Cross-Species Comparisons of Nanoparticle Interactions with Innate Immune Systems: A Methodological Review
Abstract
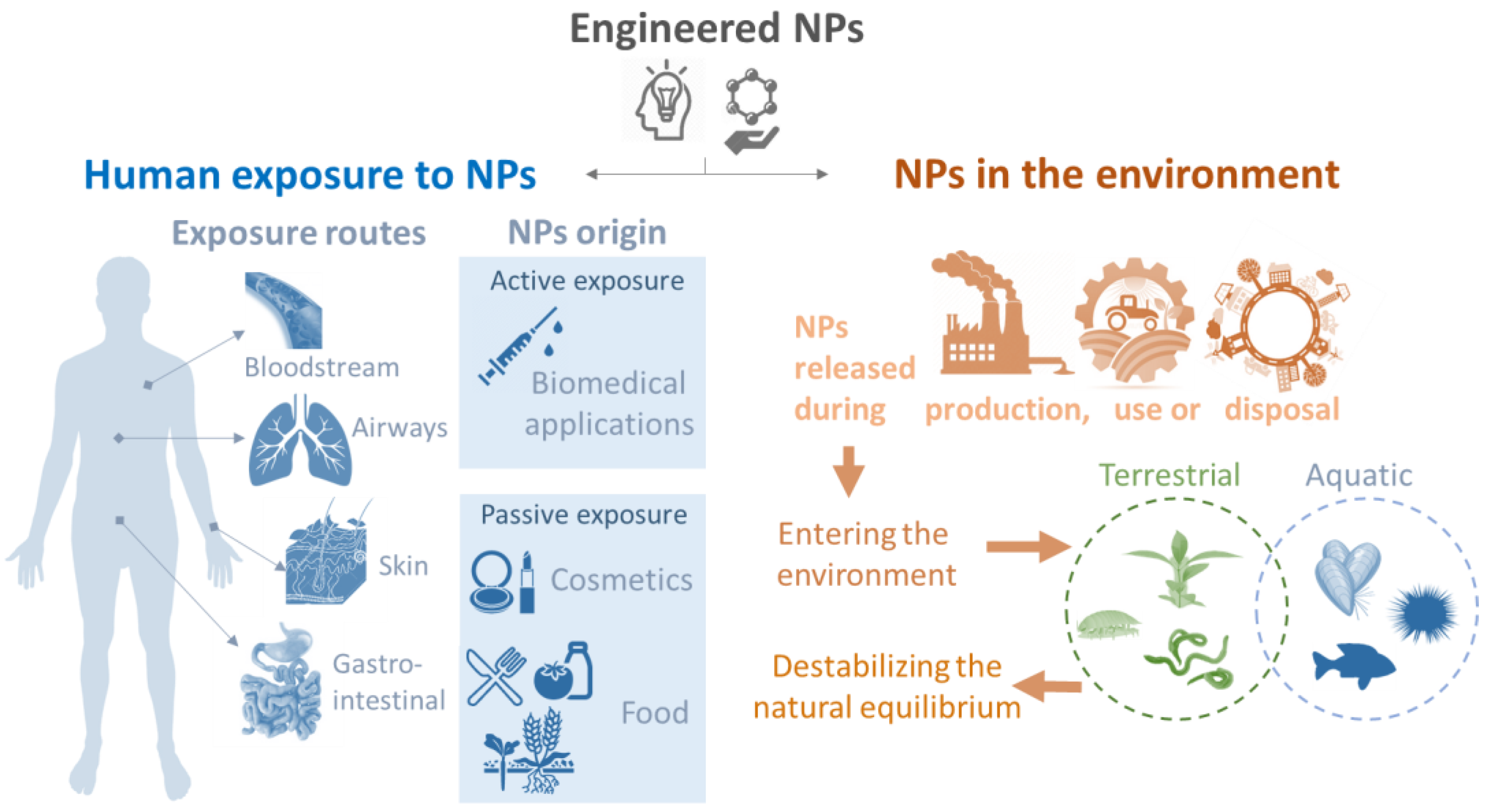
1. General Introduction: The Need for Studying Nanoparticle–Immune System Interactions
2. Short Description of the Innate Immune System for the Models of Interest
2.1. Generalities and Conserved Innate Immune Traits across the Selected Models
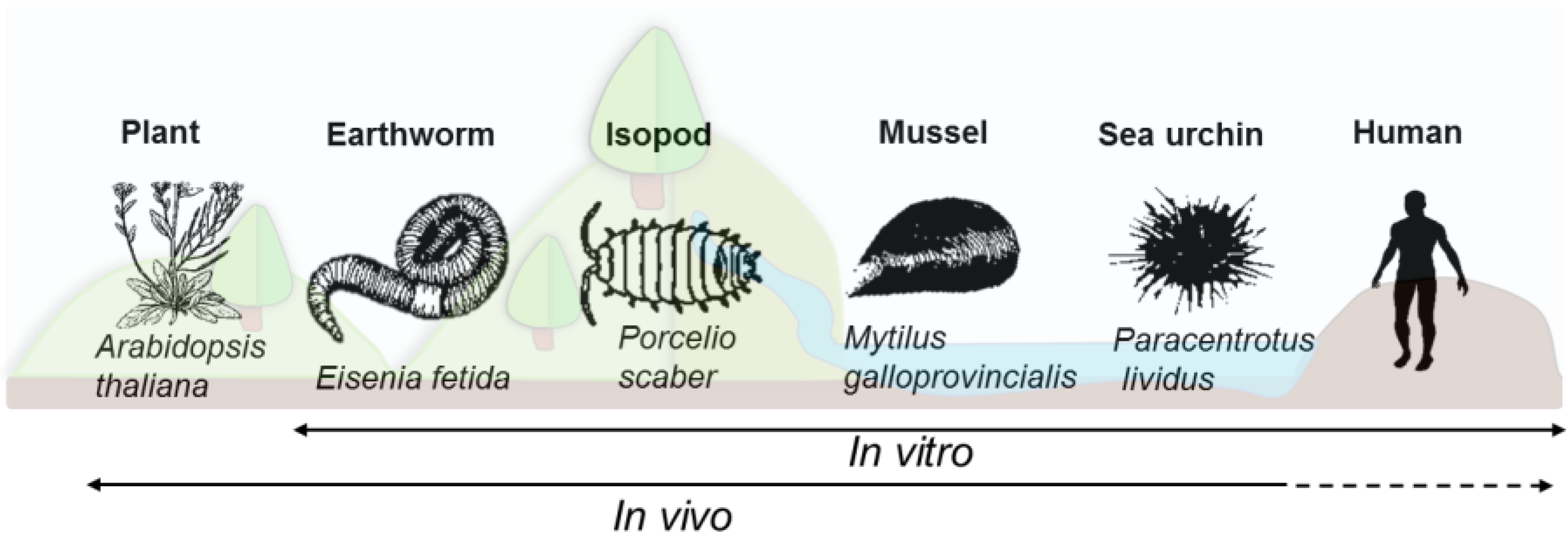
2.2. Model Specific Immune System Characteristics
2.2.1. Plants
2.2.2. Earthworms
2.2.3. Isopods
2.2.4. Mussels
2.2.5. Sea Urchins
2.2.6. Human Cells
3. Parameters Assessed: From NPs to Innate Immune Responses
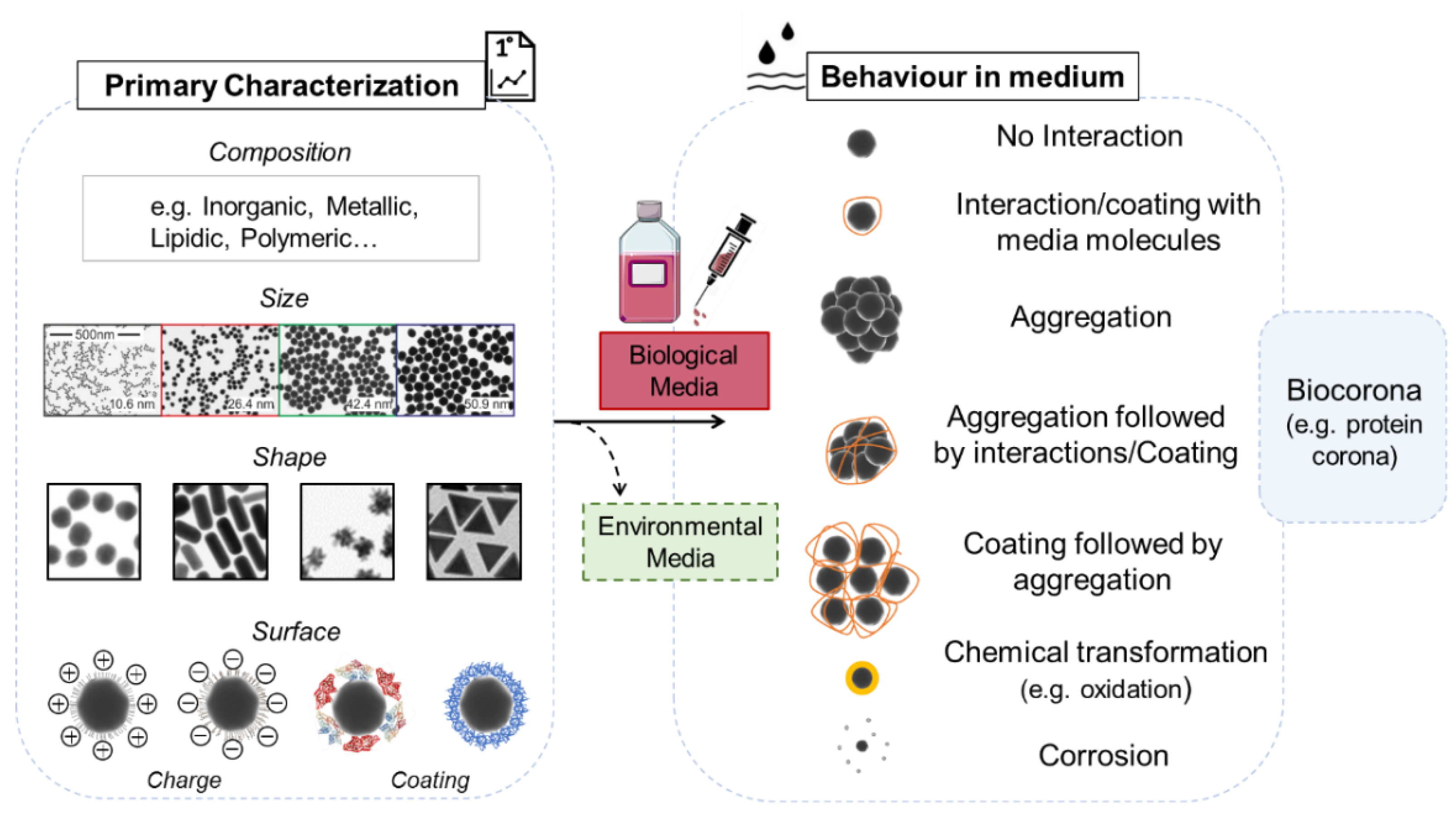
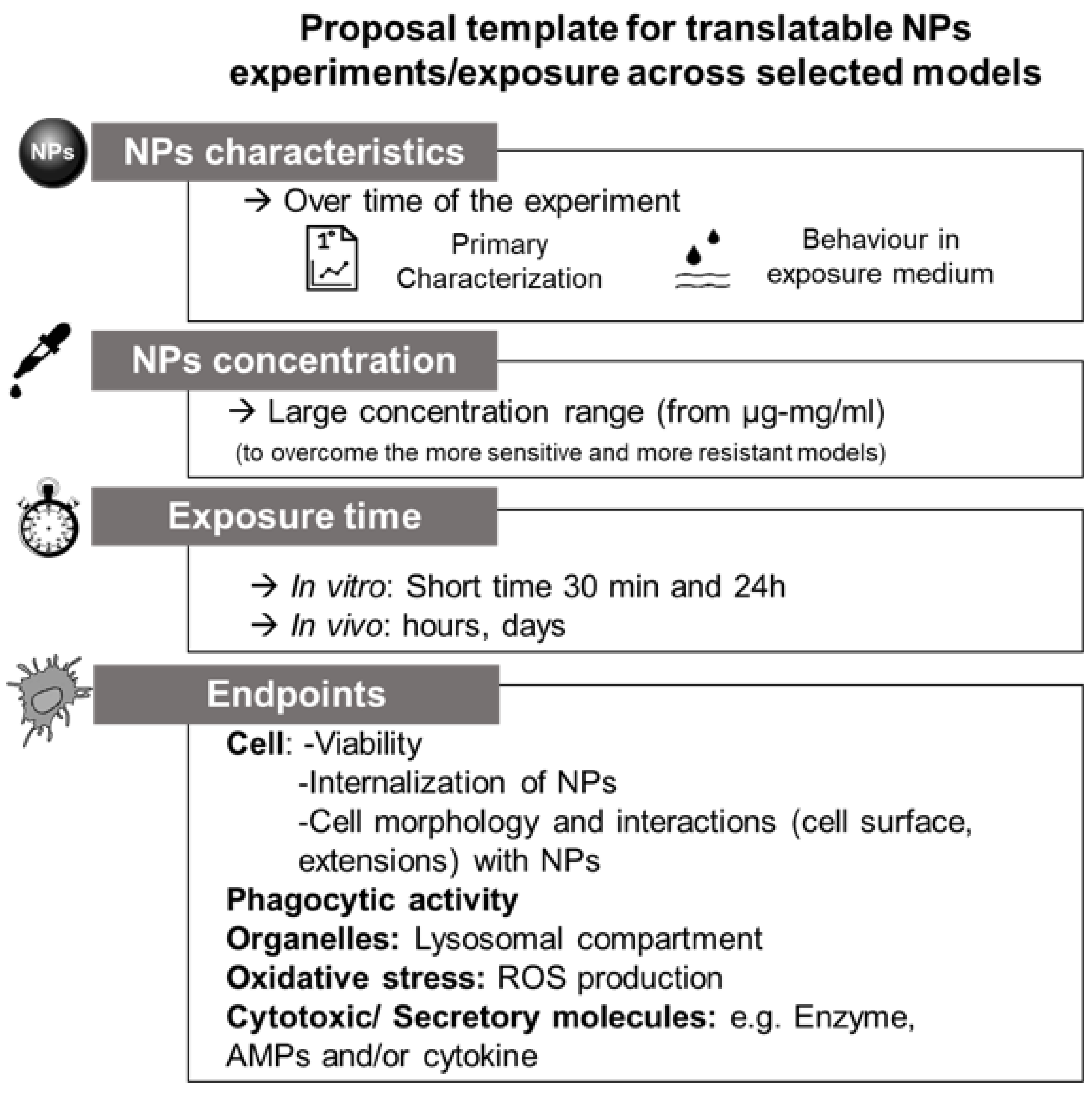
3.1. NPs: What to Consider When You Use a Biological System?
3.1.1. Primary Characterization
3.1.2. Behavior in Medium
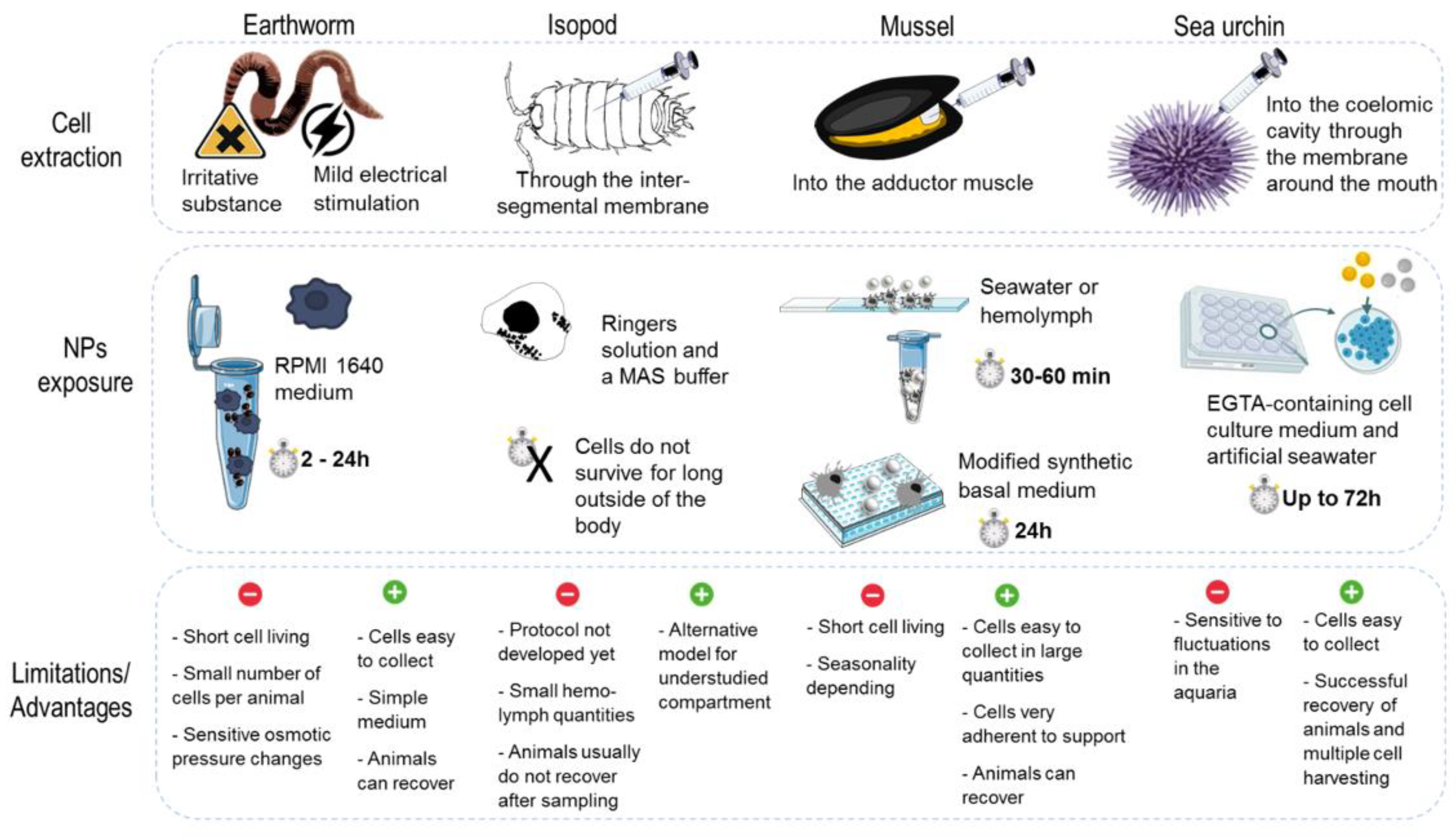
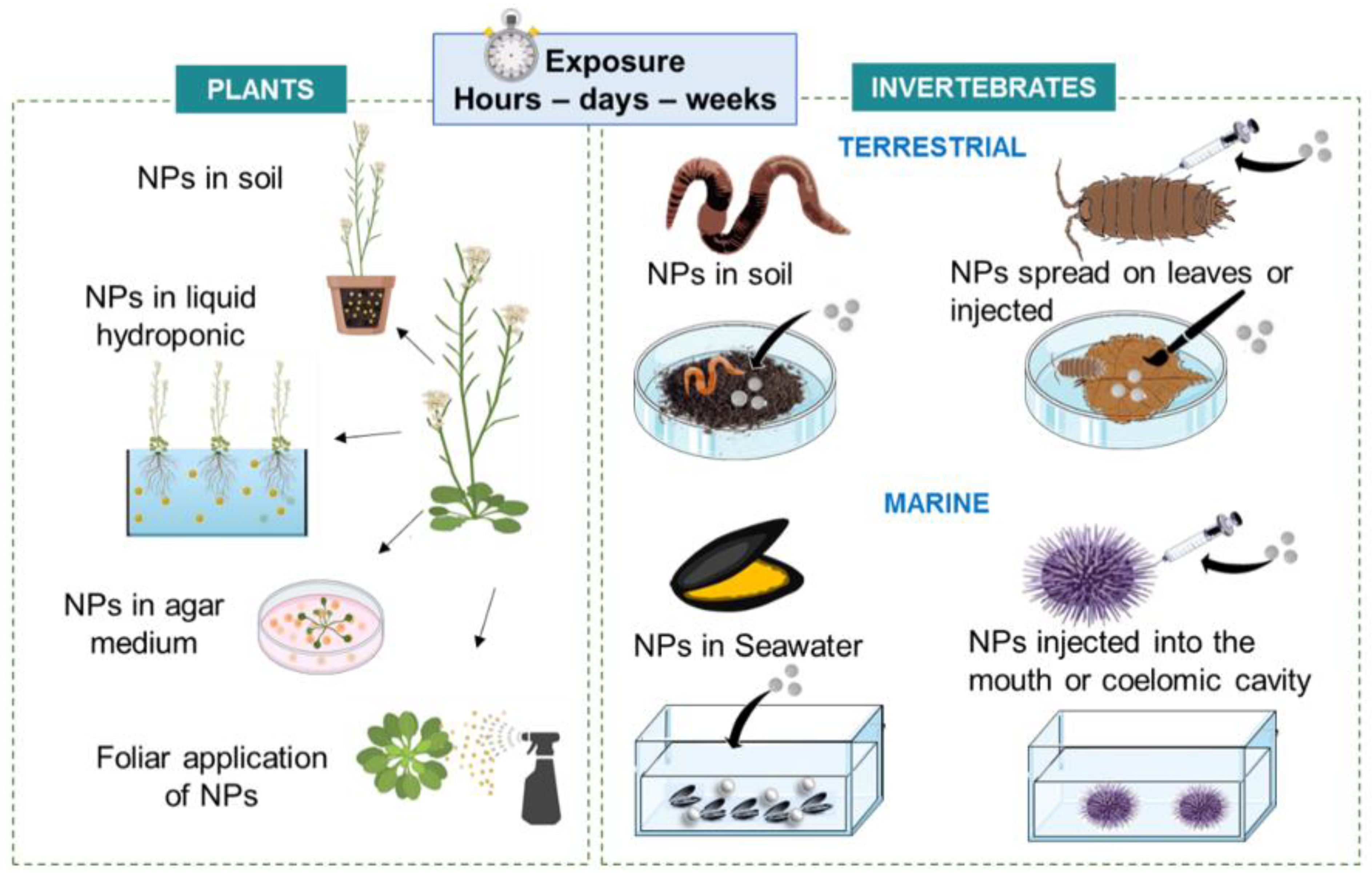
3.2. Models, Cell Culture and Mode of Exposure
3.2.1. Nonmammalian In Vitro Assays
3.2.2. Human Cell Models
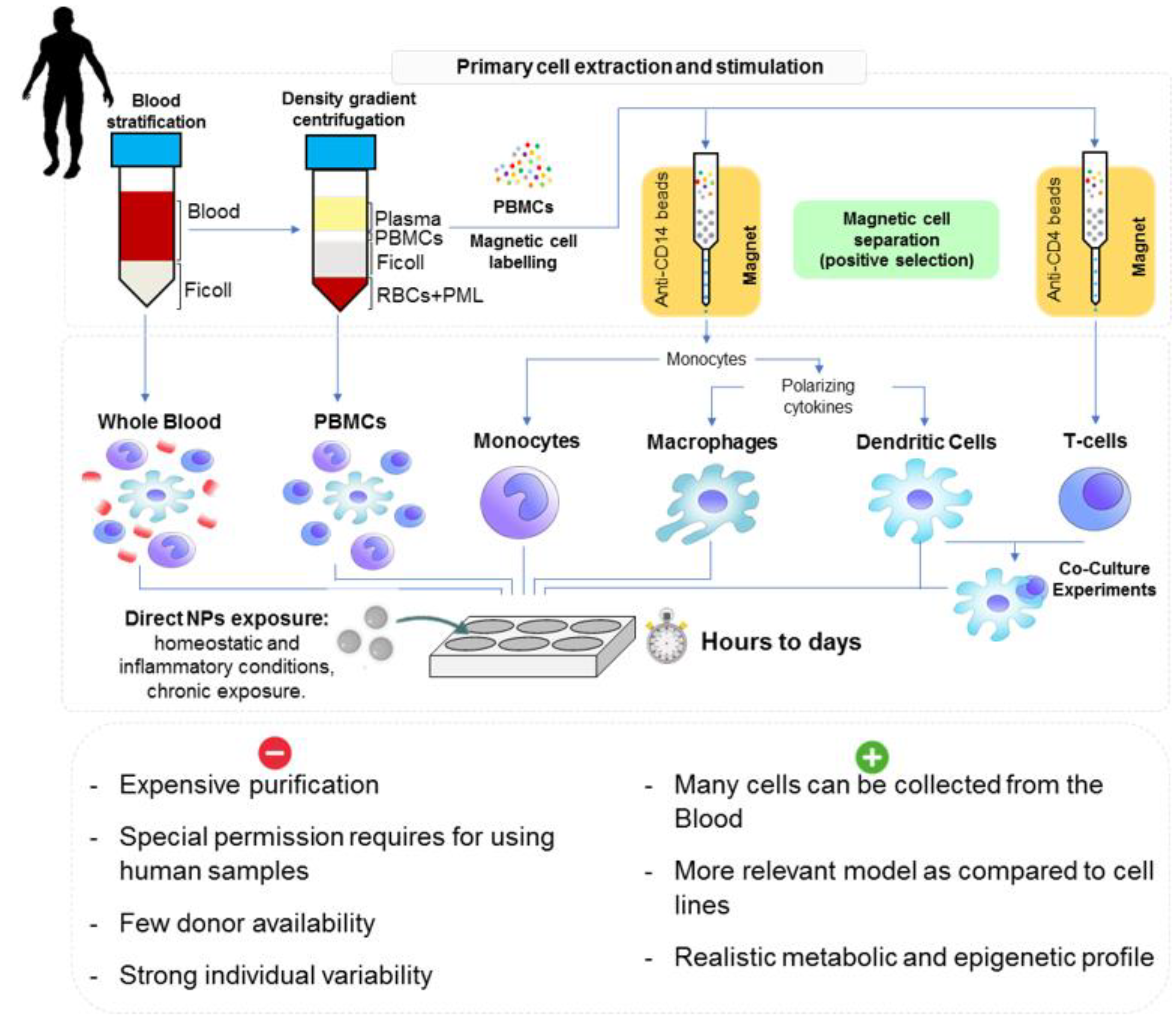
3.3. Whole Model Exposure Experiments
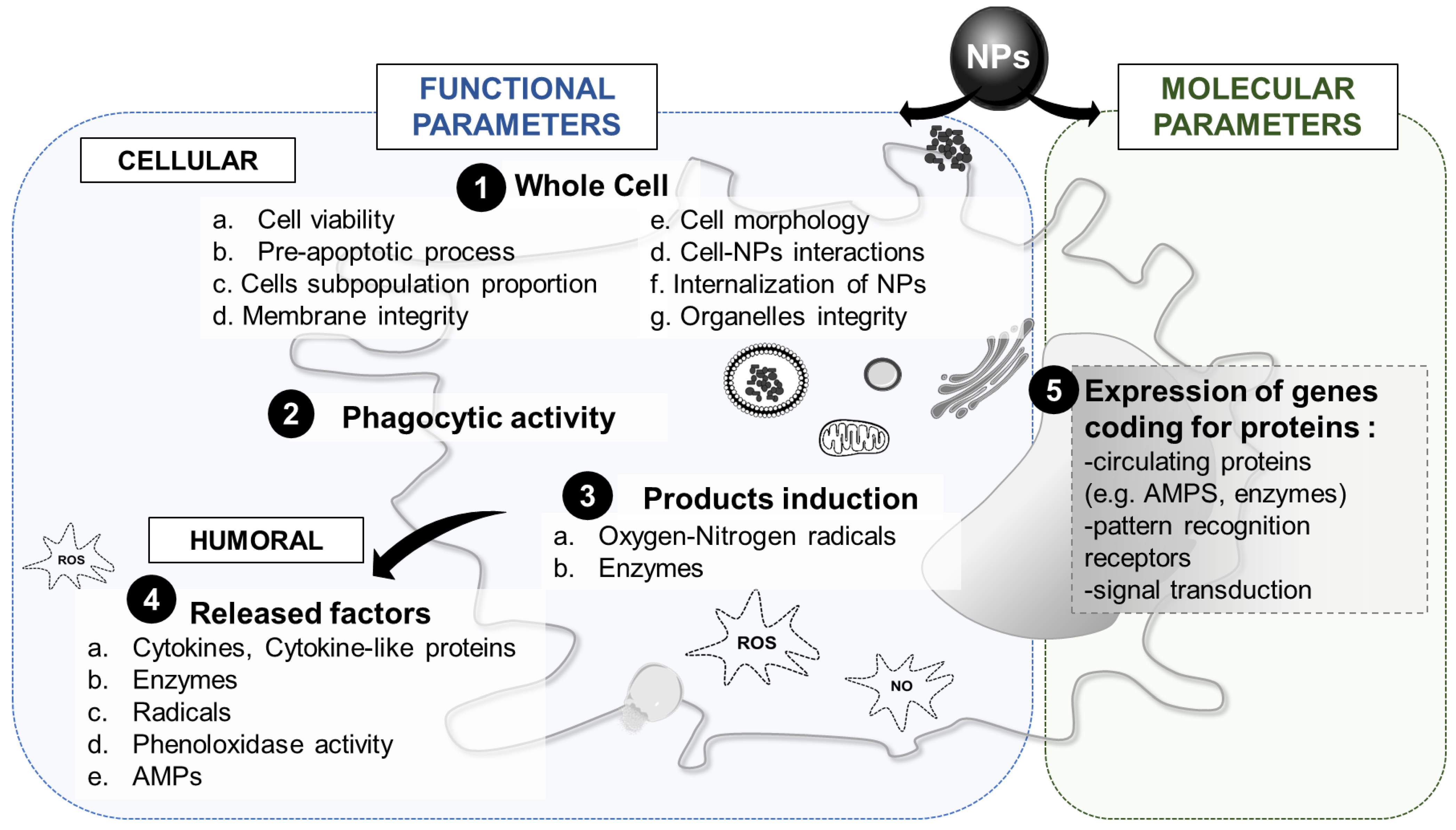
3.4. Innate Immune Parameters of Interest
3.4.1. Whole Cell Response
3.4.2. Phagocytic Activity
3.4.3. Cytotoxic Factors
3.4.4. Humoral Factors
3.4.5. Molecular Response
4. Proposal for Future Cross-Species Evaluations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Casals, E.; Gonzalez, E.; Puntes, V.F. Reactivity of Inorganic Nanoparticles in Biological Environments: Insights into Nanotoxicity Mechanisms. J. Phys. D Appl. Phys. 2012, 45, 443001. [Google Scholar] [CrossRef]
- Aitken, R.J.; Chaudhry, M.Q.; Boxall, A.B.A.; Hull, M. Manufacture and Use of Nanomaterials: Current Status in the UK and Global Trends. Occup. Med. 2006, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdorster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V. Safe Handling of Nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.; Riediker, M. Use of Nanoparticles in Swiss Industry: A Targeted Survey. Environ. Sci. Technol. 2008, 42, 2253–2260. [Google Scholar] [CrossRef]
