Hydrothermal Synthesis of Iridium-Substituted NaTaO3 Perovskites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterisation
3. Results
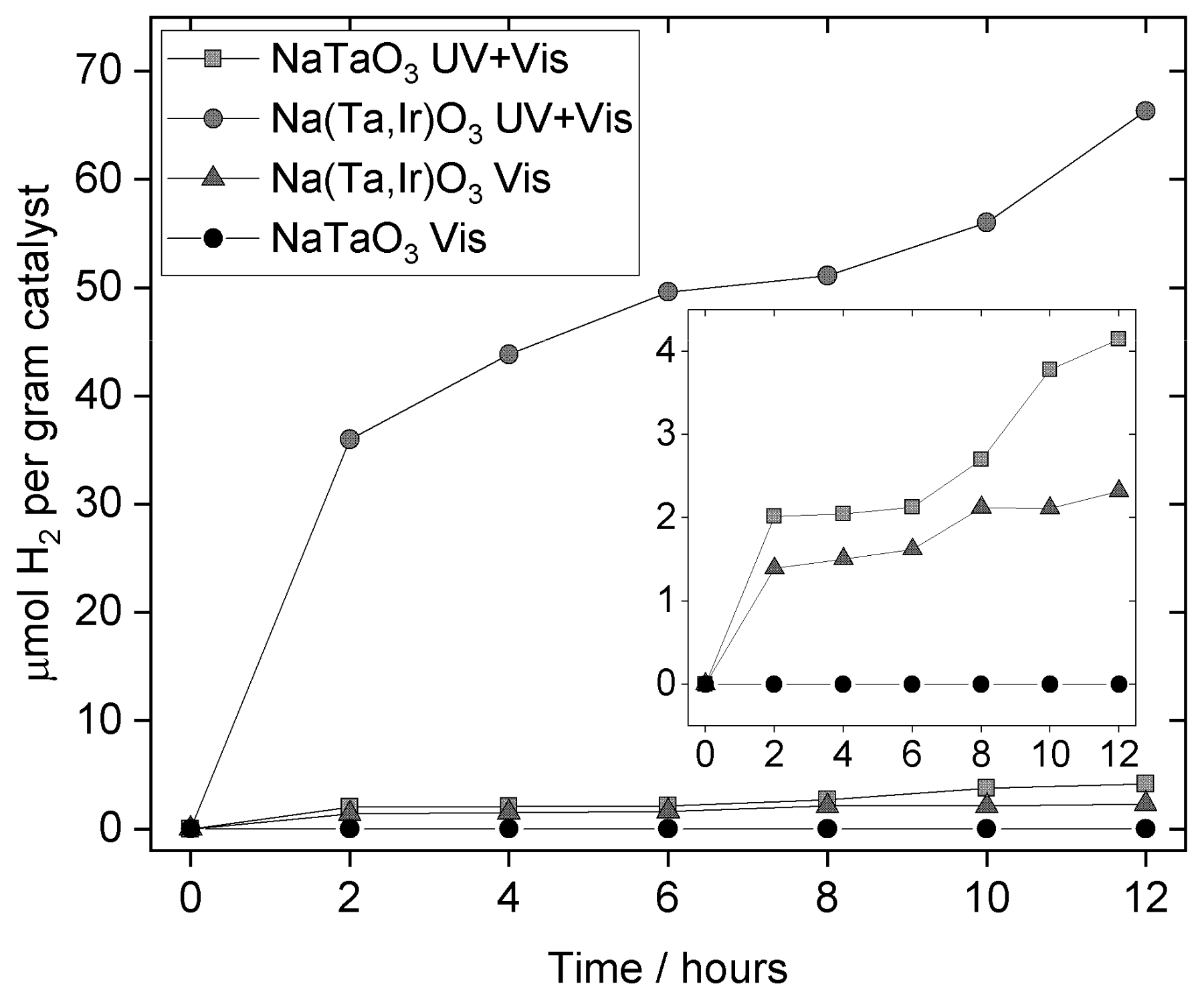
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, R.I. Perovskite Oxides Prepared by Hydrothermal and Solvothermal Synthesis: A Review of Crystallisation, Chemistry, and Compositions. Chem. Eur. J. 2020, 26, 9041–9069. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Aoki, M.; Teranishi, R.; Kaneko, K.; Takesada, M.; Moriwake, H.; Takashima, H.; Hakuta, Y. Atomic-Scale Observation of Titanium-Ion Shifts in Barium Titanate Nanoparticles: Implications for Ferroelectric Applications. ACS Appl. Nano Mater. 2019, 2, 5761–5768. [Google Scholar] [CrossRef]
- Morita, T. Piezoelectric Materials Synthesized by the Hydrothermal Method and Their Applications. Materials 2010, 3, 5236–5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardar, K.; Lees, M.R.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Direct Hydrothermal Synthesis and Physical Properties of Rare-Earth and Yttrium Orthochromite Perovskites. Chem. Mat. 2011, 23, 48–56. [Google Scholar] [CrossRef]
- Diodati, S.; Walton, R.I.; Mascotto, S.; Gross, S. Low-Temperature wet chemistry synthetic approaches towards ferrites. Inorg. Chem. Front. 2020, 7, 3282–3314. [Google Scholar] [CrossRef]
- Eckert, J.O.; HungHouston, C.C.; Gersten, B.L.; Lencka, M.M.; Riman, R.E. Kinetics and mechanisms of hydrothermal synthesis of barium titanate. J. Amer. Ceram. Soc. 1996, 79, 2929–2939. [Google Scholar] [CrossRef]
- Sōmiya, S.; Roy, R. Hydrothermal synthesis of fine oxide powders. Bull. Mater. Sci. 2000, 23, 453–460. [Google Scholar] [CrossRef]
- Riman, R.E.; Suchanek, W.L.; Lencka, M.M. Hydrothermal crystallization of ceramics. Ann. Chim. Sci. Mater. 2002, 27, 15–36. [Google Scholar] [CrossRef]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Komarneni, S. Nanophase materials by hydrothermal, microwave-hydrothermal and microwave-solvothermal methods. Curr. Sci. 2003, 85, 1730–1734. [Google Scholar]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Kumada, N. Preparation and crystal structure of new inorganic compounds by hydrothermal reaction. J. Ceram. Soc. Jap. 2013, 121, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Li, J.; Huang, X.; Tan, Y. Synthesis and enhanced photocatalytic activity of regularly shaped Cu2O nanowire polyhedra. Nano Res. 2011, 4, 448–459. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Guo, X.; Du, B.; Hu, X.; Yang, X.; He, Y.; Zhou, Y.; Zang, Z. Low-operating temperature ammonia sensor based on Cu2O nanoparticles decorated with p-type MoS2 nanosheets. J. Mater. Chem. C 2021, 9, 4838–4846. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.W.; Wu, X.D.; Han, Q.F.; Wang, X. Graphene Oxide-MnO2 Nanocomposites for Supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef]
- Modeshia, D.R.; Darton, R.J.; Ashbrook, S.E.; Walton, R.I. Control of polymorphism in NaNbO3 by hydrothermal synthesis. Chem. Commun. 2009, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Skjaervø, S.L.; Sommer, S.; Norby, P.; Bojesen, E.D.; Grande, T.; Iversen, B.B.; Einarsrud, M.A. Formation mechanism and growth of MNbO3, M = K, Na by insitu X-ray diffraction. J. Amer. Ceram. Soc. 2017, 100, 3835–3842. [Google Scholar] [CrossRef]
- Skjaervø, S.L.; Wells, K.H.; Sommer, S.; Vu, T.D.; Tolchard, J.R.; van Beek, W.; Grande, T.; Iversen, B.B.; Einarsrud, M.A. Rationalization of Hydrothermal Synthesis of NaNbO3 by Rapid in Situ Time-Resolved Synchrotron X-ray Diffraction. Cryst. Growth Des. 2018, 18, 770–774. [Google Scholar] [CrossRef]
- Song, H.W.; Ma, W.H. Hydrothermal synthesis of submicron NaNbO3 powders. Ceram. Int. 2011, 37, 877–882. [Google Scholar] [CrossRef]
