Graphene Oxide Layer-by-Layer Films for Sensors and Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Preparation
2.2. Characterization Techniques
3. Results
3.1. UV-Visible Characterization of GO Aqueous Solution and Cast Films
3.2. Buildup of GO Based Layer-by-Layer Films
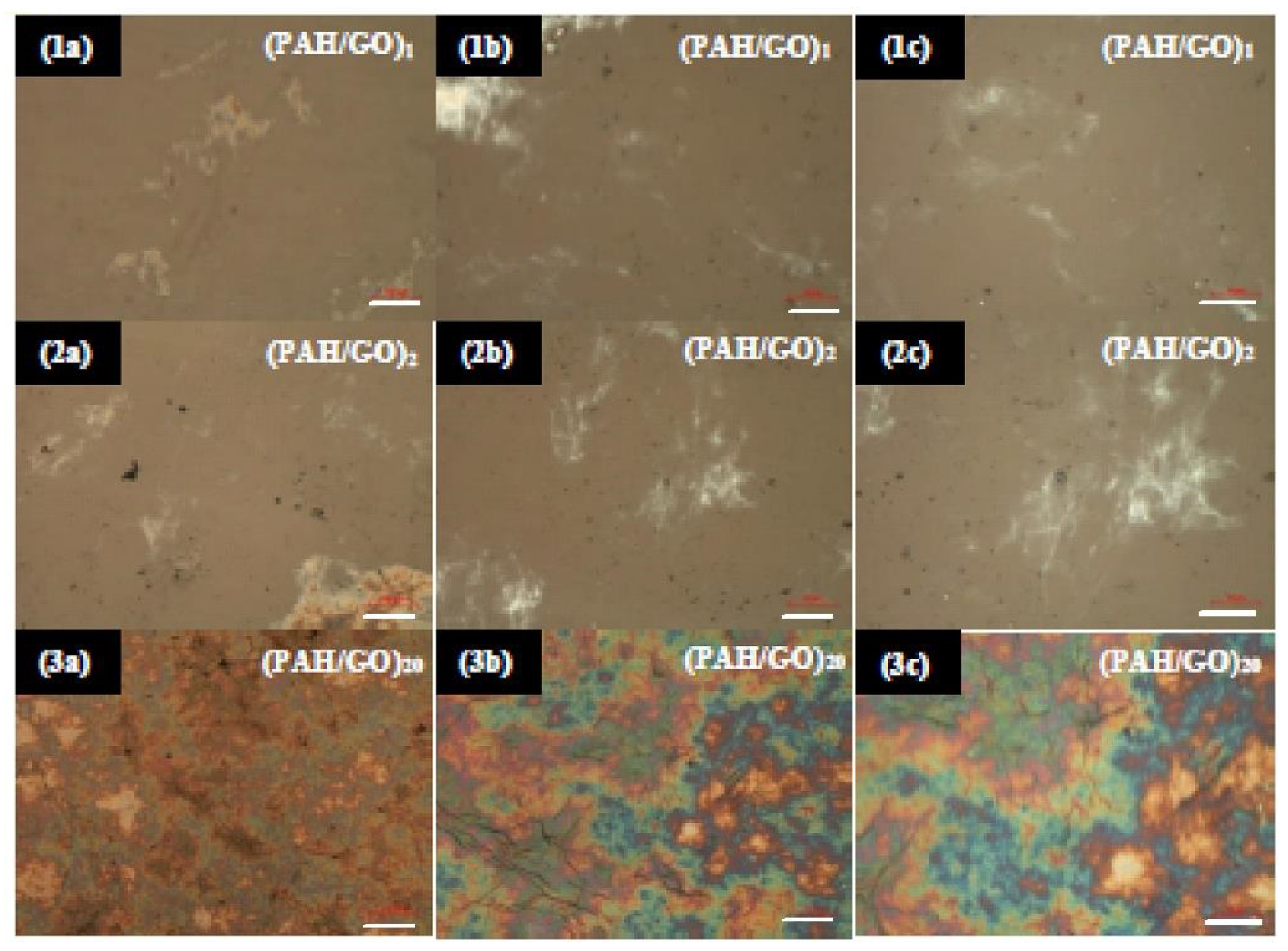
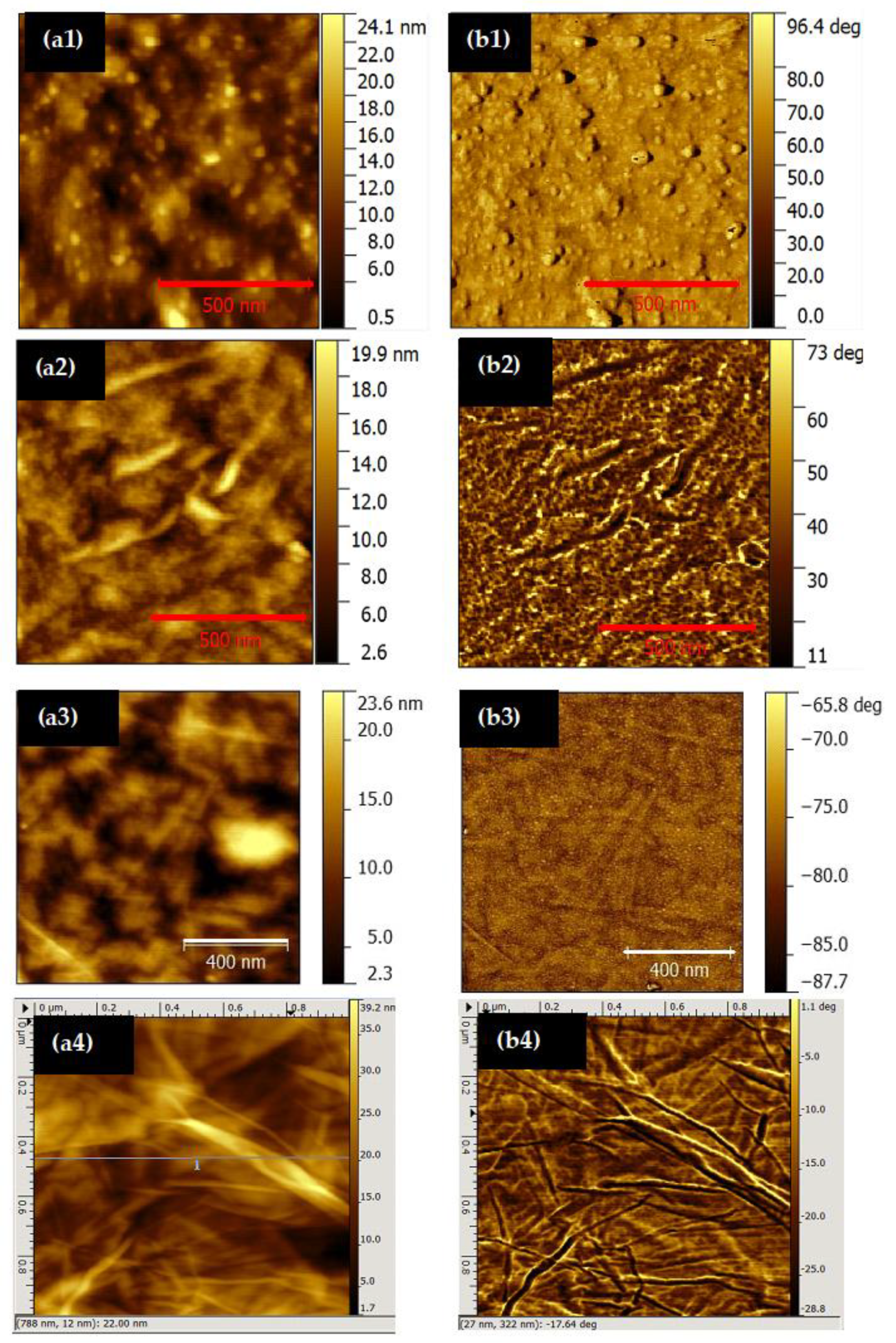
3.3. Surface Characterization
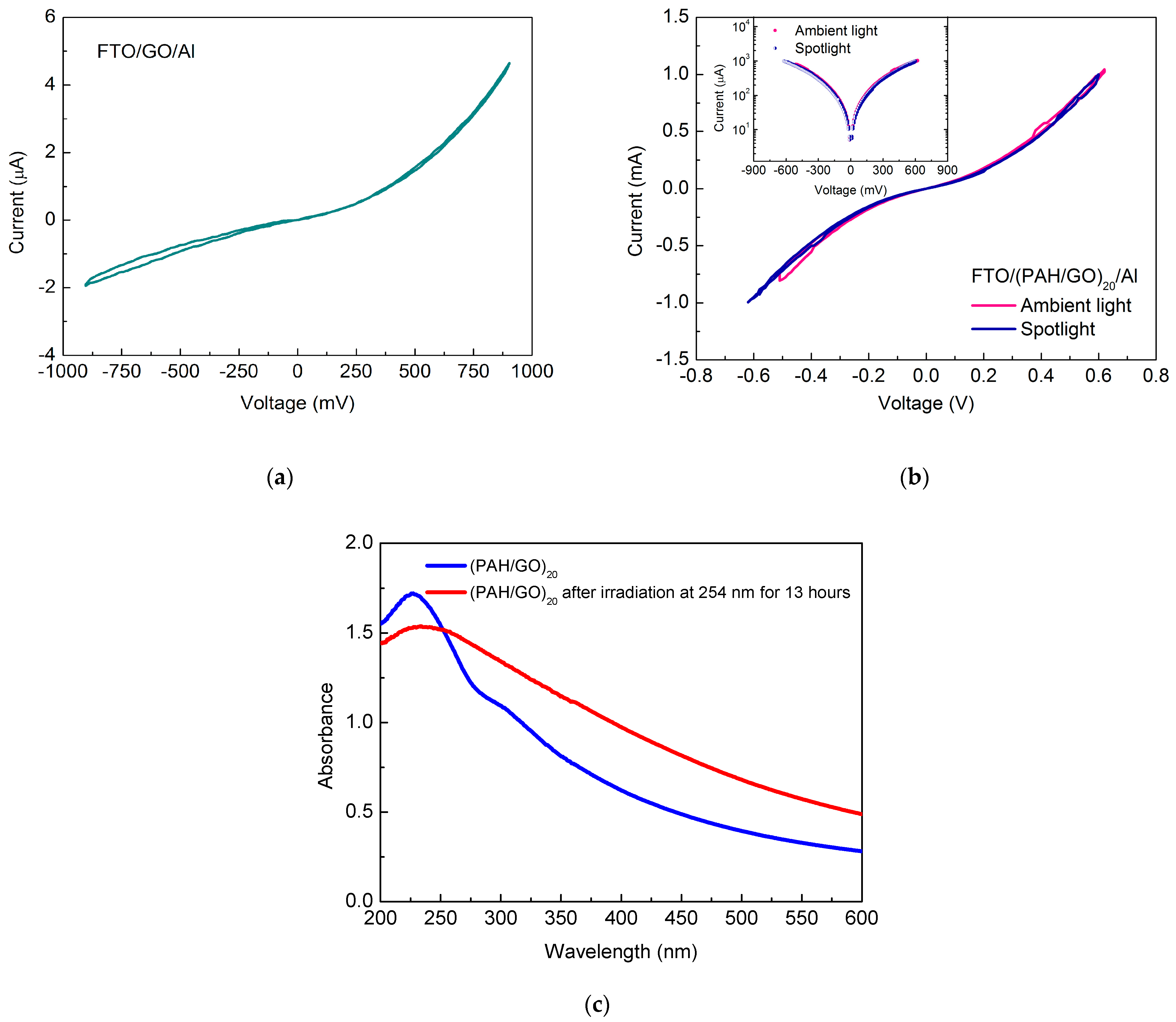
3.4. Electrical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Singh, A.K.; Hussain, S.; Hui, K.N.; Hui, K.S.; Eom, J.; Jung, J.; Singh, J. Graphene: Synthesis, properties and application in transparent electronic devices. Rev. Adv. Sci. Eng. 2013, 2, 238–258. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C.; Jiang, B.; Iocozzia, J.; He, C.; Shi, D.; Jiang, T.; Lin, Z. Graphene-based materials with tailored nanostructures for energy conversion and storage. Mater. Sci. Eng. R Rep. 2016, 102, 1–72. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, S.; Zhou, X.; Huang, X.; Zhang, Q.; Boey, F.; Zhang, H. Electrochemical deposition of ZnO nanorods on transparent reduced graphene oxide electrodes for hybrid solar cells. Small 2010, 6, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Le Xie, J.; Guo, C.; Li, C.M. Construction of one-dimensional nanostructures on graphene for efficient energy conversion and storage. Energy Environ. Sci. 2014, 7, 2559–2579. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Chi, H.; Liu, Y.; Hou, C.L.; Fang, D. Recent development of graphene materials applied in polymer solar cell. Renew. Sustain. Energy Rev. 2015, 43, 973–980. [Google Scholar] [CrossRef]