- Bogart, L.K.; Pourroy, G.; Murphy, C.J.; Puntes, V.; Pellegrino, T.; Rosenblum, D.; Peer, D. Nanoparticles for Imaging, Sensing, and Therapeutic Intervention. ACS Nano 2014, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Banach, M. Silver Nanoparticles—A Material of the Future…? Open Chem. 2016, 14. [Google Scholar] [CrossRef]
- Boraschi, D. From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology. Vaccines 2015, 10, 930–939. [Google Scholar] [CrossRef]
- Lehner, R. Intelligent Nanomaterials for Medicine: Carrier Platforms and Targeting Strategies in the Context of Clinical Application. Nanomedicine 2013, 16, 742–757. [Google Scholar] [CrossRef]
- Boraschi, D. Interaction of Engineered Nanomaterials with the Immune System: Health-Related Safety and Possible Benefits. Curr. Opin. Toxicol. 2018, 10, 74–83. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current Status and Future Prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.; McNeil, S. Immunological Properties of Engineered Nanomaterials. Nat. Nanotechnol. 2007, 2, 10. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better Safe than Sorry: Understanding the Toxicological Properties of Inorganic Nanoparticles Manufactured for Biomedical Applications. Adv. Drug Deliv. Rev. 2010, 13, 362–374. [Google Scholar] [CrossRef]
- Jiang, Z.; Jacob, J.A.; Li, J.; Wu, X.; Wei, G.; Vimalanathan, A.; Mani, R.; Nainangu, P.; Rajadurai, U.M.; Chen, B. Influence of Diet and Dietary Nanoparticles on Gut Dysbiosis. Microb. Pathog. 2018, 118, 61–65. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Lahive, E.; Schultz, C.L. Nanomaterial Transformations in the Environment: Effects of Changing Exposure Forms on Bioaccumulation and Toxicity. Small 2020, 16. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Bundschuh, M. Nanoparticles in the Environment: Where Do We Come from, Where Do We Go To? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Graca, B.; Zgrundo, A.; Zakrzewska, D.; Rzodkiewicz, M.; Karczewski, J. Origin and Fate of Nanoparticles in Marine Water-Preliminary Results. Chemosphere 2018, 206, 359–368. [Google Scholar] [CrossRef]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment. Int. J. Environ. Res. Public Health 2015, 12, 5581–5602. [Google Scholar] [CrossRef]
- Rocha, T.L.; Gomes, T.; Sousa, V.S.; Mestre, N.C.; Bebianno, M.J. Ecotoxicological Impact of Engineered Nanomaterials in Bivalve Molluscs: An Overview. Mar. Environ. Res. 2015, 111, 74–88. [Google Scholar] [CrossRef]
- Boraschi, D.; Alijagic, A.; Auguste, M.; Barbero, F.; Ferrari, E.; Hernadi, S.; Mayall, C.; Michelini, S.; Pacheco, N.I.N.; Prinelli, A.; et al. Addressing Nanomaterial Immunosafety by Evaluating Innate Immunity across Living Species. Small 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Swartzwelter, B.J.; Fux, A.C.; Johnson, L.; Swart, E.; Hofer, S.; Hofstätter, N.; Geppert, M.; Italiani, P.; Boraschi, D.; Duschl, A.; et al. The Impact of Nanoparticles on Innate Immune Activation by Live Bacteria. Int. J. Mol. Sci. 2020, 23, 9695. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Duschl, A. Nanoparticles and the Immune System: Safety and Effects; Elsevier/AP: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-408085-0. [Google Scholar]
- Mitchell-Olds, T. Arabidopsis Thaliana and Its Wild Relatives: A Model System for Ecology and Evolution. Trends Ecol. Evol. 2001, 16. [Google Scholar] [CrossRef]