- Kumada, N.; Dong, Q.; Yonesaki, Y.; Takei, T.; Kinomura, N. Hydrothermal synthesis of NaNbO3-morphology change by starting compounds. J. Ceram. Soc. Jap. 2011, 119, 483–485. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.D.; Wang, J.H.; Wang, H.L.; Wu, Z.J.; Wu, H.P. Hydrothermal synthesis of morphology-controlled KNbO3, NaNbO3, and (K,Na)NbO3 powders. Ceram. Int. 2017, 43, 7222–7230. [Google Scholar] [CrossRef]
- Nakashima, K.; Toshima, Y.; Kobayashi, Y.; Kakihana, M. Effects of raw materials on NaNbO3 nanocube synthesis via the solvothermal method. J. As. Ceram. Soc. 2019, 7, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Kanie, K.; Numamoto, Y.; Tsukamoto, S.; Takahashi, H.; Mizutani, H.; Terabe, A.; Nakaya, M.; Tani, J.; Muramatsu, A. Hydrothermal Synthesis of Sodium and Potassium Niobates Fine Particles and Their Application to Lead-Free Piezoelectric Material. Mater. Trans. 2011, 52, 2119–2125. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zhu, G.S.; Chao, X.L.; Wei, L.L.; Yang, Z.P. Properties of NaNbO3 powders and ceramics prepared by hydrothermal reaction. Mater. Chem. Phys. 2011, 126, 183–187. [Google Scholar] [CrossRef]
- Fukada, M.; Shibata, K.; Imai, T.; Yamazoe, S.; Hosokawa, S.; Wada, T. Fabrication of lead-free piezoelectric NaNbO3 ceramics at low temperature using NaNbO3 nanoparticles synthesized by solvothermal method. J. Ceram. Soc. Jap. 2013, 121, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.F.; Li, X.K.; Wang, D.F.; Yuan, Y.P.; Zou, Z.G.; Ye, J.H. NaNbO3 Nanostructures: Facile Synthesis, Characterization, and Their Photocatalytic Properties. Catal. Lett. 2009, 132, 205–212. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A Review on Visible Light Active Perovskite-Based Photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polat, O.; Durmus, Z.; Coskun, F.M.; Coskun, M.; Turut, A. Engineering the band gap of LaCrO3 doping with transition metals (Co, Pd, and Ir). J. Mater. Sci. 2018, 53, 3544–3556. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; You, S.; Enrichi, F.; Vomiero, A.; Saruhan, B. Impact of Oxalate Ligand in Co-Precipitation Route on Morphological Properties and Phase Constitution of Undoped and Rh-Doped BaTiO3 Nanoparticles. Nanomaterials 2019, 9, 1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calì, E.; Kerherve, G.; Naufal, F.; Kousi, K.; Neagu, D.; Papaioannou, E.I.; Thomas, M.P.; Guiton, B.S.; Metcalfe, I.S.; Irvine, J.T.S.; et al. Exsolution of Catalytically Active Iridium Nanoparticles from Strontium Titanate. ACS Appl. Mater. Interfaces 2020, 12, 37444–37453. [Google Scholar] [CrossRef]
- Kawasaki, S.; Takahashi, R.; Akagi, K.; Yoshinobu, J.; Komori, F.; Horiba, K.; Kumigashira, H.; Iwashina, K.; Kudo, A.; Lippmaa, M. Electronic Structure and Photoelectrochemical Properties of an Ir-Doped SrTiO3 Photocatalyst. J. Phys. Chem. C 2014, 118, 20222–20228. [Google Scholar] [CrossRef]
- Iwase, A.; Saito, K.; Kudo, A. Sensitization of NaMO3 (M: Nb and Ta) Photocatalysts with Wide Band Gaps to Visible Light by Ir Doping. Bull. Chem. Soc. Jap. 2009, 82, 514–518. [Google Scholar] [CrossRef]
- Kudo, A.; Yoshino, S.; Tsuchiya, T.; Udagawa, Y.; Takahashi, Y.; Yamaguchi, M.; Ogasawara, I.; Matsumoto, H.; Iwase, A. Z-scheme photocatalyst systems employing Rh- and Ir-doped metal oxide materials for water splitting under visible light irradiation. Farad. Disc. 2019, 215, 313–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardar, K.; Fisher, J.; Thompsett, D.; Lees, M.R.; Clarkson, G.J.; Sloan, J.; Kashtiban, R.J.; Walton, R.I. Structural variety in iridate oxides and hydroxides from hydrothermal synthesis. Chem. Sci. 2011, 2, 1573–1578. [Google Scholar] [CrossRef]
- Toby, B.H.; von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Peters, J.J.P. clTEM. Available online: https://jjppeters.github.io/clTEM/ (accessed on 19 March 2021).