- Patil, K.; Rashidi, S.; Wang, H.; Wei, W. Recent progress of graphene-based photoelectrode materials for dye-sensitized solar cells. Int. J. Photoenergy 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Liu, B.; Gao, H.; Zhang, Y.; Yan, H.; Song, X. Graphene/Si Schottky solar cells: A review of recent advances and prospects. RSC Adv. 2019, 9, 863–877. [Google Scholar] [CrossRef]
- Das, S.; Pandey, D.; Thomas, J.; Roy, T. The Role of Graphene and other 2D materials in solar photovoltaics. Adv. Mater. 2019, 31, e1802722. [Google Scholar] [CrossRef]
- Goyenola, C.; Stafström, S.; Hultman, L.; Gueorguiev, A.G.K. Structural patterns arising during synthetic growth of fullerene-like sulfocarbide. J. Phys. Chem. C 2012, 116, 21124–21131. [Google Scholar] [CrossRef]
- Schmidt, S.; Greczynski, G.; Goyenola, C.; Gueorguiev, G.; Czigány, Z.; Jensen, J.; Ivanov, I.G.; Hultman, L. CFx thin solid films deposited by high power impulse magnetron sputtering: Synthesis and characterization. Surf. Coat. Technol. 2011, 206, 646–653. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2011, 25, 15–34. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nat. Cell Biol. 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Marques, I.; Magalhâes-Mota, G.; Pires, F.; Sério, S.; Ribeiro, P.A.; Raposo, M. Detection of traces of triclosan in water. Appl. Surf. Sci. 2017, 421, 142–147. [Google Scholar] [CrossRef]
- Raposo, M.; Oliveira, O.N., Jr.; Dhanabalan, A. Langmuir-Blodgett (LB) and self-assembled (SA) films. In Handbook of Surfaces and Interfaces of Materials; Nalwa, H.S., Ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The future of layer-by-layer assembly: A tribute to ACS Nano associate editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-X.; Miao, J.; Liu, B. Layer-by-layer self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J. Am. Chem. Soc. 2014, 136, 1559–1569. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Li, M.; Yu, X.; Liu, H. A new biocomputing platform based on potential- and pH-sensitive bioelectrocatalysis and layer-by-layer films assembled with graphene derivatives and weak polyelectrolyte. Electrochim. Acta 2016, 220, 562–572. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Feng, L.; Li, X.; An, Q. A scalable interfacial engineering strategy for a finely tunable, homogeneous MoS2/rGO-based HER catalytic structure. Adv. Mater. Interfaces 2020, 7, 1902022. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Gong, J.; Li, Z.; Zhang, J. Hybrid poly(allylamine hydrochloride)–graphene oxide microcapsules: Preparation, characterization and application in textiles with controlled release behavior. Mater. Adv. 2020, 1, 804–813. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Lutkenhaus, J.L.; Siqueira, J.R. Building up nanostructured layer-by-layer films combining reduced graphene oxide-manganese dioxide nanocomposite in supercapacitor electrodes. Thin Solid Films 2021, 718, 138483. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Molinnus, D.; Beging, S.; Siqueira, J.R., Jr.; Schöning, M.J. Biosensor based on self-assembled films of graphene oxide and polyaniline using a field-effect device platform. Phys. Status Solidi A 2021, 2000747. [Google Scholar] [CrossRef]
- Magro, C.; Zagalo, P.; Pereira-Da-Silva, J.; Mateus, E.P.; Ribeiro, A.B.; Ribeiro, P.; Raposo, M. Polyelectrolyte based sensors as key to achieve quantitative electronic tongues: Detection of triclosan on aqueous environmental matrices. Nanomaterials 2020, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Eden, S.; Limao-Vieira, P.; Hoffmann, S.V.; Mason, N. VUV photoabsorption in CF3X (X = Cl, Br, I) fluoro-alkanes. Chem. Phys. 2006, 323, 313–333. [Google Scholar] [CrossRef]
- Ferreira, Q.; Gomes, P.J.; Ribeiro, P.A.; Jones, N.C.; Hoffmann, S.V.; Mason, N.J.; Oliveira, O.N.; Raposo, M. Determination of Degree of Ionization of Poly(allylamine hydrochloride) (PAH) and Poly[1-[4-(3-carboxy-4 hydroxyphenylazo)benzene sulfonamido]-1,2-ethanediyl, sodium salt] (PAZO) in layer-by-layer films using vacuum photoabsorption spectroscopy. Langmuir 2012, 29, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.J.; Ferraria, A.M.; Rego, A.; Hoffmann, S.V.; Ribeiro, P.A.; Raposo, M. Energy thresholds of DNA damage induced by uv radiation: An XPS study. J. Phys. Chem. B 2015, 119, 5404–5411. [Google Scholar] [CrossRef]
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 9 June 2021).
- Meriga, V.; Valligatla, S.; Sundaresan, S.; Cahill, C.; Dhanak, V.R.; Chakraborty, A.K. Optical, electrical, and electrochemical properties of graphene based water soluble polyaniline composites. J. Appl. Polym. Sci. 2015, 132, 42766. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds; Wiley: New York, NY, USA, 1991; ISBN 0471 63404 2. [Google Scholar]
- Duarte, A.A.; Gomes, P.J.; Ribeiro, J.H.F.; Ribeiro, P.A.; Hoffmann, S.V.; Mason, N.J.; Oliveira, O.N.; Raposo, M. Characterization of PAH/DPPG layer-by-layer films by VUV spectroscopy. Eur. Phys. J. E-Soft Matter 2013, 36, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, Y.; Yin, G.; Lin, Y. Carbon nanotubes decorated with Pt nanoparticles via electrostatic self-assembly: A highly active oxygen reduction electrocatalyst. J. Mater. Chem. 2010, 20, 2826–2830. [Google Scholar] [CrossRef]