- Bevan, M.; Walsh, S. The Arabidopsis Genome: A Foundation for Plant Research. Genome Res. 2005, 15, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- The Arabidopsis Genome Iniative. Analysis of the Genome Sequence of the Flowering Plant Arabidopsis Thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- OECD. Test No 222. Earthworm Reproduction Test (Eisenia Fetida/ Eisenia Andrei); OECD: Paris, France, 2016. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Chapman&Hall: London, UK, 1996. [Google Scholar]
- Van Gestel, C.A.M.; Loureiro, S.; Zidar, P. Terrestrial Isopods as Model Organisms in Soil Ecotoxicology: A Review. Zookeys 2018, 801, 127–162. [Google Scholar] [CrossRef]
- Malev, O. Effects of CeO2 Nanoparticles on Terrestrial Isopod Porcellio Scaber: Comparison of CeO2 Biological Potential with Other Nanoparticles. Arch. Environ. Contam. Toxicol. 2017, 72, 303–311. [Google Scholar] [CrossRef]
- Novak, S.; Romih, T.; Dra, B.; Ferraris, P.; Sorieul, S.; Zieba, M.; Sebastian, V.; Arruebo, M.; Ho, S.B. The in Vivo Effects of Silver Nanoparticles on Terrestrial Isopods, Porcellio Scaber, Depend on a Dynamic Interplay between Shape, Size and Nanoparticle Dissolution Properties. Analyst 2019, 2. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue Mussels (Mytilus Edulis spp.) as Sentinel Organisms in Coastal Pollution Monitoring: A Review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Fernández Robledo, J.A.; Yadavalli, R.; Allam, B.; Pales Espinosa, E.; Gerdol, M.; Greco, S.; Stevick, R.J.; Gómez-Chiarri, M.; Zhang, Y.; Heil, C.A.; et al. From the Raw Bar to the Bench: Bivalves as Models for Human Health. Dev. Comp. Immunol. 2019, 92, 260–282. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Lun, C.M.; Smith, L.C. SpTransformer Proteins from the Purple Sea Urchin Opsonize Bacteria, Augment Phagocytosis, and Retard Bacterial Growth. PLoS ONE 2018, 13, e0196890. [Google Scholar] [CrossRef]
- Pandora-H2020. Available online: https://www.Pandora-H2020.Eu/ (accessed on 16 February 2021).