- Knight, K.S.; Kennedy, B.J. Phase coexistence in NaTaO3 at room temperature; a high resolution neutron powder diffraction study. Solid State Sci. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Dent, A.J.; Cibin, G.; Ramos, S.; Smith, A.D.; Scott, S.M.; Varandas, L.; Pearson, M.R.; Krumpa, N.A.; Jones, C.P.; Robbins, P.E. B18: A core XAS spectroscopy beamline for Diamond. J. Phys. Conf. Ser. 2009, 190, 012039. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.H.; Liferovich, R.P. A structural study of the perovskite series Ca1−xNaxTi1−xTaxO3. J. Solid State Chem. 2004, 177, 4420–4427. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gao, Y.; Su, Y.G.; Meng, Y.; Wang, S.W.; Jia, Q.Y.; Wang, X.J. Preparation and Photocatalytic Mechanism of Vanadium Doped NaTaO3 Nanoparticles. Integr. Ferroelectr. 2011, 127, 106–115. [Google Scholar] [CrossRef]
- Gao, R.; Zhou, S.X.; Li, W.; Chen, M.; Wu, L.M. Facile synthesis of uniform and well-defined single-crystal sodium tantalate cubes and their assembly into oriented two-dimensional nanofilm. Cryst. Eng. Commun. 2012, 14, 7031–7035. [Google Scholar] [CrossRef]