- Bédard, M.F.; Sadasivan, S.; Sukhorukov, G.B.; Skirtach, A. Assembling polyelectrolytes and porphyrins into hollow capsules with laser-responsive oxidative properties. J. Mater. Chem. 2009, 19, 2226–2233. [Google Scholar] [CrossRef]
- Ferreira, Q.; Ribeiro, P.A.; Raposo, M. Villain’s fractal growth of poly[1-[4-(3-carboxy-4-hydroxyphenylazo) benzenesulfonamido]-1,2-ethanediyl, sodium salt] J-aggregates onto layer-by-layer films and its effect on film absorbance spectrum. J. Appl. Phys. 2013, 113, 243508. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Chhowalla, M.; Cho, K.; Chabal, Y.J. Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 2010, 9, 840–845. [Google Scholar] [CrossRef]
- Rotariu, L.; Istrate, O.-M.; Bala, C. Poly(allylamine hydrochloride) modified screen-printed carbon electrode for sensitive and selective detection of NADH. Sens. Actuators B Chem. 2014, 191, 491–497. [Google Scholar] [CrossRef]
- Cornejo, J.J.M.; Matsuoka, E.; Daiguji, H. Size control of hollow poly-allylamine hydrochloride/poly-sodium styrene sulfonate microcapsules using the bubble template method. Soft Matter 2011, 7, 1897–1902. [Google Scholar] [CrossRef]
- Duarte, A.A.; Rego, A.; Salerno, M.; Ribeiro, P.A.; El Bari, N.; Bouchikhi, B.; Raposo, M. DPPG liposomes adsorbed on polymer cushions: Effect of roughness on amount, surface composition and topography. J. Phys. Chem. B 2015, 119, 8544–8552. [Google Scholar] [CrossRef]