- Pinsino, A.; Bastús, N.G.; Busquets-Fité, M.; Canesi, L.; Cesaroni, P.; Drobne, D.; Duschl, A.; Ewart, M.-A.; Gispert, I.; Horejs-Hoeck, J.; et al. Probing the Immune Responses to Nanoparticles across Environmental Species. A Perspective of the EU Horizon 2020 Project PANDORA. Environ. Sci. Nano 2020, 7, 3216–3232. [Google Scholar] [CrossRef]
- Soderhall, K. Invertebrate Immunity. Advances in Experimental Medicine and Biology; Landes Bioscience and Springer Science+Business Media, LLC: New York, NY, USA, 2010; ISBN 978-1-4419-8058-8. [Google Scholar]
- Canesi, L.; Procházková, P. The Invertebrate Immune System as a Model for Investigating the Environmental Impact of Nanoparticles. In Nanoparticles and the Immune System Safety and Effects; Boraschi, D., Duschl, A., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 91–112. [Google Scholar]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.E. The Complement System. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Bilej, M.; Baetselier, P.D.; Dijck, E.V.; Stijlemans, B.; Colige, A.; Beschin, A. Distinct Carbohydrate Recognition Domains of an Invertebrate Defense Molecule Recognize Gram-Negative and Gram-Positive Bacteria. J. Biol. Chem. 2001, 276, 45840–45847. [Google Scholar] [CrossRef]
- Soderhall, K.; Cerenius, L. Role of the Prophenoloxidase-Activating System in Invertebrate Immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Jaenicke, E.; Fraune, S.; May, S.; Irmak, P.; Augustin, R.; Meesters, C.; Decker, H.; Zimmer, M. Is Activated Hemocyanin Instead of Phenoloxidase Involved in Immune Response in Woodlice? Dev. Comp. Immunol. 2009, 33, 1055–1063. [Google Scholar] [CrossRef]
- Motion, G.B.; Huitema, E. Nuclear Processes Associated with Plant Immunity and Pathogen Susceptibility. Brief Funct. Genom. 2015, 14, 243–252. [Google Scholar] [CrossRef]
- Coll, N.; Epple, P.; Dangl, J. Programmed Cell Death in the Plant Immune System. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef]
- Newman, M.-A. MAMP (Microbe-Associated Molecular Pattern) Triggered Immunity in Plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Miescher-Institut, F.; Box, P.O. FLS2: An LRR Receptor–like Kinase Involved in the Perception of the Bacterial Elicitor Flagellin in Arabidopsis. Mol. Cell 2000, 5, 1003–1011. [Google Scholar]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the Plant Immune System from Dissection to Deployment. Science 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Gust, A.A.; Pruitt, R.; Nürnberger, T. Sensing Danger: Key to Activating Plant Immunity. Trends Plant Sci. 2017, 22, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Sukarta, O.C.A. Structure-Informed Insights for NLR Functioning in Plant Immunity. Semin. Cell Dev. Biol. 2016, 56, 134–149. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J.; Nürnberger, T.; Joosten, M.H.A.J. Of PAMPs and Effectors: The Blurred PTI-ETI Dichotomy. Plant. Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, Z.; Chen, W.; Glazebrook, J.; Chang, H.-S.; Han, B.; Zhu, T.; Zou, G.; Katagiri, F. Quantitative Nature of Arabidopsis Responses during Compatible and Incompatible Interactions with the Bacterial Pathogen Pseudomonas Syringae. Plant Cell 2003, 15, 317–330. [Google Scholar] [CrossRef]
- Bigeard, J. Signaling Mechanisms in Pattern-Triggered Immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Chagas, F.O.; Pessotti, R.D.C.; Caraballo-Rodrıguez, A.M.; Pupo, M.T. Chemical Signaling Involved in Plant–Microbe Interactions. Chem. Soc. Rev. 2018, 47, 1652–1704. [Google Scholar] [CrossRef]
- Halim, V.A.; Vess, A.; Scheel, D.; Rosahl, S. The Role of Salicylic Acid and Jasmonic Acid in Pathogen Defence. Plant Biol. 2006, 8, 307–313. [Google Scholar] [CrossRef]
- Kachroo, P.; Kachroo, A. The Roles of Salicylic Acid and Jasmonic Acid in Plant Immunity. In Molecular Plant Immunity; Sessa, G., Ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 55–79. ISBN 978-1-118-48143-1. [Google Scholar]
- Bilej, M.; Procházková, P.; Silerova, M.; Joskova, R. Earthworm immunity. In Invertebrate Immunity; Landes Bioscience and Springer Science: New York, NY, USA, 2010; pp. 66–79. [Google Scholar]
- Cooper, E.L.; Kauschke, E.; Cossarizza, A. Digging for Innate Immunity since Darwin and Metchnikoff. BioEssays 2002, 24, 319–333. [Google Scholar] [CrossRef]
- Molnár, L. Cold-Stress Induced Formation of Calcium and Phosphorous Rich Chloragocyte Granules (Chloragosomes) in the Earthworm Eisenia Fetida. Comp. Biochem. Physiol. 2012, 11, 199–209. [Google Scholar] [CrossRef]
- Beschin, A.; Bilej, M.; Hanssens, F.; Raymakers, J.; Dyck, E.V.; Revets, H.; Brys, L.; Gomez, J.; Baetselier, P.D.; Timmermans, M. Identification and Cloning of a Glucan- and Lipopolysaccharide- Binding Protein from Eisenia Foetida Earthworm Involved in the Activation of Prophenoloxidase Cascade. J. Biol. Chem. 1998, 273, 24948–24954. [Google Scholar] [CrossRef]
- Škanta, F.; Roubalová, R.; Dvořák, J.; Procházková, P.; Bilej, M. Molecular Cloning and Expression of TLR in the Eisenia Andrei Earthworm. Dev. Comp. Immunol. 2013, 41, 694–702. [Google Scholar] [CrossRef]
- Škanta, F. LBP/BPI Homologue in Eisenia Andrei Earthworms. Dev. Comp. Immunol. 2016, 54, 1–6. [Google Scholar] [CrossRef]
- Silerova, M.; Prochazkova, P.; Joskova, R.; Josens, G.; Beschin, A.; De Baetselier, P.; Bilej, M. Comparative Study of the CCF-like Pattern Recognition Protein in Different Lumbricid Species. Dev. Comp. Immunol. 2006, 30, 765–771. [Google Scholar] [CrossRef]
- Cotuk, A.; Dales, R.P. Lyzomyme Activity in the Coelomic Fluid and Coelomocytes of the Earthworm Eisenia Foetida Sav. in Relation to Bacterial Infection. Comp. Biochem. Physiol. 1984, 78A, 469–474. [Google Scholar] [CrossRef]
- Joskova, R.; Šilerová, M. Identification and Cloning of an Invertebrate-Type Lysozyme from Eisenia Andrei. Dev. Comp. Immunol. 2009, 33, 932–938. [Google Scholar] [CrossRef]
- Lassegues, M.; Milochau’, A.; Doignon, F.; Pasquier, L.D.; Valembois, P. Sequence and Expression of an Eisenia-Fetida-Derived CDNA Clone That Encodes the 40-KDa Fetidin Antibacterial Protein. JBIC J. Biol. Inorg. Chem. 1997, 246, 756–762. [Google Scholar]
- Sekizawa, Y.; Hagiwara, K.; Nakajima, T.; Kobayashi, H. A Novel Protein, Lysenin, That Causes Contraction of the Isolated Rat Aorta: Its Purification from the Coelomic Fluid of the Earthworm Eisenia Foetida. Biomed. Res. 1996, 17, 197–203. [Google Scholar] [CrossRef]
- Chevalier, F.; Herbinière-Gaboreau, J.; Bertaux, J.; Raimond, M.; Morel, F.; Bouchon, D.; Grève, P.; Braquart-Varnier, C. The Immune Cellular Effectors of Terrestrial Isopod Armadillidium Vulgare: Meeting with Their Invaders, Wolbachia. PLoS ONE 2011, 6, e18531. [Google Scholar] [CrossRef] [PubMed]
- Kostanjšek, R. Pathogenesis, Tissue Distribution and Host Response to Rhabdochlamydia Porcellionis Infection in Rough Woodlouse Porcellio Scaber. J. Invertebr. Pathol. 2015, 125, 56–67. [Google Scholar] [CrossRef]
