystals: Interband Critical Points under Dry N2 and Optical Degradation under Humid Air. J. Phys. Chem. C 2021, 125, 4938. [Google Scholar] [CrossRef]
- Bass, K.K.; McAnally, R.E.; Zhou, S.; Djurovich, P.I.; Thompson, M.E.; Melot, B.C. Influence of moisture on the preparation, crystal structure, and photophysical properties of organohalide perovskites. Chem. Commun. 2014, 50, 15819–15822. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- You, J.; Yang, Y.M.; Hong, Z.; Song, T.B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.H.; Li, G.; et al. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef]
- Eperon, G.E.; Habisreutinger, S.N.; Leijtens, T.; Bruijnaers, B.J.; van Franeker, J.J.; deQuilettes, D.W.; Pathak, S.; Sutton, R.J.; Grancini, G.; Ginger, D.S.; et al. The Importance of Moisture in Hybrid Lead Halide Perovskite Thin Film Fabrication. ACS Nano 2015, 9, 9380–9393. [Google Scholar] [CrossRef]
- Pathak, S.; Sepe, A.; Sadhanala, A.; Deschler, F.; Haghighirad, A.; Sakai, N.; Goedel, K.C.; Stranks, S.D.; Noel, N.; Price, M.; et al. Atmospheric Influence upon Crystallization and Electronic Disorder and Its Impact on the Photophysical Properties of Organic-Inorganic Perovskite Solar Cells. ACS Nano 2015, 9, 2311–2320. [Google Scholar] [CrossRef]
- Ko, H.S.; Lee, J.W.; Park, N.G. 15.76% efficiency perovskite solar cells prepared under high relative humidity: Importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815. [Google Scholar] [CrossRef]
- Sci, E.E. The synergistic effect of H2O and DMF towards stable and 20% efficiency inverted perovskite solar cells. Energy Environ. Sci. 2017, 10, 808–817. [Google Scholar]
- Zhang, W.; Xiong, J.; Li, J.; Daoud, W.A. Mechanism of Water Effect on Enhancing the Photovoltaic Performance of Triple-Cation Hybrid Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 12699–12708. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Ma, Z.; Strzalka, J.; Ren, Y.; Lin, K.F.; Wang, L.; Zhou, H.; Jiang, Z.; Chen, W. Mild water intake orients crystal formation imparting high tolerance on unencapsulated halide perovskite solar cells. Cell Rep. Phys. Sci. 2021, 2, 100395. [Google Scholar] [CrossRef]
- Hui, W.; Chao, L.; Lu, H.; Xia, F.; Wei, Q.; Su, Z.; Niu, T.; Tao, L.; Du, B.; Li, D.; et al. Stabilizing black-phase formamidinium perovskite formation at room temperature and high humidity. Science 2021, 371, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Cordero, F.; Craciun, F.; Trequattrini, F.; Imperatori, P.; Paoletti, A.M.; Pennesi, G. Competition between Polar and Antiferrodistortive Modes and Correlated Dynamics of the Methylammonium Molecules in MAPbI3. J. Phys. Chem. Lett. 2018, 9, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Cordero, F.; Craciun, F.; Trequattrini, F.; Generosi, A.; Paci, B.; Paoletti, A.M.; Pennesi, G. Stability of Cubic FAPbI3 from X-ray Diffraction, Anelastic, and Dielectric Measurements. J. Phys. Chem. Lett. 2019, 10, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Cordero, F.; Trequattrini, F.; Craciun, F.; Paoletti, A.M.; Pennesi, G.; Zanotti, G. Cation reorientation and octahedral tilting in the metal-organic perovskites MAPI and FAPI. J. Alloys Compd. 2021, 867, 158210. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Wozny, S.; Alkurd, N.R.; Yang, M.; Kovarik, L.; Holesinger, T.G.; Al-Jassim, M.; Zhu, K.; Zhou, W.; Berry, J.J. Effect of Water Vapor, Temperature, and Rapid Annealing on Formamidinium Lead Triiodide Perovskite Crystallization. ACS Energy Lett. 2016, 1, 155–161. [Google Scholar] [CrossRef]