- Maji, P.; Chatterjee, S.; Das, S. Study on charge transportation and scaling behavior of CsPbI3 microwires. Ceram. Int. 2019, 45, 6012–6020. [Google Scholar] [CrossRef]
- Carreira, D.; Ribeiro, P.; Raposo, M.; Sério, S. Engineering of TiO2 or ZnO-graphene oxide nanoheterojunctions for hybrid solar cells devices. Photonics 2021, 8, 75. [Google Scholar] [CrossRef]

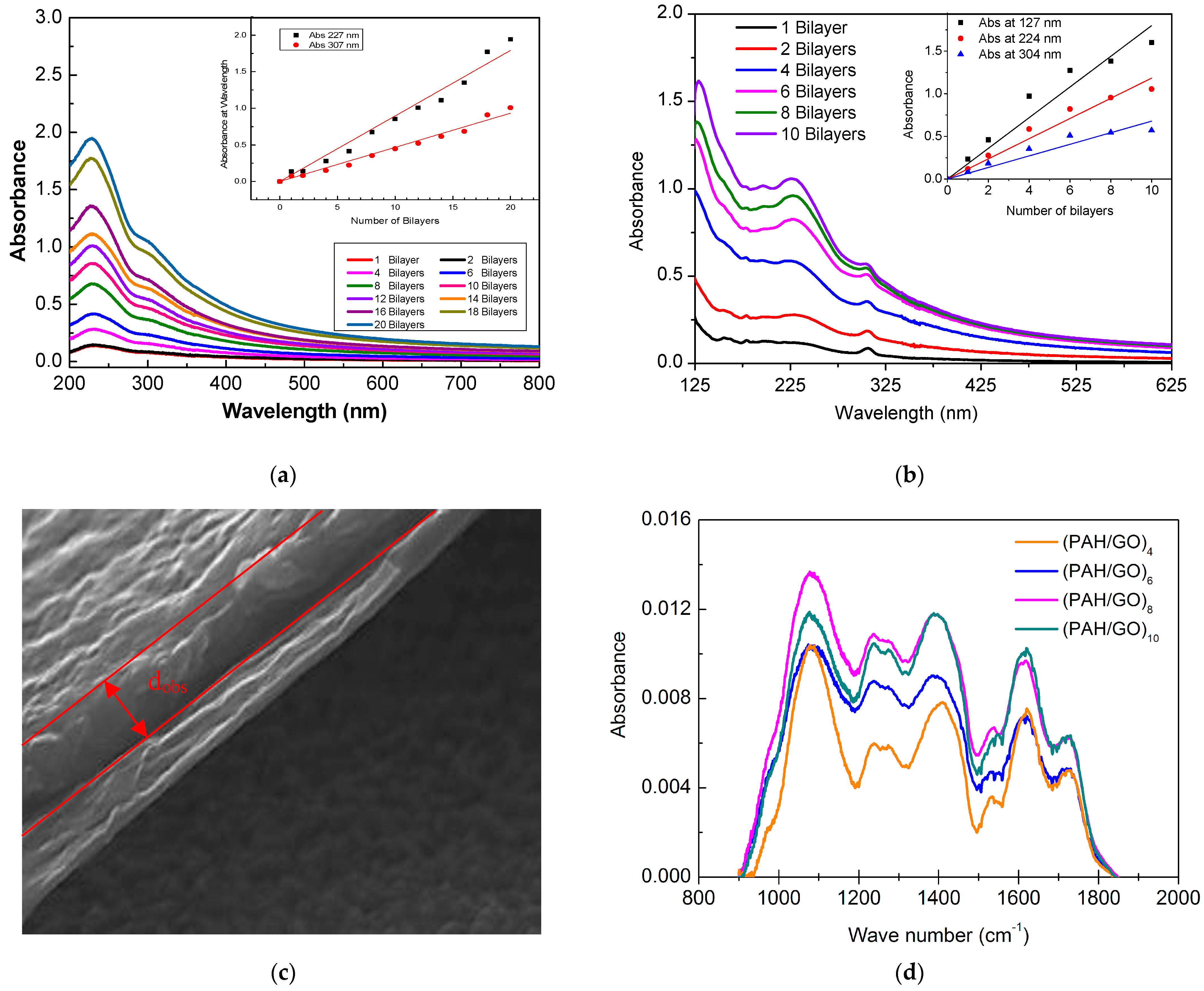

| Films | Band Position (nm) | Band Energy (eV) | FWHH (nm) | Assignments to Electronic Transitions |
|---|---|---|---|---|
| GO Cast Films | 146 ± 4 | 8.5 ± 0.3 | 50 ± 10 | π-π* of the carbonyl groups (aldehydes and ketones) |
| 210 ± 20 | 5.9 ± 0.6 | 84 | π-π* of the aromatic ring (phenol); n–π* of the carboxylic group | |
| 247.3 ± 0.2 | 5.0 ± 0.8 | 49.5 ± 5.1 | π-π* of the aromatic ring (benzene) | |
| 299 ± 4 | 4.2 ± 0.1 | 55.4 ± 3.2 | n-π* of carbonyl group | |
| PAH/GO LbL films | 127.8 ± 0.4 | 9.7 | 24.0 ± 0.8 | ---- |
| 149.2 ± 2.3 | 8.3 | 33.2 ± 4.1 | π-π* of the carbonyl groups (aldehydes and ketones) | |
| 178 ± 1 | 7 | --- | nN-3pa of amine; π-π* of the aromatic ring (phenol); n-π* of the carboxylic group | |
| 196.1 ± 0.7 | 6.3 | 9.3 ± 2.2 | nN-3sa of amine; π-π* of the aromatic ring (phenol); n-π* of the carboxylic group | |
| 199.1 ± 8.7 | 6.2 | 14.1 ± 6.9 | π-π* of the aromatic ring (benzene); | |
| 225.3 ± 0.1 | 5.5 | 81.2 ± 0.4 | π-π* of carbonyl group | |
| 305.1 ± 0.2 | 4.1 | 34.4 ± 0.7 | n-π* of carbonyl group |
| Peak Position (cm−1) | Peak Position in Literature (cm−1) | Assignments | |
|---|---|---|---|
| 972 | 990.7 | Vibrational modes of R-CH=CH2 groups [29]. | PAH |
| 1074.9 | 1088; 1048; 1080 | Stretching C-N bonds of PAH, stretching vibrations C-O bonds of the C-O-C epoxide groups of GO and with vibrations C-OH groups of the COOH carboxyl groups of GO. | PAH, GO |