- Grewe, T.; Meier, K.; Tuysuz, H. Photocatalytic hydrogen production over various sodium tantalates. Catal. Today 2014, 225, 142–148. [Google Scholar] [CrossRef]
- Wang, X.J.; Bai, H.L.; Meng, Y.; Zhao, Y.H.; Tang, C.H.; Gao, Y. Synthesis and Optical Properties of Bi3+ Doped NaTaO3 Nano-Size Photocatalysts. J. Nanosci. Nanotechnol. 2010, 10, 1788–1793. [Google Scholar] [CrossRef]
- Liu, Y.L.; Su, Y.G.; Han, H.; Wang, X.J. Hydrothermal Preparation of Copper Doped NaTaO3 Nanoparticles and Study on the Photocatalytic Mechanism. J. Nanosci. Nanotechnol. 2013, 13, 853–857. [Google Scholar] [CrossRef]
- Sardar, K.; Petrucco, E.; Hiley, C.I.; Sharman, J.D.B.; Wells, P.P.; Russell, A.E.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Water-Splitting Electrocatalysis in Acid Conditions Using Ruthenate-Iridate Pyrochlores. Angew. Chem. Int. Edit. 2014, 53, 10960–10964. [Google Scholar] [CrossRef] [Green Version]
- Müller-Buschbaum, H. On the crystal chemistry of Oxoiridates. Z. Anorg. Allg. Chem. 2005, 631, 1005–1028. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Highly efficient decomposition of pure water into H2 and O2 over NaTaO3 photocatalysts. Catal. Lett. 1999, 58, 153–155. [Google Scholar] [CrossRef]
- Kumada, N.; Morozumi, Y.; Yonesaki, Y.; Takei, T.; Kinomura, N.; Hayashi, T. Preparation of Na0.5Bi0.5TiO3 by hydrothermal reaction. J. Ceram. Soc. Jap. 2008, 116, 1238–1240. [Google Scholar] [CrossRef] [Green Version]
- Handoko, A.D.; Goh, G.K.L.; Chew, R.X. Piezoelectrically active hydrothermal KNbO3 thin films. Cryst. Eng. Commun. 2012, 14, 421–427. [Google Scholar] [CrossRef]
- O’Brien, A.; Woodward, D.I.; Sardar, K.; Walton, R.I.; Thomas, P.A. Inference of oxygen vacancies in hydrothermal Na0.5Bi0.5TiO3. Appl. Phys. Lett. 2012, 101, 142902. [Google Scholar] [CrossRef]
- Singh, H.P. Determination of thermal expansion of germanium, rhodium and iridium by X-rays. Acta Crystallogr. Sect. A 1968, 24, 469–471. [Google Scholar] [CrossRef]
