Electronic Structure and d-Band Center Control Engineering over Ni-Doped CoP3 Nanowall Arrays for Boosting Hydrogen Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
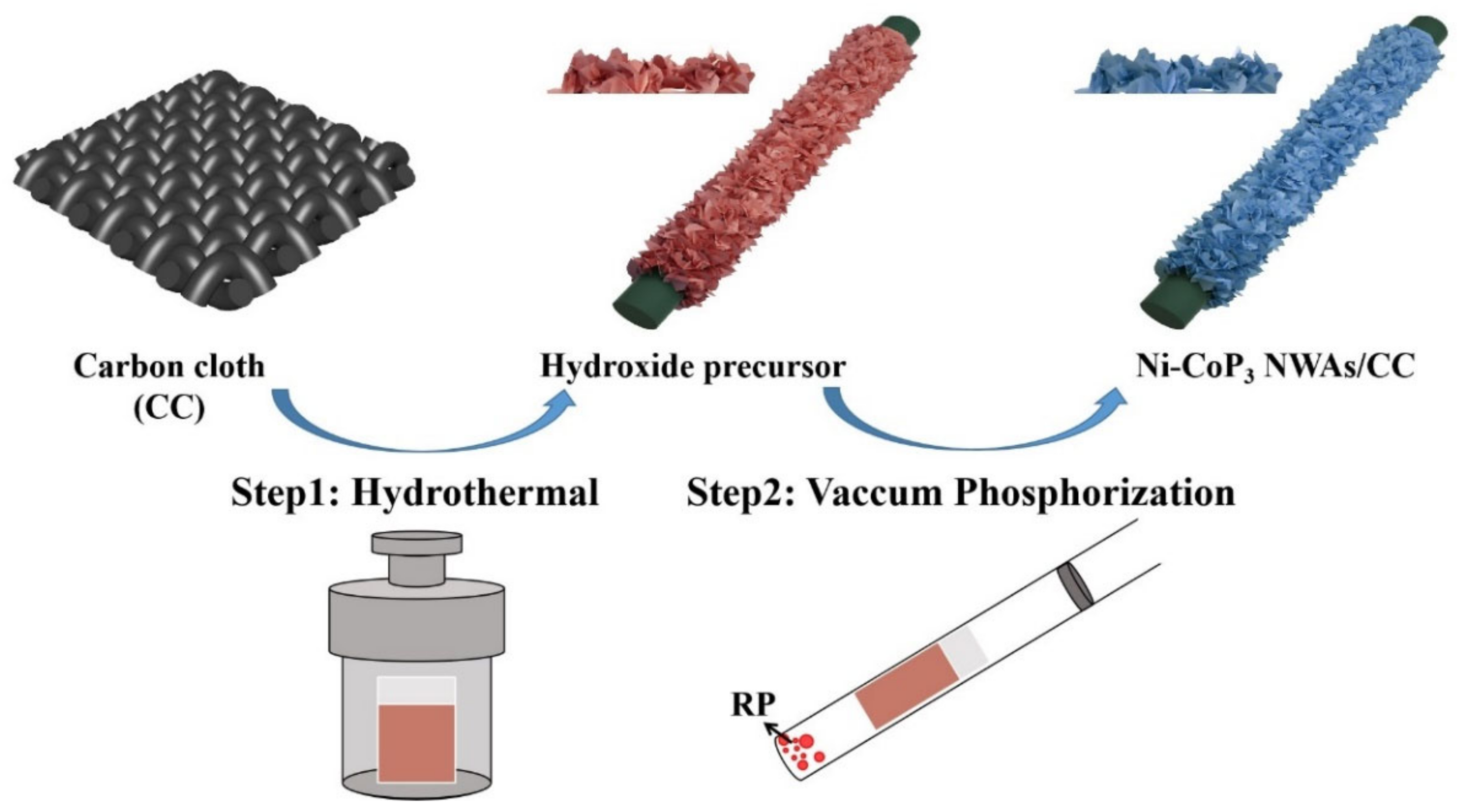
2.2. Synthesis of Ni-Doped CoP3 NWAs
2.3. Materials Characterization
2.4. Electrochemical Measurements
2.5. Theoretical Calculation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorffff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Sun, H.M.; Yan, Z.H.; Liu, F.M.; Xu, W.C.; Cheng, F.Y.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Gusnao, R.; Sofer, Z.; Pumera, M. Metal phosphorous Trichalcogenides (MPCh(3)): From synthesis to contemporary energy challenges. Angew. Chem. Int. Ed. 2019, 58, 9326–9337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Yang, S.X.; Li, H.Y.; Zan, Y.X.; Li, X.Y.; Zhu, Y.; Dou, M.L.; Wang, F. Sustainable carbonaceous materials derived from biomass as metal-free electrocatalysts. Adv. Mater. 2019, 31, 1805718. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, X.; Liu, Y.P.; Ai, X.; Asefa, T.; Zou, X.X. Active site engineering in porous electrocatalysts. Adv. Mater. 2020, 32, 2002435. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Plebanski, M. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Dai, Q.; Wang, M.; Qu, L.; Dai, L. Earth-abundant carbon catalysts for renewable generation of clean energy from sunlight and water. Nano Energy 2017, 41, 367–376. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.H.; Jiao, L.F. Multifunctional transition metal-based phosphides in energy-related electrocatalysis. Adv. Energy Mater. 2019, 10, 1902104. [Google Scholar] [CrossRef]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like NiCoP for highly efficient overall water splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sheng, M.; Bhushan, M.W.; Sun, Y. Hierarchically porous urchin-like Ni2P superstructures supported on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. ACS Catal. 2016, 6, 714–721. [Google Scholar] [CrossRef]
- Wu, T.; Pi, M.; Zhang, D.; Chen, S. 3D structured porous CoP3 nanoneedle arrays as an efficient bifunctional electrocatalyst for the evolution reaction of hydrogen and oxygen. J. Mater. Chem. A 2016, 4, 14539–14544. [Google Scholar] [CrossRef]
- Men, Y.; Tan, Y.; Li, P.; Cao, X.; Jia, S.; Wang, J.; Chen, S.; Luo, W. Tailoring the 3d-orbital electron filling degree of metal center to boost alkaline hydrogen evolution electrocatalysis. Appl. Catal. B: Environ. 2021, 284, 119718. [Google Scholar] [CrossRef]
- Geng, S.; Tian, F.Y.; Li, M.G.; Guo, X.; Yu, Y.S.; Yang, W.W.; Hou, Y.L. Hole-rich CoP nanosheets with an optimized d-band center for enhancing pH-universal hydrogen evolution electrocatalysis. J. Mater. Chem. A 2021, 9, 8561–8567. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Liu, J. CoP2 nanoparticles on reduced graphene oxide sheets as a super-efficient bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2016, 4, 4686–4690. [Google Scholar] [CrossRef]