- Liu, H. Phenoloxidase Is an Important Component of the Defense against Aeromonas Hydrophila Infection in a Crustacean, Pacifastacus Leniusculus. J. Biol. Chem. 2007, 282, 33593–33598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Ginger Extract Extends the Lifespan of Drosophila Melanogaster through Antioxidation and Ameliorating Metabolic Dysfunction. J. Funct. Foods 2018, 49, 295–305. [Google Scholar] [CrossRef]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-Mediated Immunity in Arthropods: Hematopoiesis, Coagulation, Melanization and Opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Wu, S.-H.; Lai, C.-Y.; Lee, C.-Y. Demonstration of Nitric Oxide Synthase Activity in Crustacean Hemocytes and Anti-Microbial Activity of Hemocyte-Derived Nitric Oxide. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Rosa, R.; Barracco, M. Antimicrobial Peptides in Crustaceans. Invert. Surviv. J. 2010, 7, 262–284. [Google Scholar]
- Chevalier, F.; Herbinière-Gaboreau, J.; Charif, D.; Mitta, G.; Gavory, F.; Wincker, P.; Grève, P.; Braquart-Varnier, C.; Bouchon, D. Feminizing Wolbachia: A Transcriptomics Approach with Insights on the Immune Response Genes in Armadillidium Vulgare. BMC Microbiol. 2012, 12, S1. [Google Scholar] [CrossRef]
- Canesi, L.; Pruzzo, C. Specificity of Innate Immunity in bivalves: A Lesson From Bacteria. In Lessons in Immunity: From Single-Cell Organisms to Mammals; Ballarin, L., Cammarata, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 79–92. [Google Scholar]
- Gerdol., M.; Gomez-Chiari, M.; Castillo, M.G.; Figueras, A.; Fiorito, G.; Moreira, R.; Novoa, B.; Pallavicini, A.; Ponte, G.; Roumbedakis, K.; et al. Immunity in Molluscs: Recognition and Effector Mechanisms, with a Focus on Bivalvia. In Advances in Comparative Immunology; Cooper, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 225–342. [Google Scholar]
- Pezzati, E.; Canesi, L.; Damonte, G.; Salis, A.; Marsano, F.; Grande, C.; Vezzulli, L.; Pruzzo, C. Susceptibility of V Ibrio Aestuarianu s 01/032 to the Antibacterial Activity of M Ytilus Haemolymph: Identification of a Serum Opsonin Involved in Mannose-Sensitive Interactions: Vibrio Aestuarianus and Bivalve Haemocytes. Environ. Microbiol. 2015, 17, 4271–4279. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Qiu, L.; Zhang, H. Bivalve immunity. In Invertebrate Immunity; Landes Bioscience and Springer Science: New York, NY, USA, 2010. [Google Scholar]
- Allam, B.; Raftos, D. Immune Responses to Infectious Diseases in Bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef]
- Luna-Acosta, A.; Breitwieser, M.; Renault, T.; Thomas-Guyon, H. Recent Findings on Phenoloxidases in Bivalves. Mar. Pollut. Bull. 2017, 122, 5–16. [Google Scholar] [CrossRef]
- Pinsino, A. Sea Urchin Immune Cells as Sentinels of Environmental Stress. Dev. Comp. Immunol. 2015, 49, 198–205. [Google Scholar] [CrossRef]
- Smith, L.C.; Arizza, V.; Hudgell, M.A.B.; Barone, G.; Bodnar, A.G.; Buckley, K.M.; Cunsolo, V.; Dheilly, N.M.; Franchi, N.; Fugmann, S.D.; et al. Echinodermata: The complex immune system in echinoderms. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing AG: New York, NY, USA, 2018; pp. 409–501. [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. The Genome of the Sea Urchin Strongylocentrotus Purpuratus. Science 2006, 10, 941–952. [Google Scholar] [CrossRef]
- Rast, J.P.; Smith, L.C.; Loza-Coll, M.; Hibino, T.; Litman, G.W. Genomic Insights into the Immune System of the Sea Urchin. Science 2006, 314, 952–956. [Google Scholar] [CrossRef]
- Echinoderm Antimicrobial peptides: The ancient arms of the Deuterostome inna immune system. In Lessons in Immunity: From Single Cell Organisms to Mammals; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 145–153.
- Schillaci, D.; Arizza, V.; Parrinello, N.; Di Stefano, V.; Fanara, S.; Muccilli, V.; Cunsolo, V.; Haagensen, J.J.A.; Molin, S. Antimicrobial and Antistaphylococcal Biofilm Activity from the Sea Urchin Paracentrotus Lividus: Antimicrobial and Antistaphylococcal Biofilm Activity. J. Appl. Microbiol. 2010, 108, 17–24. [Google Scholar] [CrossRef]
- Alijagic, A. Gold Nanoparticles Coated with Polyvinylpyrrolidone and Sea Urchin Extracellular Molecules Induce Transient Immune Activation. J. Hazard. Mater. 2021, 402, 123793. [Google Scholar] [CrossRef]
- Liu, M.-C.; Liao, W.-Y.; Buckley, K.M.; Yang, S.Y.; Rast, J.P.; Fugmann, S.D. AID/APOBEC-like Cytidine Deaminases Are Ancient Innate Immune Mediators in Invertebrates. Nat. Commun. 2018, 9, 1948. [Google Scholar] [CrossRef]
- Chernecky, C.C.; Berger, B.J. Laboratory Tests and Diagnostic Procedures-E-Book; Elsevier Health Science: St. Louis, MO, USA, 2012. [Google Scholar]
- Tsou, C.-L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical Roles for CCR2 and MCP-3 in Monocyte Mobilization from Bone Marrow and Recruitment to Inflammatory Sites. J. Clin. Investig. 2007, 117, 902–909. [Google Scholar] [CrossRef]
- Italiani, P. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Mowat, A.M. Constant Replenishment from Circulating Monocytes Maintains the Macrophage Pool in Adult Intestine. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Seif, M.; Dembek, A.; Cavelius, C.; Huwer, H.; Kraegeloh, A.; Kiemer, A.K. M2 Polarization Enhances Silica Nanoparticle Uptake by Macrophages. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janewayr, C.A. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nat. Cell Biol. 1997, 388, 4. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 4. [Google Scholar] [CrossRef]
- Peiser, L.; Mukhopadhyay, S.; Gordon, S. Scavenger Receptors in Innate Immunity. Curr. Opin. Immunol. 2002, 14, 123–128. [Google Scholar] [CrossRef]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.C.; Gordon, S. Dectin-1 Is A Major β-Glucan Receptor On Macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential Roles of MDA5 and RIG-I Helicases in the Recognition of RNA Viruses. Nat. Cell Biol. 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Martinon, F. NLRs Join TLRs as Innate Sensors of Pathogens. Trends Immunol. 2005, 26, 447–454. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Barbero, F.; Moriones, O.H.; Bastús, N.G.; Puntes, V. Dynamic Equilibrium in the Cetyltrimethylammonium Bromide–Au Nanoparticle Bilayer, and the Consequent Impact on the Formation of the Nanoparticle Protein Corona. Bioconjugate Chem. 2019, 30, 2917–2930. [Google Scholar] [CrossRef]
- Li, Y.; Boraschi, D. Endotoxin Contamination: A Key Element in the Interpretation of Nanosafety Studies. Nanomedicine 2016, 11, 269–287. [Google Scholar] [CrossRef]
- Treuel, L.; Docter, D.; Maskos, M.; Stauber, R.H. Protein Corona-from Molecular Adsorption to Physiological Complexity. Beilstein J. Nanotechnol. 2015, 6, 857–873. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle–Cell Interactions: Molecular Structure of the Protein Corona and Cellular Outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle Size and Surface Properties Determine the Protein Corona with Possible Implications for Biological Impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical−Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. Nat. Nanotech. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Silvio, D.D. Effect of Protein Corona Magnetite Nanoparticles Derived from Bread in Vitro Digestion on Caco-2 Cells Morphology and Uptake. Int. J. Biochem. 2016, 75, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjugate Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Miclaus, T.; Scavenius, C.; Kwiatkowska, K.; Sobota, A. Species Differences Take Shape at Nanoparticles: Protein Corona Made of the Native Repertoire Assists Cellular Interaction. Environ. Sci. Technol. 2013, 47, 14367–14375. [Google Scholar] [CrossRef]