- Kanhere, P.; Zheng, J.; Chen, Z. Visible light driven photocatalytic hydrogen evolution and photophysical properties of Bi3+ doped NaTaO3. Int. J. Hydro. Ener. 2012, 37, 4889–4896. [Google Scholar] [CrossRef]
- Onishi, H. Sodium Tantalate Photocatalysts Doped with Metal Cations: Why Are They Active for Water Splitting? ChemSusChem 2019, 12, 1825–1834. [Google Scholar] [CrossRef]
- Alves, G.A.S.; Centurion, H.A.; Sambrano, J.R.; Ferrer, M.M.; Gonçalves, R.V. Band Gap Narrowing of Bi-Doped NaTaO3 for Photocatalytic Hydrogen Evolution under Simulated Sunlight: A Pseudocubic Phase Induced by Doping. ACS Appl. Energy Mater. 2021, 4, 671–679. [Google Scholar] [CrossRef]
- Suzuki, S.; Matsumoto, H.; Iwase, A.; Kudo, A. Enhanced H2 evolution over an Ir-doped SrTiO3 photocatalyst by loading of an Ir cocatalyst using visible light up to 800 nm. Chem. Commun. 2018, 54, 10606–10609. [Google Scholar] [CrossRef]
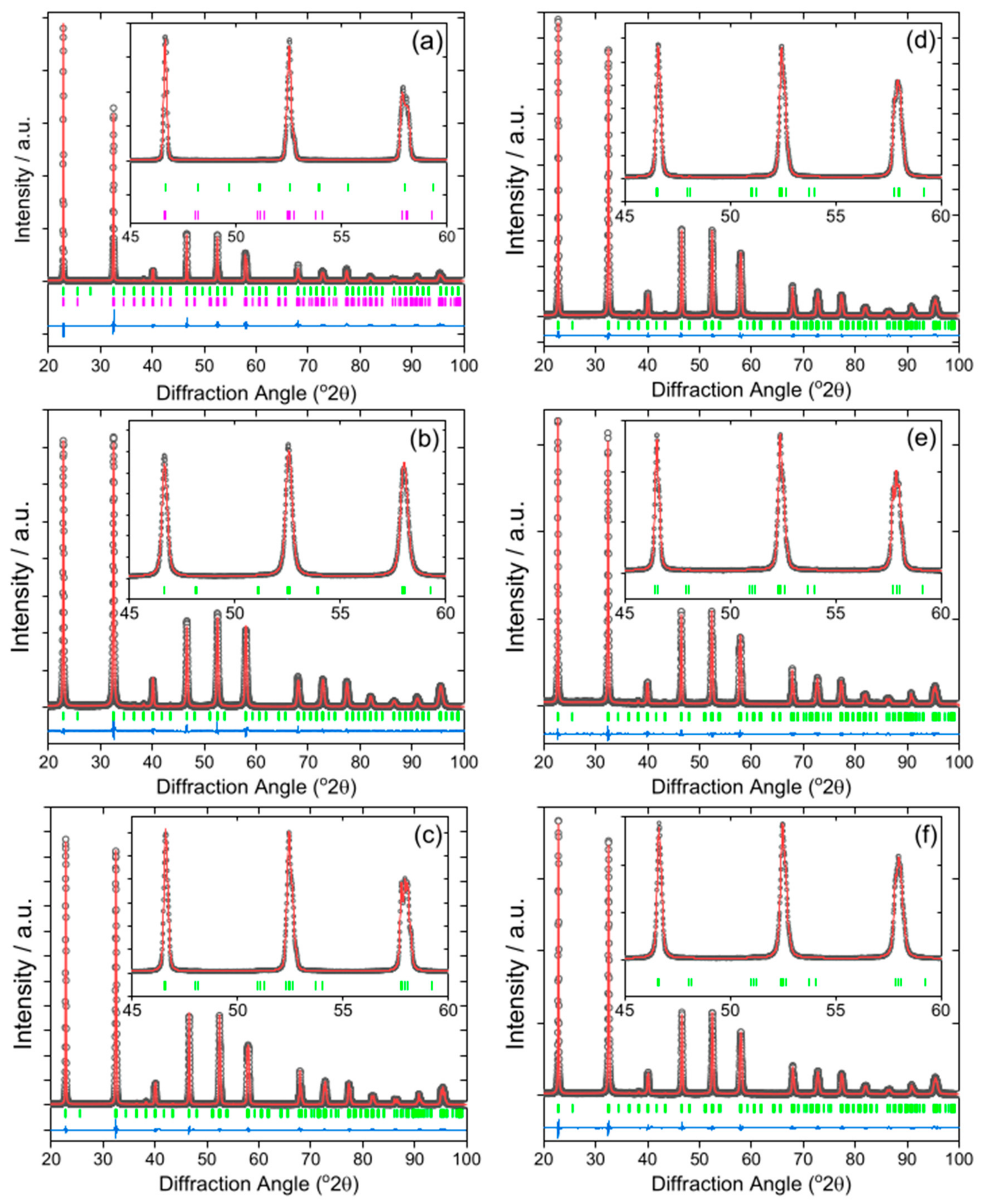