- Liu, W.; Geng, P.; Li, S.Q.; Liu, W.H.; Fan, D.Y.; Lu, H.D.; Li, Z.H.; Liu, Y.P. Tuning electronic configuration of WP2 nanosheet arrays via nickel doping for high-efficiency hydrogen evolution reaction. J. Energy Chem. 2021, 55, 17–24. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Lee, J.S. Sulfur and nitrogen dual-doped molybdenum phosphide nanocrystallites as an active and stable hydrogen evolution reaction electrocatalyst in acidic and alkaline media. ACS Catal. 2017, 7, 3030–3038. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Russo, P.A.; Pinna, N. Recent advances in multimetal and doped transition-metal phosphides for the hydrogen evolution reaction at different pH values. ACS Appl. Mater. Interfaces 2021, 13, 22077–22097. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, R.; Lu, W.; He, L.; Jiang, X.; Asiri, A.M.; Sun, X. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation. Adv. Mater. 2017, 29, 1602441. [Google Scholar] [CrossRef]
- Liu, T.T.; Ma, X.; Liu, D.N.; Hao, S.; Du, G.; Ma, Y.J.; Asiri, A.M.; Sun, X.P.; Chen, L. Mn doping of CoP nanosheets array: An efficient electrocatalyst for hydrogen evolution reaction with enhanced activity at all pH values. ACS Catal. 2017, 7, 98–102. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Lin, Y.; Liu, C. Metal doping effect of the M-Co2P/nitrogendoped carbon nanotubes (M = Fe, Ni, Cu) hydrogen evolution hybrid catalysts. ACS Appl. Mater. Inter. 2016, 8, 13890–13901. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Cheng, Y.F.; He, J.R.; Yu, B.; Wang, B.; Zhang, X.J.; Li, W.X.; Ranadoss, M.; Zhang, W.L.; Yang, D.X. Vertical V-doped CoP nanowall arrays as a highly efficient and stable electrocatalyst for the hydrogen evolution reaction at all pH values. ACS Appl. Energy Mater. 2020, 3, 1027–1035. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Popczun, E.J.; McEnaney, J.M.; Schaak, R.E. Nanostructured Co2P electrocatalyst for the hydrogen evolution reaction and direct comparison with morphologically equivalent CoP. Chem. Mater. 2015, 27, 3769–3774. [Google Scholar] [CrossRef]
- Popczun, E.J.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 5531–5534. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Xu, S.; Kong, D.; Cha, J.J.; Zheng, G.; Hsu, P.C.; Yan, K.; Bradshaw, D.; Prinz, F.B.; et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhuang, Z.W.; Cao, X.; Zhang, C.; Peng, Q.; Chen, C.; Li, Y.D. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 2020, 49, 2215–2264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.S.; Zhao, P.X.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, K.; Lin, Y.; Cao, X.; Cheng, Y.; Liu, S.; Zeng, L.; Cheong, W.; Zhao, D.; Wu, K.; et al. Electronic structure and d-band center control engineering over M-doped CoP (M = Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production. Nano Energy 2019, 56, 411–419. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 2014, 126, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Andrew, P.G.; Stephen, D.W.; Cavell, G.; Arthur, M. Examination of the bonding in binary transition-metal monophosphides MP (M) Cr, Mn, Fe, Co) by X-ray photoelectron spectroscopy. Inorg. Chem. 2005, 44, 8988–8998. [Google Scholar]
- Pi, M.; Guo, W.; Wu, T.; Wang, X.; Zhang, D.; Wang, S.; Chen, S. Pulsed laser deposition-assisted synthesis of porous WP2 nanosheet arrays integrated on graphite paper as a 3D flexible cathode for efficient hydrogen evolution. J. Power Sources 2017, 364, 253–257. [Google Scholar] [CrossRef]
- Wu, T.; Dang, Y.; He, J.; Li, T.; Qu, G.; Gao, Y.; Tan, F. Synthesis and mechanism investigation of three-dimensional porous CoP3 nanoplate arrays as efficient hydrogen evolution reaction electrocatalyst. Appl. Surf. Sci. 2019, 494, 179–186. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. 2014, 126, 9731–9735. [Google Scholar] [CrossRef]