| 1236.5 1272.6–1343.5 | 1230; 1227; 1226 | Asymmetric stretching vibrations of the epoxide group C-O-C. Stretching of C-OH bonds. | GO |
| 1548.6–1574.7 | 1500–1600 | sp2 group hybridized at the C=C bonds in the vibrational plane (GO) and mainly with the torsion in the N-H oscillation and the vibration in the stretching C-N of the carbonate group (PAH). | |
| 1619.6 | 1632; 1600–1650; 1628 and 1644; 1622 | Stretching at the C=C bond of the aromatic ring, the C=O vibrations in the ketone (GO) groups, the vibrations due to torsion at the N-H bonds in the primary amines and with the stretching vibrations of the carbonate groups. | |
| 1725.2 | 1723 and 1731; 1650–1750 | C=O stretch of the carbonyl group; Vibrations on the COOH carboxyl group. | |
| 2847.7 | 2847 | Symmetrical stretching vibrations of C-H (CH2), (PAH). | PAH |
| 2930.1 | 2921 | Asymmetric stretching vibrations of C-H (CH2), respectively (PAH). | PAH |
| 3085.9 | 3060 | asymmetric N-H stretching vibrations of the -NH3+ group (PAH) | |
| 3255.4 | 3250; 3050–3800; | O-H stretching at hydroxyl group notably phenol, C-OH (GO). | |
| 3397.9–3587.2 | 3050–3800 | O-H stretch in the hydroxyl group namely phenol, C-OH, which are also attributed to the COOH and H2O groups. |
| Area (μm2) | Sa (nm) | Sq (nm) | Stacks Width (nm) | Stacks Length (nm) | Height (nm) | SA (nm2) | |
|---|---|---|---|---|---|---|---|
| PAH | 4.08 | 3.03 | 3.81 | 31 | |||
| 1.02 | 2.68 | 3.41 | 27 | 18–190 | |||
| GO | 4.03 | 2.32 | 3.04 | 18 | |||
| 1.03 | 1.88 | 2.39 | 28–105 | 120–822 | 18 | ||
| 0.29 | 1.61 | 2.05 | |||||
| (PAH/GO)2 | 4.05 | 2.65 | 3.34 | 21 | |||