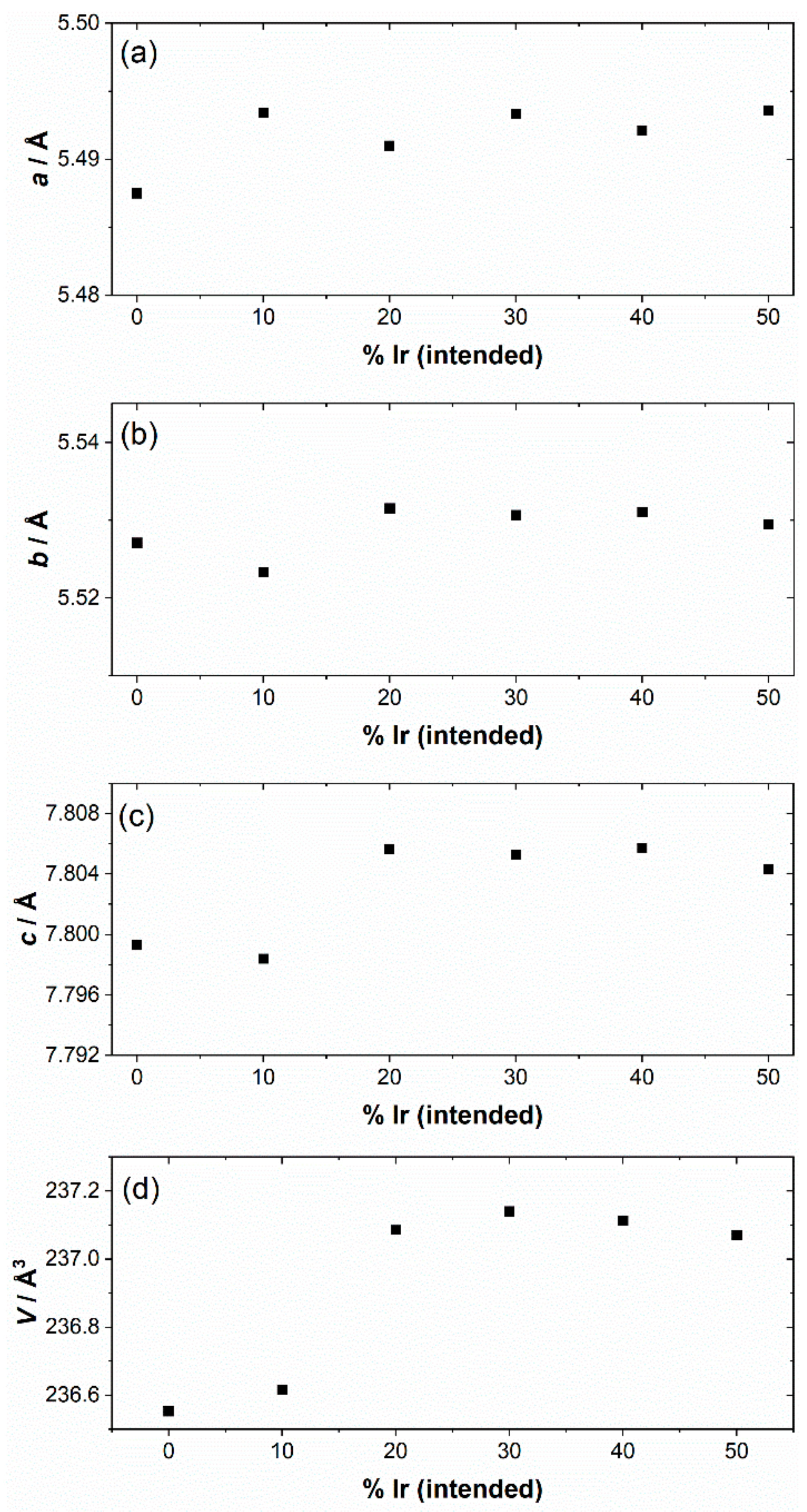
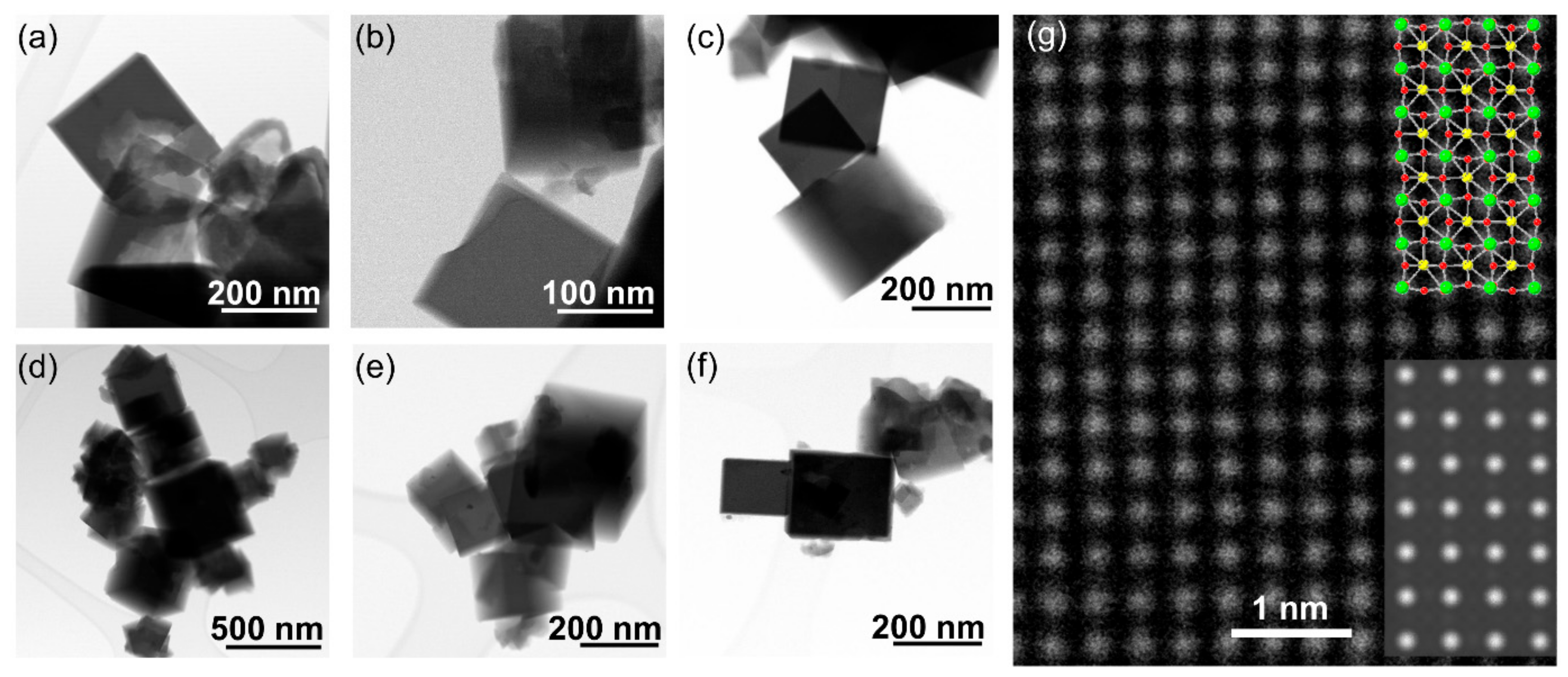
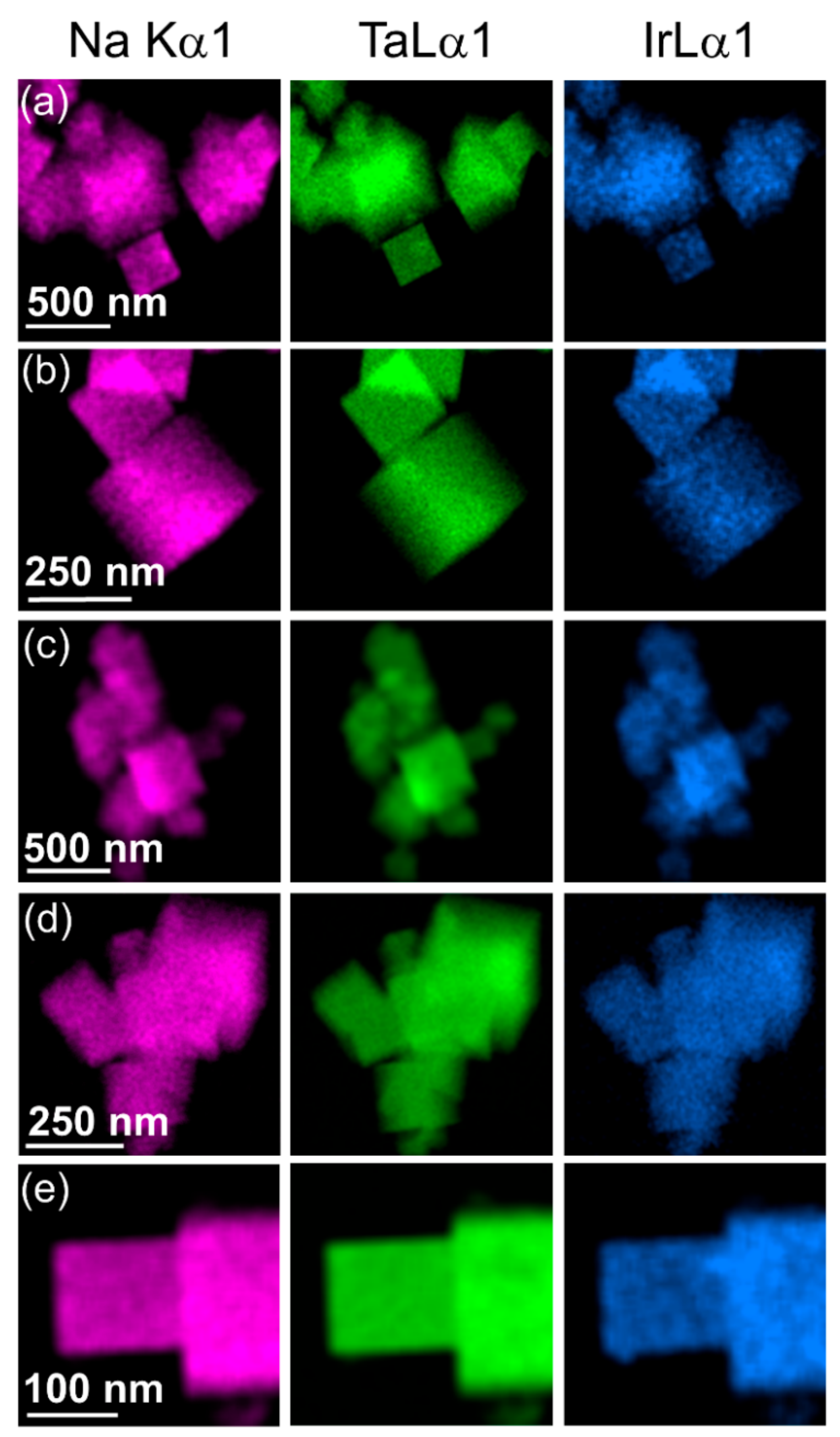
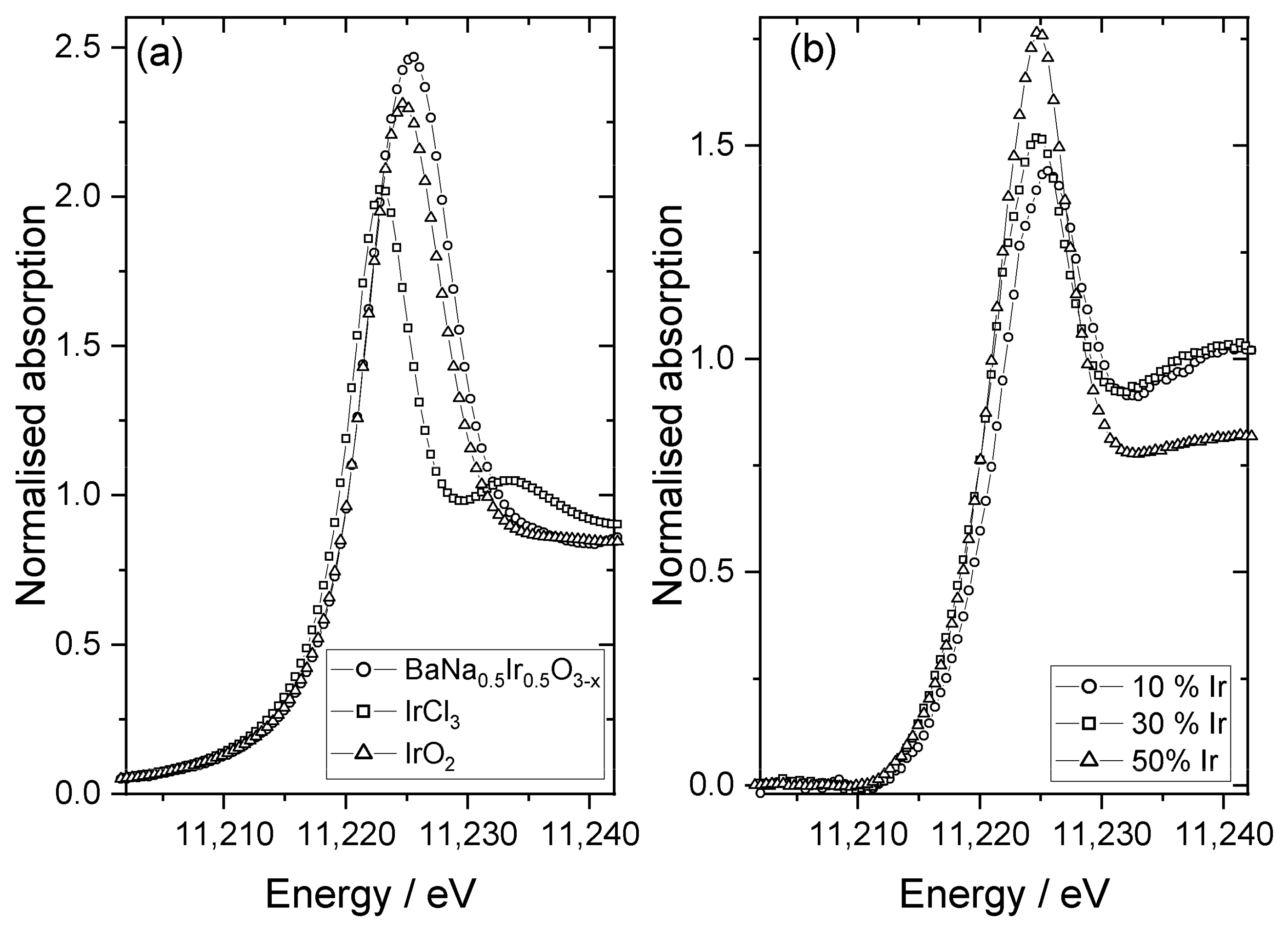
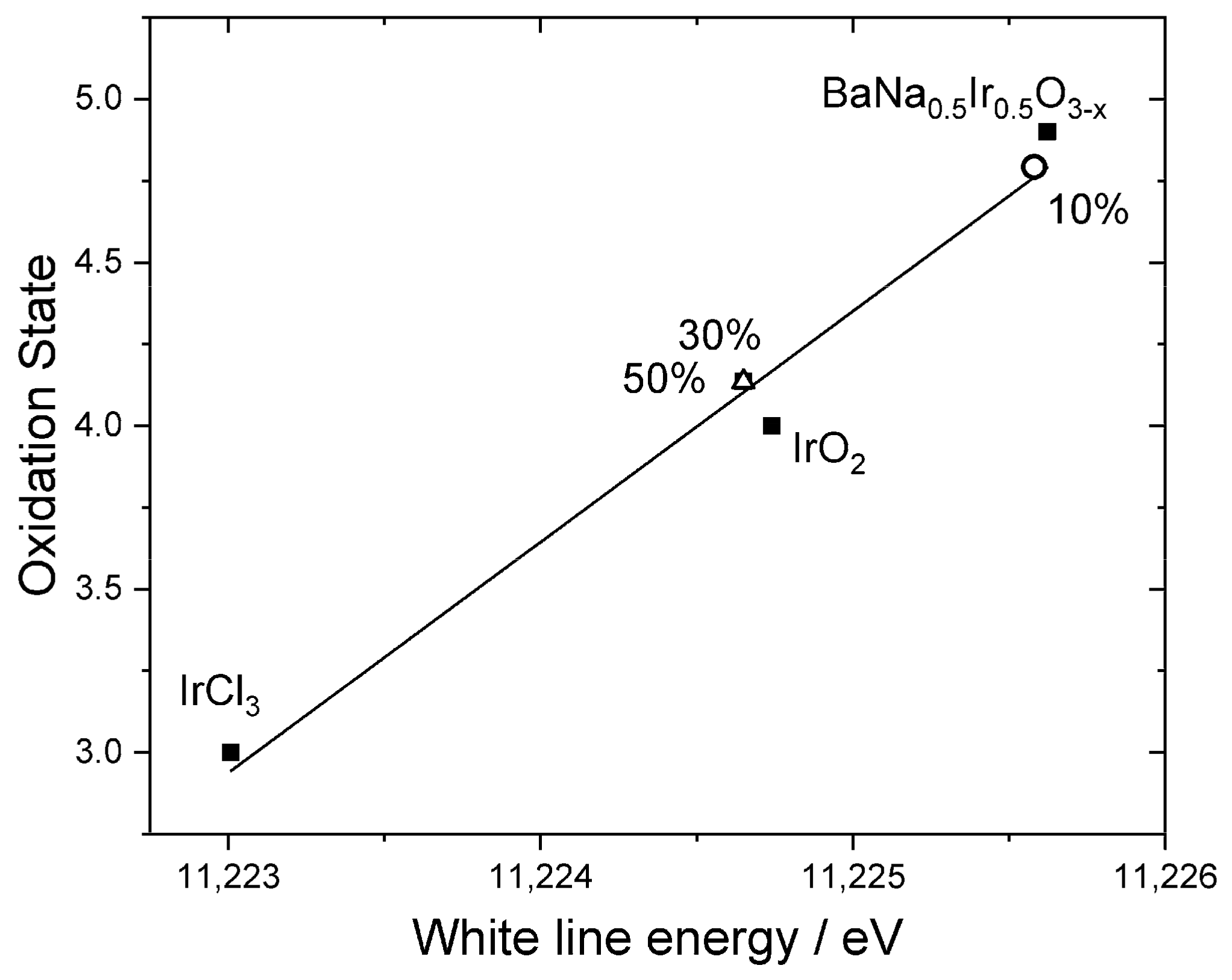
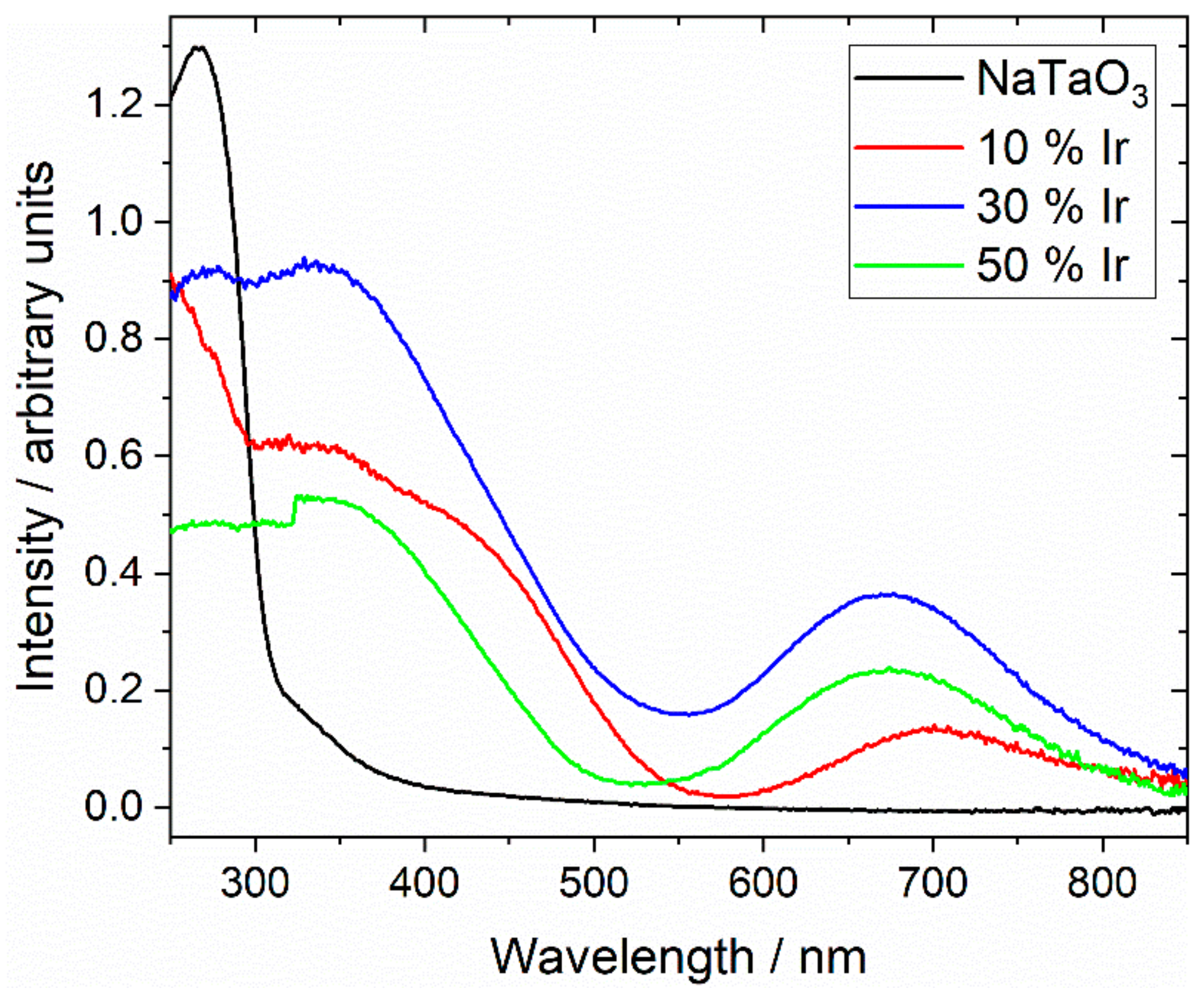
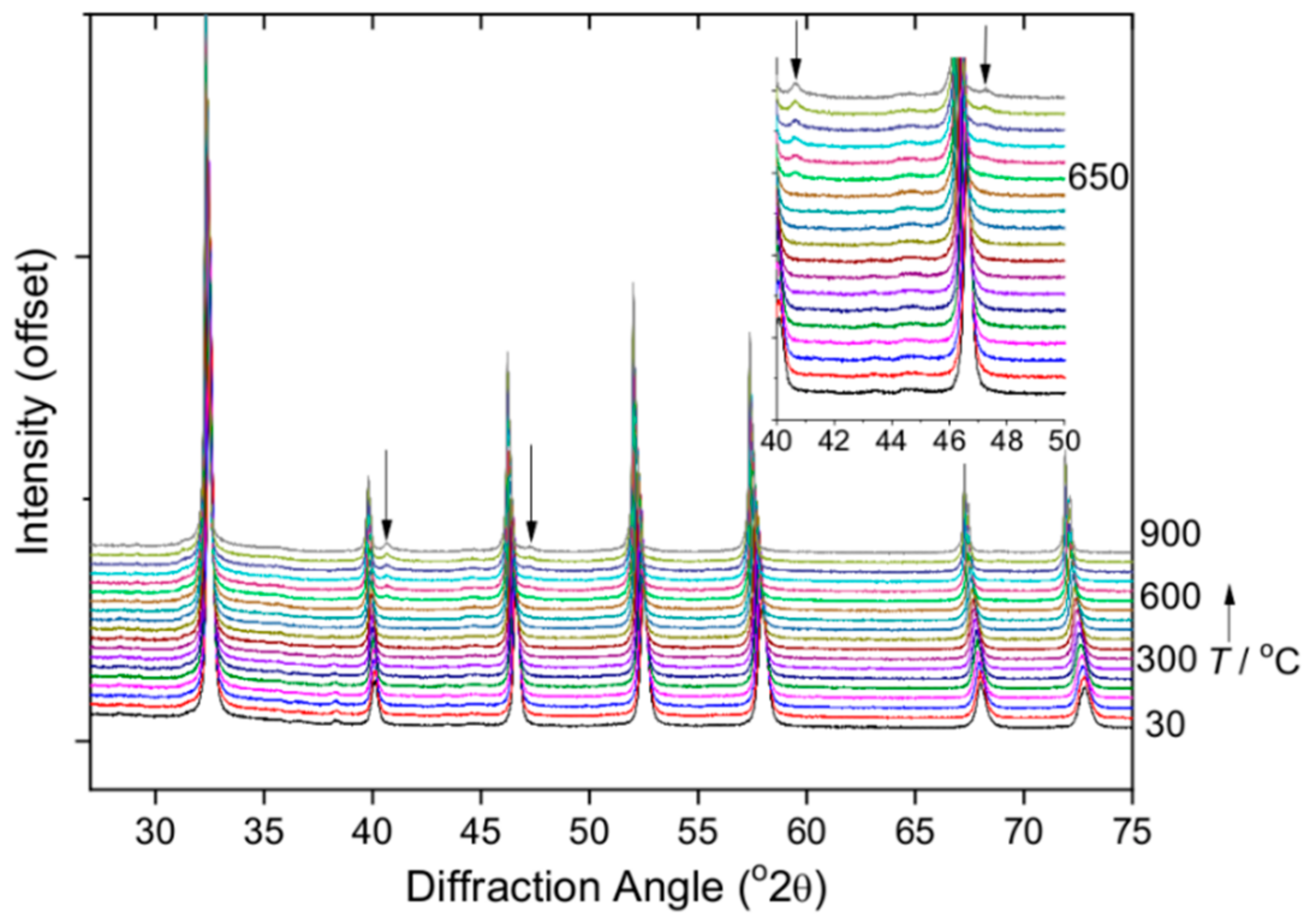
| Material | Lattice Parameters | ||
|---|---|---|---|
| a/Å | b/Å | c/Å | |
| NaTaO3 [41] | 5.48109(9) | 5.52351(9) | 7.79483(12) |
| NaTaO3 a | 5.48750(5) | 5.52711(6) | 7.7993(1) |
| NaTaO3-10% Ir | 5.4934(1) | 5.5233(1) | 7.7984(2) |
| NaTaO3-20% Ir | 5.49099(4) | 5.53154(5) | 7.8056(6) |
| NaTaO3-30% Ir | 5.49335(6) | 5.53067(9) | 7.8053(1) |
| NaTaO3-40% Ir | 5.49212(8) | 5.53101(9) | 7.8057(1) |
| NaTaO3-50% Ir | 5.4936(1) | 5.5295(1) | 7.8043(1) |
| EDS Results | |||
|---|---|---|---|
| Intended Ir Substitution | Tantalum/% | Iridium/% | Determined Formula |
| 10% | 97.6 | 2.4 | NaTa0.98Ir0.02O3 |
| 20% | 95.3 | 4.7 | NaTa0.95Ir0.05O3 |
| 30% | 91.6 | 8.4 | NaTa0.92Ir0.08O3 |
| 40% | 87.8 | 12.2 | NaTa0.88Ir0.12O3 |