- Kakekhani, A.; Katti, R.N.; Rappe, A.M. Water in hybrid perovskites: Bulk MAPbI3 degradation via super-hydrous state. APL Mater. 2019, 7, 041112. [Google Scholar] [CrossRef]
- Cordero, F.; Bella, L.D.; Corvasce, F.; Latino, P.M.; Morbidini, A. An insert for anelastic spectroscopy measurements from 80 K to 1100 K. Meas. Sci. Technol. 2009, 20, 015702. [Google Scholar] [CrossRef]
- Chen, T.; Foley, B.J.; Park, C.; Brown, C.M.; Harriger, L.W.; Lee, J.; Ruff, J.; Yoon, M.; Choi, J.J.; Lee, S.H. Entropy-Driven Structural Transition and Kinetic Trapping in Formamidinium Lead Iodide Perovskite. Sci. Adv. 2016, 2, e1601650. [Google Scholar] [CrossRef]
- Becker, M.; Klüner, T.; Wark, M. Formation of hybrid ABX3 perovskite compounds for solar cell application: First-principles calculations of effective ionic radii and determination of tolerance factors. Dalton Trans. 2017, 46, 3500–3509. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Megaw, H.D. Origin of Ferroelectricity in Barium Titanate and Other Perovskite-Type Crystals. Acta Cryst. 1952, 5, 739–749. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Cordero, F.; Craciun, F.; Trequattrini, F. Ionic Mobility and Phase Transitions in Perovskite Oxides for Energy Applications. Challenges 2017, 8, 5. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kafalas, J.A.; Longo, J.M. High Pressure Synthesis. In Preparative Methods in Solid State Chemistry; Hagenmuller, P., Ed.; Academic Press: London, UK, 1972. [Google Scholar]
- Deng, Z.; Kieslich, G.; Bristowe, P.D.; Cheetham, A.K.; Sun, S. Octahedral connectivity and its role in determining the phase stabilities and electronic structures of low-dimensional, perovskite-related iodoplumbates. APL Mater. 2018, 6, 114202. [Google Scholar] [CrossRef]
- Ramsdell, L.S. Studies on silicon carbide. Am. Mineral. 1947, 32, 64–82. [Google Scholar]
- Gratia, P.; Zimmermann, I.; Schouwink, P.; Yum, J.H.; Audinot, J.N.; Sivula, K.; Wirtz, T.; Nazeeruddin, M.K. The Many Faces of Mixed Ion Perovskites: Unraveling and Understanding the Crystallization Process. ACS Energy Lett. 2017, 2, 2686–2693. [Google Scholar] [CrossRef]
- Szostak, R.; Marchezi, P.E.; dos Santos Marques, A.; da Silva, J.C.; de Holanda, M.S.; Soares, M.M.; Tolentino, H.C.N.; Nogueira, A.F. Exploring the formation of formamidinium-based hybrid perovskites by antisolvent methods: In situ GIWAXS measurements during spin coating. Sustain. Energy Fuels 2019, 3, 2287–2297. [Google Scholar] [CrossRef]
- Dang, H.X.; Wang, K.; Ghasemi, M.; Tang, M.C.; Bastiani, M.D.; Aydin, E.; Dauzon, E.; Barrit, D.; Peng, J.; Smilgies, D.M.; et al. Multi-cation Synergy Suppresses Phase Segregation in Mixed-Halide Perovskites. Joule 2019, 3, 1746–1764. [Google Scholar] [CrossRef]
- Tanaka, H.; Oku, T.; Ueoka, N. Structural stabilities of organic-inorganic perovskite crystals. Jpn. J. Appl. Phys. 2018, 57, 08RE12. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef]
- Rosales, B.A.; Mundt, L.E.; Allen, T.G.; Moore, D.T.; Prince, K.J.; Wolden, C.A.; Rumbles, G.; Schelhas, L.T.; Wheeler, L.M. Reversible multicolor chromism in layered formamidinium metal halide perovskites. Nat. Commun. 2020, 11, 5234. [Google Scholar] [CrossRef]
- Flores-Livas, J.; Tomerini, D.; Amsler, M.; Boziki, A.; Rothlisberger, U.; Goedecker, S. Emergence of hidden phases of methylammonium lead iodide (CH3NH3PbI3) upon compression. Phys. Rev. Mater. 2018, 2, 085201. [Google Scholar] [CrossRef]