| 1.12 | 2.50 | 3.12 | 43–135 | 222–670 | 19 | 23–50 | |
| 0.28 | 1.62 | 2.04 | |||||
| (PAH/GO)20 # | 4.04 | 4.75 | 5.74 | ||||
| 1.03 | 4.54 | 5.76 | 13.3–87 | 200–800 | 31 | ||
| (PAH/GO)20 * | 4.24 | 3.63 | 4.50 | 26 | |||
| 1.01 | 2.17 | 2.74 | 11–62 | 281–564 | 17 |
| GO | PAH/GO | |
|---|---|---|
| (4.49 ± 0.11) × 105 | (2.584 ± 0.072) × 109 | |
| 0.0321 ± 0.0007 | 0.042 ± 0.002 | |
| 11.4 | 3 × 10−3 | |
| (1.25 ± 0.42) × 105 | (7.2 ± 0.3) × 107 | |
| 0.0052 ± 0.0002 | 0.006 ± 0.002 | |
| 6.65 | 1.3 × 10−2 | |
| (8.29 ± 0.14) × 104 | (6.2 ± 1.6) × 106 | |
| 0.00037 ± 0.00001 | 0.00043 ± 0.00009 | |
| 0.72 | 1.1 × 10−2 | |
| R2 | 0.99991 | 0.99943 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, I.C.C.; Sério, S.; Ferreira, Q.; Jones, N.C.; Hoffmann, S.V.; Ribeiro, P.A.; Raposo, M. Graphene Oxide Layer-by-Layer Films for Sensors and Devices. Nanomaterials 2021, 11, 1556. https://doi.org/10.3390/nano11061556
Assunção ICC, Sério S, Ferreira Q, Jones NC, Hoffmann SV, Ribeiro PA, Raposo M. Graphene Oxide Layer-by-Layer Films for Sensors and Devices. Nanomaterials. 2021; 11(6):1556. https://doi.org/10.3390/nano11061556
Chicago/Turabian StyleAssunção, Ivan C. C., Susana Sério, Quirina Ferreira, Nykola C. Jones, Søren V. Hoffmann, Paulo A. Ribeiro, and Maria Raposo. 2021. "Graphene Oxide Layer-by-Layer Films for Sensors and Devices" Nanomaterials 11, no. 6: 1556. https://doi.org/10.3390/nano11061556
APA StyleAssunção, I. C. C., Sério, S., Ferreira, Q., Jones, N. C., Hoffmann, S. V., Ribeiro, P. A., & Raposo, M. (2021). Graphene Oxide Layer-by-Layer Films for Sensors and Devices. Nanomaterials, 11(6), 1556. https://doi.org/10.3390/nano11061556