- Canesi, L.; Balbi, T.; Fabbri, R.; Salis, A.; Damonte, G.; Volland, M.; Blasco, J. Biomolecular Coronas in Invertebrate Species: Implications in the Environmental Impact of Nanoparticles. NanoImpact 2017, 8, 89–98. [Google Scholar] [CrossRef]
- Marques-Santos, L.F.; Grassi, G.; Bergami, E.; Faleri, C.; Balbi, T.; Salis, A.; Damonte, G.; Canesi, L.; Corsi, I. Cationic Polystyrene Nanoparticle and the Sea Urchin Immune System: Biocorona Formation, Cell Toxicity, and Multixenobiotic Resistance Phenotype. Nanotoxicology 2018, 12, 847–867. [Google Scholar] [CrossRef]
- Grassi, G.; Landi, C.; Della Torre, C.; Bergami, E.; Bini, L.; Corsi, I. Proteomic Profile of the Hard Corona of Charged Polystyrene Nanoparticles Exposed to Sea Urchin Paracentrotus Lividus Coelomic Fluid Highlights Potential Drivers of Toxicity. Environ. Sci. Nano 2019, 6, 2937–2947. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Nowack, B. Nanosilver Revisited Downstream. Science 2010, 330, 1054–1055. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of Nanosilver History: Implications for Policy Makers. Policy Anal. 2011, 45, 1177–1183. [Google Scholar]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling Nanomaterial Environmental Fate in Aquatic Systems. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef]
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting Their Toxicity to Daphnia Magna—A Call for Updating Toxicity Testing Policies. Proteomics 2020, 20, 1800412. [Google Scholar] [CrossRef]
- Saavedra, J.; Stoll, S.; Slaveykova, V.I. Influence of Nanoplastic Surface Charge on Eco-Corona Formation, Aggregation and Toxicity to Freshwater Zooplankton. Environ. Pollut. 2019, 252, 715–722. [Google Scholar] [CrossRef]
- Barbero, F.; Mayall, C.; Drobne, D.; Saiz-Poseu, J.; Bastús, N.G.; Puntes, V. Formation and Evolution of the Nanoparticle Environmental Corona: The Case of Au and Humic Acid. Sci. Total Environ. 2021, 768, 144792. [Google Scholar] [CrossRef]
- Batley, G.E.; Kirby, J.K.; Mclaughlin, M.J. Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments. Accounts Chem. Res. 2013, 46, 854–862. [Google Scholar] [CrossRef]
- Nowack, B.; Rose, J. Potential Scenarios for Nanomaterial Release and Subsequent Alteration in the Environment. Environ. Toxicol. Chem. 2011, 31, 50–59. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M.; Baalousha, M.; Chen, J.; Chaudry, Q.; Von der kammer, F.; Kuhlbusch, T.A.J.; Lead, J.; Nickel, C.; Quik, J.T.K.; Renker, M.; et al. A Review of the Properties and Processes Determining the Fate of Engineered Nanomaterials in the Aquatic Environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084–2134. [Google Scholar] [CrossRef]
- Svendsen, C.; Spurgeon, D.J.; Hankard, P.K.; Weeks, J.M. A Review of Lysosomal Membrane Stability Measured by Neutral Red Retention: Is It a Workable Earthworm Biomarker? Ecotoxicol. Environ. Saf. 2004, 57, 20–29. [Google Scholar] [CrossRef]
- Eyambe, G.S.; Goven, A.J.; Fitzpatrick, L.C.; Venables, B.J.; Cooper, E.L. A Non-Invasive Technique for Sequential Collection of Earthworm (Lumbricus Terrestris) Leukocytes during Subchronic Immunotoxicity Studies. Lab. Anim. 1991, 25, 61–67. [Google Scholar] [CrossRef]
- Garcia-Velasco, N. Selection of an Optimal Culture Medium and the Most Responsive Viability Assay to Assess AgNPs Toxicity with Primary Cultures of Eisenia Fetida Coelomocytes. Ecotoxicol. Environ. Saf. 2019, 183, 109545. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Y.; Li, M.; Ji, F.; Hu, C.; Cui, Y. Evaluation of Complex Toxicity of Canbon Nanotubes and Sodium Pentachlorophenol Based on Earthworm Coelomocytes Test. PLoS ONE 2017, 12, e0170092. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y. Time-Course Profiling of Molecular Stress Responses to Silver Nanoparticles in the Earthworm Eisenia Fetida. Ecotoxicol. Environ. Saf. 2013, 98, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Semerad, J.; Pacheco, N.I.N.; Grasserova, A.; Prochazkova, P.; Pivokonsky, M.; Pivokonska, L.; Cajthaml, T. In Vitro Study of the Toxicity Mechanisms of Nanoscale Zero-Valent Iron (NZVI) and Released Iron Ions Using Earthworm Cells. Nanomaterials 2020, 10, 2189. [Google Scholar] [CrossRef]
- Pacheco, N.I.N.; Roubalova, R.; Semerad, J.; Grasserova, A.; Benada, O.; Kofronova, O.; Cajthaml, T.; Dvorak, J.; Bilej, M.; Prochazkova, P. In Vitro Interactions of TiO2 Nanoparticles with Earthworm Coelomocytes: Immunotoxicity Assessment. Nanomaterials 2021, 11, 250. [Google Scholar] [CrossRef]
- Swart, E.; Dvorak, J.; Hernádi, S.; Goodall, T.; Kille, P.; Spurgeon, D.; Svendsen, C.; Prochazkova, P. The Effects of In Vivo Exposure to Copper Oxide Nanoparticles on the Gut Microbiome, Host Immunity, and Susceptibility to a Bacterial Infection in Earthworms. Nanomaterials 2020, 10, 1337. [Google Scholar] [CrossRef]
- Dolar, A. Modulations of Immune Parameters Caused by Bacterial and Viral Infections in the Terrestrial Crustacean Porcellio Scaber: Implications for Potential Markers in Environmental Research. Dev. Comp. Immunol. 2020, 113, 103789. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Balbi, T. Invertebrate Models for Investigating the Impact of Nanomaterials on Innate Immunity: The Example of the Marine Mussel Mytilus spp. CBNT 2017, 2, 77–83. [Google Scholar] [CrossRef]
- Barrick, A.; Guillet, C.; Mouneyrac, C.; Châtel, A. Investigating the Establishment of Primary Cultures of Hemocytes from Mytilus Edulis. Cytotechnology 2018, 70, 1205–1220. [Google Scholar] [CrossRef]
- Katsumiti, A.; Tomovska, R.; Cajaraville, M.P. Intracellular Localization and Toxicity of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets to Mussel Hemocytes in Vitro. Aquat. Toxicol. 2017, 188, 138–147. [Google Scholar] [CrossRef]
- Sendra, M.; Volland, M.; Balbi, T.; Fabbri, R.; Yeste, M.P.; Gatica, J.M.; Canesi, L.; Blasco, J. Cytotoxicity of CeO2 Nanoparticles Using in Vitro Assay with Mytilus Galloprovincialis Hemocytes: Relevance of Zeta Potential, Shape and Biocorona Formation. Aquat. Toxicol. 2018, 200, 13–20. [Google Scholar] [CrossRef]
- Balbi, T.; Fabbri, R.; Montagna, M.; Camisassi, G.; Canesi, L. Seasonal Variability of Different Biomarkers in Mussels (Mytilus Galloprovincialis) Farmed at Different Sites of the Gulf of La Spezia, Ligurian Sea, Italy. Mar. Pollut. Bull. 2017, 116, 348–356. [Google Scholar] [CrossRef]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Cytotoxicity and Cellular Mechanisms Involved in the Toxicity of CdS Quantum Dots in Hemocytes and Gill Cells of the Mussel Mytilus Galloprovincialis. Aquat. Toxicol. 2014, 153, 39–52. [Google Scholar] [CrossRef]
- Canesi, L.; Auguste, M.; Bebianno, M.J. Sublethal Effects of Nanoparticles on Aquatic Invertebrates, from Molecular to Organism Level. In Ecotoxicology of Nanoparticles in Aquatic Systems; Blasco, J., Corsi, I., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 38–61. [Google Scholar]