- Palosz, B.; Steurer, W.; Schulz, H. The structure of PbI2 polytypes 2H and 4H: A study of the 2H–4H transition. J. Phys. Condens. Matter 1990, 2, 5285. [Google Scholar] [CrossRef]
- Brenner, T.M.; Rakita, Y.; Orr, Y.; Klein, E.; Feldman, I.; Elbaum, M.; Cahen, D.; Hodes, G. Conversion of Single Crystalline PbI2 to CH3NH3PbI3: Structural Relations and Transformation Dynamics. Chem. Mater. 2016, 28, 6501–6510. [Google Scholar] [CrossRef]
- Barrit, D.; Cheng, P.; Darabi, K.; Tang, M.C.; Smilgies, D.M.; Liu, S.F.; Anthopoulos, T.D.; Zhao, K.; Amassian, A. Room-Temperature Partial Conversion of α-FAPbI3 Perovskite Phase via PbI2 Solvation Enables High-Performance Solar Cells. Adv. Funct. Mater. 2020, 30, 1907442. [Google Scholar] [CrossRef]
- Zhang, L.; Sit, P.H.L. Ab Initio Study of Interaction of Water, Hydroxyl Radicals, and Hydroxide Ions with CH3NH3PbI3 and CH3NH3PbBr3 Surfaces. J. Phys. Chem. C 2015, 119, 22370–22378. [Google Scholar] [CrossRef]
- Wu, C.G.; Chiang, C.H.; Tseng, Z.L.; Nazeeruddin, M.K.; Hagfeldt, A.; Grätzel, M. High efficiency stable inverted perovskite solar cells without current hysteresis. Energy Environ. Sci. 2015, 8, 2725–2733. [Google Scholar] [CrossRef]
- Mosconi, E.; Azpiroz, J.M.; Angelis, F.D. Ab Initio Molecular Dynamics Simulations of Methylammonium Lead Iodide Perovskite Degradation by Water. Chem. Mater. 2015, 27, 4885–4892. [Google Scholar] [CrossRef]
- Caddeo, C.; Saba, M.I.; Meloni, S.; Filippetti, A.; Mattoni, A. Collective Molecular Mechanisms in the CH3NH3PbI3 Dissolution by Liquid Water. ACS Nano 2017, 11, 9183–9190. [Google Scholar] [CrossRef] [PubMed]
- Akbali, B.; Topcu, G.; Guner, T.; Ozcan, M.; Demir, M.M.; Sahin, H. CsPbBr3 perovskites: Theoretical and experimental investigation on water-assisted transition from nanowire formation to degradation. Phys. Rev. Mater. 2018, 2, 034601. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in Situ Techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Imler, G.H.; Li, X.; Xu, B.; Dobereiner, G.E.; Dai, H.L.; Rao, Y.; Wayland, B.B. Solid state transformation of the crystalline monohydrate (CH3NH3)PbI3(H2O) to the (CH3NH3)PbI3 perovskite. Chem. Commun. 2015, 51, 11290–11292. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.R.; Robertson, K.N.; Cameron, T.S.; Knop, O. Alkylammonium lead halides. Part 1. Isolated ions in (CH3NH3)4PbI6·2H2O. Can. J. Chem. 1987, 65, 1042–1046. [Google Scholar] [CrossRef]
- Hall, G.N.; Stuckelberger, M.; Nietzold, T.; Hartman, J.; Park, J.S.; Werner, J.; Niesen, B.; Cummings, M.L.; Rose, V.; Ballif, C.; et al. The Role of Water in the Reversible Optoelectronic Degradation of Hybrid Perovskites at Low Pressure. J. Phys. Chem. C 2017, 121, 25659–25665. [Google Scholar] [CrossRef]
- Jong, U.G.; Yu, C.J.; Ri, G.C.; McMahon, A.P.; Harrison, N.M.; Barnes, P.F.; Walsh, A. Influence of water intercalation and hydration on chemical decomposition and ion transport in methylammonium lead halide perovskites. J. Mater. Chem. A 2018, 6, 1067–1074. [Google Scholar] [CrossRef]
- Kye, Y.H.; Yu, C.J.; Jong, U.G.; Chen, Y.; Walsh, A. Critical Role of Water in Defect Aggregation and Chemical Degradation of Perovskite Solar Cells. J. Phys. Chem. Lett. 2018, 9, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Waghmare, U.V. Effect of humidity on the orientational ordering of CH3 in methylammonium lead iodide. Bull. Mater. Sci. 2020, 43, 304. [Google Scholar] [CrossRef]