| 50% | 85.1 | 14.9 | NaTa0.85Ir0.15O3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnett, D.L.; Vincent, C.D.; Clayton, J.A.; Kashtiban, R.J.; Walton, R.I. Hydrothermal Synthesis of Iridium-Substituted NaTaO3 Perovskites. Nanomaterials 2021, 11, 1537. https://doi.org/10.3390/nano11061537
Burnett DL, Vincent CD, Clayton JA, Kashtiban RJ, Walton RI. Hydrothermal Synthesis of Iridium-Substituted NaTaO3 Perovskites. Nanomaterials. 2021; 11(6):1537. https://doi.org/10.3390/nano11061537
Chicago/Turabian StyleBurnett, David L., Christopher D. Vincent, Jasmine A. Clayton, Reza J. Kashtiban, and Richard I. Walton. 2021. "Hydrothermal Synthesis of Iridium-Substituted NaTaO3 Perovskites" Nanomaterials 11, no. 6: 1537. https://doi.org/10.3390/nano11061537
APA StyleBurnett, D. L., Vincent, C. D., Clayton, J. A., Kashtiban, R. J., & Walton, R. I. (2021). Hydrothermal Synthesis of Iridium-Substituted NaTaO3 Perovskites. Nanomaterials, 11(6), 1537. https://doi.org/10.3390/nano11061537