- Wilson, A.D.; Newell, R.H.; McNevin, M.J.; Muckerman, J.T.; Dubois, M.R.; Dubois, D.L. Hydrogen oxidation and production using nickel-based molecular catalysts with positioned proton relays. J. Am. Chem. Soc. 2006, 128, 358–366. [Google Scholar] [CrossRef]
- Barton, B.E.; Rauchfuss, T.B. Hydride-containing models for the active site of the nickel-iron hydrogenases. J. Am. Chem. Soc. 2010, 132, 14877–14885. [Google Scholar] [CrossRef]
- Skúlason, E.; Karlberg, G.S.; Rossmeisl, J.; Bligaard, T.; Greeley, J.; Jónsson, H.; Nørskov, J.K. Density functional theory calculations for the hydrogen evolution reaction in an electrochemical double layer on the Pt(111) electrode. Phys. Chem. Chem. Phys. 2007, 9, 3241–3250. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.B.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K. Large-scale, density functional theory-based screening of alloys for hydrogen evolution. Surf. Sci. 2007, 601, 1590–1598. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tsai, C.; Chan, K.; Benck, J.D.; Nørskov, J.K.; Abild-Pedersen, F.; Jaramillo, T.F. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ. Sci. 2015, 8, 3022–3029. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.Y.; Wang, H.; Jiao, Y.; Hu, Z.; Zheng, Y.; Zheng, L.; Mao, J.; Liu, H.; Du, X.W.; et al. Activating cobalt(II) oxide nanorods for efficient electrocatalysis by strain engineering. Nat. Commun. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Ling, T.; Vasileff, A.; Qiao, S.Z. S-NiFe2O4 ultra-small nanoparticle built nanosheets for efficient water splitting in alkaline and neutral pH. Nano Energy 2017, 40, 264–273. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S.Z. The hydrogen evolution reaction in alkaline solution: From theory, single crystal models, to practical electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 7568–7579. [Google Scholar] [CrossRef] [PubMed]
- Staszak-Jirkovsky, J.; Malliakas, C.D.; Lopes, P.P.; Danilovic, N.; Kota, S.S.; Chang, K.C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.R.; Kanatzidis, M.G.; et al. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197–203. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Yu, S.; Wen, T.; Zhu, X.; Yang, F.; Sun, X.; Wang, X.; Hu, W. Ternary NiCo2Px nanowires as pH-universal electrocatalysts for highly efficient hydrogen evolution reaction. Adv. Mater. 2017, 29, 1605502. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Wu, T.; Xu, M.; Zhou, D.; Xiao, Z. Electronic Structure and d-Band Center Control Engineering over Ni-Doped CoP3 Nanowall Arrays for Boosting Hydrogen Production. Nanomaterials 2021, 11, 1595. https://doi.org/10.3390/nano11061595
Qi J, Wu T, Xu M, Zhou D, Xiao Z. Electronic Structure and d-Band Center Control Engineering over Ni-Doped CoP3 Nanowall Arrays for Boosting Hydrogen Production. Nanomaterials. 2021; 11(6):1595. https://doi.org/10.3390/nano11061595
Chicago/Turabian StyleQi, Jing, Tianli Wu, Mengyao Xu, Dan Zhou, and Zhubing Xiao. 2021. "Electronic Structure and d-Band Center Control Engineering over Ni-Doped CoP3 Nanowall Arrays for Boosting Hydrogen Production" Nanomaterials 11, no. 6: 1595. https://doi.org/10.3390/nano11061595
APA StyleQi, J., Wu, T., Xu, M., Zhou, D., & Xiao, Z. (2021). Electronic Structure and d-Band Center Control Engineering over Ni-Doped CoP3 Nanowall Arrays for Boosting Hydrogen Production. Nanomaterials, 11(6), 1595. https://doi.org/10.3390/nano11061595