- Dastidar, S.; Hawley, C.J.; Dillon, A.D.; Gutierrez-Perez, A.; Spanier, J.E.; Fafarman, A.T. Quantitative Phase-Change Thermodynamics and Metastability of Perovskite-Phase Cesium Lead Iodide. J. Phys. Chem. Lett. 2017, 8, 1278–1282. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved Phase Stability of Formamidinium Lead Triiodide Perovskite by Strain Relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Nowick, A.S.; Berry, B.S. Anelastic Relaxation in Crystalline Solids; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Benoit, W. Mechanical Spectroscopy Q−1 2001: With Applications to Materials Science; Trans Tech Publications: Totton, UK, 2001; Chapter 5.1. [Google Scholar]
- Müller, C.; Glaser, T.; Plogmeyer, M.; Sendner, M.; Döring, S.; Bakulin, A.A.; Brzuska, C.; Scheer, R.; Pshenichnikov, M.S.; Kowalsky, W.; et al. Water Infiltration in Methylammonium Lead Iodide Perovskite: Fast and Inconspicuous. Chem. Mater. 2015, 27, 7835–7841. [Google Scholar] [CrossRef]
- Huang, J.; Tan, S.; Lund, P.D.; Zhou, H. Impact of H2O on organic-inorganic hybrid perovskite solar cells. Energy Environ. Sci. 2017, 10, 2284–2311. [Google Scholar] [CrossRef]
- Murugadoss, G.; Arunachalam, P.; Panda, S.K.; Kumar, M.R.; Jothiramalingam, R.; Al-lohedan, H.; Wasmiah, M.D. Crystal stabilization of a-FAPbI3 perovskite by rapid annealing method in Industrial scale. J. Mater. Res. Technol. 2021, 12, 1924–1930. [Google Scholar] [CrossRef]
- Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. NH2CH-NH2PbI3: An Alternative Organolead Iodide Perovskite Sensitizer for Mesoscopic Solar Cells. Chem. Mater. 2014, 26, 1485–1491. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Thermal degradation of formamidinium based lead halide perovskites into sym-triazine and hydrogen cyanide observed by coupled thermogravimetry-mass spectrometry analysis. J. Mater. Chem. A 2019, 7, 16912–16919. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Yeghishyan, A.V.; Moskovskikh, D.O.; Kapaldo, J.; Mintairov, A.; Mukasyan, A.S. Mechanochemical synthesis of methylammonium lead iodide perovskite. J. Mater. Sci. 2016, 51, 9123–9130. [Google Scholar] [CrossRef]
- Camus, C.; Kaspari, C.; Rappich, J.; Blank, V.; Nickel, N. Going beyond Alchemy: In-situ Analysis of Perovskite Growth by Optical Reflectance. In Proceedings of the 47th IEEE Photovoltaic Specialists Conference, Calgary, AB, Canada, 15 June–21 August 2020. [Google Scholar]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Matsushima, T.; Fujihara, T.; Potscavage, W.J., Jr.; Adachi, C. Degradation Mechanisms of Solution-Processed Planar Perovskite Solar Cells: Thermally Stimulated Current Measurement for Analysis of Carrier Traps. Adv. Mater. 2016, 28, 466–471. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, F.; Craciun, F.; Paoletti, A.M.; Zanotti, G. Structural Transitions and Stability of FAPbI3 and MAPbI3: The Role of Interstitial Water. Nanomaterials 2021, 11, 1610. https://doi.org/10.3390/nano11061610
Cordero F, Craciun F, Paoletti AM, Zanotti G. Structural Transitions and Stability of FAPbI3 and MAPbI3: The Role of Interstitial Water. Nanomaterials. 2021; 11(6):1610. https://doi.org/10.3390/nano11061610
Chicago/Turabian StyleCordero, Francesco, Floriana Craciun, Anna Maria Paoletti, and Gloria Zanotti. 2021. "Structural Transitions and Stability of FAPbI3 and MAPbI3: The Role of Interstitial Water" Nanomaterials 11, no. 6: 1610. https://doi.org/10.3390/nano11061610
APA StyleCordero, F., Craciun, F., Paoletti, A. M., & Zanotti, G. (2021). Structural Transitions and Stability of FAPbI3 and MAPbI3: The Role of Interstitial Water. Nanomaterials, 11(6), 1610. https://doi.org/10.3390/nano11061610