- Kádár, E.; Lowe, D.M.; Solé, M.; Fisher, A.S.; Jha, A.N.; Readman, J.W.; Hutchinson, T.H. Uptake and Biological Responses to Nano-Fe versus Soluble FeCl3 in Excised Mussel Gills. Anal. Bioanal. Chem. 2010, 396, 657–666. [Google Scholar] [CrossRef]
- Pinsino, A.; Alijagic, A. Sea Urchin Paracentrotus Lividus Immune Cells in Culture: Formulation of the Appropriate Harvesting and Culture Media and Maintenance Conditions. Biol. Open 2019, 8, 7. [Google Scholar] [CrossRef]
- Alijagic, A.; Gaglio, D.; Napodano, E.; Russo, R.; Costa, C.; Benada, O.; Kofronova, O.; Pinsino, A. Titanium Dioxide Nanoparticles Temporarily Influence the Sea Urchin Immunological State Suppressing Inflammatory-Relate Gene Transcription and Boosting Antioxidant Metabolic Activity. J. Hazard. Mater. 2020, 11. [Google Scholar] [CrossRef]
- Nurnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate Immunity in Plants and Animals: Striking Similarities and Obvious Differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar] [CrossRef]
- Michelini, S.; Barbero, F.; Prinelli, A.; Steiner, P.; Weiss, R.; Verwanger, T.; Andosch, A.; Lütz-Meindl, U.; Puntes, V.F.; Drobne, D.; et al. Gold Nanoparticles (AuNPs) Impair LPS-Driven Immune Responses by Promoting a Tolerogenic-like Dendritic Cell Phenotype with Altered Endosomal Structures. Nanoscale 2021. [Google Scholar] [CrossRef]
- Koeffler, H.P. Human Myeloid Leukemia Cell Lines: A Review. Blood 1980, 56, 344–350. [Google Scholar] [CrossRef]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Current in Vitro Methods in Nanoparticle Risk Assessment: Limitations and Challenges. Eur. J. Pharm. Biopharm. 2009, 72, 370–377. [Google Scholar] [CrossRef]
- Pott, G.; Chan, E.; Dinarello, C.A.; Shapiro, L. A-1-Antitrypsin Is an Endogenous Inhibitor of Proinflammatory Cytokine Production in Whole Blood. J. Leukoc. Biol. 2009, 85, 11. [Google Scholar] [CrossRef] [PubMed]
- Beguin, Y.; Noizat-Pirenne, F.; Pirenne, J.; Gathy, R.; Dehart, I.; Igot, D.; Baudrihaye, M.; Delacroix, D.; Franchimontl, P. Direct stimulation of cytokines (il-lp, tnf-a, il-6, il-2, ifn-y and gm-csf) in whole blood. I. Comparison with isolated pbmc stimulation. Cytokine 1992, 4, 239–248. [Google Scholar]
- Kiertscher, S.M.; Roth, M.D. Human CD14 + Leukocytes Acquire the Phenotype and Function of Antigen-Presenting Dendritic Cells When Cultured in GM-CSF and IL-4. J. Leukoc. Biol. 1996, 59, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, I.A. Leukoreduction System Chambers Are an Efficient, Valid, and Economic Source of Functional Monocyte-Derived Dendritic Cells and Lymphocytes. Immunobiology 2013, 218, 1392–1401. [Google Scholar] [CrossRef]
- Arts, R.J.W. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef]
- Pfaller, T.; Colognato, R.; Nelissen, I.; Favilli, F.; Casals, E.; Ooms, D.; Leppens, H.; Ponti, J.; Stritzinger, R.; Puntes, V.; et al. The Suitability of Different Cellular in Vitro Immunotoxicity and Genotoxicity Methods for the Analy. Nanotoxicology 2010, 4, 52–72. [Google Scholar] [CrossRef]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef]
- Siddiqui, D.M.H.; Al-Whaibi, M.H.; Mohammad, F. Nanotechnology and Plant. Sciences: Nanoparticles and Their Impact on Plants; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-14501-3. [Google Scholar]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between Engineered Nanoparticles (ENPs) and Plants: Phytotoxicity, Uptake and Accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Koelmel, J.; Leland, T.; Wang, H.; Amarasiriwardena, D.; Xing, B. Investigation of Gold Nanoparticles Uptake and Their Tissue Level Distribution in Rice Plants by Laser Ablation-Inductively Coupled-Mass Spectrometry. Environ. Pollut. 2013, 174, 222–228. [Google Scholar] [CrossRef]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle Uptake in Plants: Gold Nanomaterial Localized in Roots of Arabidopsis Thaliana by X-Ray Computed Nanotomography and Hyperspectral Imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria Italica L.) under Field Conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef]
- Al-Khaishany, M.Y. Role of Nanoparticles in Plants. In Nanotechnology and Plant Sciences; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer International Publishing: Cham, Swizterland, 2015; pp. 19–35. ISBN 978-3-319-14501-3. [Google Scholar]
- Hayashi, Y.; Miclaus, T.; Engelmann, P.; Autrup, H.; Sutherland, D.S.; Scott-Fordsmand, J.J. Nanosilver Pathophysiology in Earthworms: Transcriptional Profiling of Secretory Proteins and the Implication for the Protein Corona. Nanotoxicology 2016, 10, 303–331. [Google Scholar] [CrossRef]
- Waalewijn-Kool, P.L.; Ortiz, M.D. Effect of Different Spiking Procedures on the Distribution and Toxicity of ZnO Nanoparticles in Soil. Ecotoxicol. 2012, 21, 1797–1804. [Google Scholar] [CrossRef]
- Boraschi, D.; Oostingh, G.J.; Casals, E.; Italiani, P.; Nelissen, I.; Puntes, V.F.; Duschl, A. Nano-Immunosafety: Issues in Assay Validation. J. Phys. Conf. Ser. 2011, 304, 9. [Google Scholar] [CrossRef]
- Moret, Y.; Moreau, J. The Immune Role of the Arthropod Exoskeleton. Invert. Surviv. J. 2012, 9, 200–206. [Google Scholar]
- Mayall, C.; Dolar, A.; Jemec Kokalj, A.; Novak, S.; Razinger, J.; Barbero, F.; Puntes, V.; Drobne, D. Stressor-Dependant Changes in Immune Parameters in the Terrestrial Isopod Crustacean, Porcellio Scaber: A Focus on Nanomaterials. Nanomaterials 2021, 11, 934. [Google Scholar] [CrossRef]
- Duroudier, N.; Katsumiti, A.; Mikolaczyk, M.; Schäfer, J.; Bilbao, E.; Cajaraville, M.P. Dietary Exposure of Mussels to PVP/PEI Coated Ag Nanoparticles Causes Ag Accumulation in Adults and Abnormal Embryo Development in Their Offspring. Sci. Total Environ. 2019, 655, 48–60. [Google Scholar] [CrossRef]
- Ward, J.E.; Kach, D.J. Marine Aggregates Facilitate Ingestion of Nanoparticles by Suspension-Feeding Bivalves. Mar. Environ. Res. 2009, 68, 137–142. [Google Scholar] [CrossRef]
- Barmo, C.; Ciacci, C.; Canonico, B.; Fabbri, R.; Cortese, K.; Balbi, T.; Marcomini, A.; Pojana, G.; Gallo, G.; Canesi, L. In Vivo Effects of N-TiO2 on Digestive Gland and Immune Function of the Marine Bivalve Mytilus Galloprovincialis. Aquat. Toxicol. 2013, 132–133, 9–18. [Google Scholar] [CrossRef]
- Auguste, M. In Vivo Immunomodulatory and Antioxidant Properties of Nanoceria (NCeO2) in the Marine Mussel Mytilus Galloprovincialis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 95–102. [Google Scholar] [CrossRef]
- Falugi, C. Toxicity of Metal Oxide Nanoparticles in Immune Cells of the Sea Urchin. Mar. Environ. Res. 2012, 76, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Pinsino, A.; Russo, R.; Bonaventura, R.; Brunelli, A.; Marcomini, A.; Matranga, V. Titanium Dioxide Nanoparticles Stimulate Sea Urchin Immune Cell Phagocytic Activity Involving TLR/P38 MAPK-Mediated Signalling Pathway. Sci. Rep. 2015, 5, 14492. [Google Scholar] [CrossRef] [PubMed]
- Chivasa, S.; Ndimba, B.K.; Simon, W.J.; Lindsey, K.; Slabas, A.R. Extracellular ATP Functions as an Endogenous External Metabolite Regulating Plant Cell Viability. Plant Cell 2005, 17, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Bigorgne, E.; Foucaud, L.; Caillet, C.; Giamberini, L.; Nahmani, J.; Thomas, F.; Rodius, F. Cellular and Molecular Responses of E. Fetida Cœlomocytes Exposed to TiO2 Nanoparticles. J. Nanopart. Res. 2012, 14, 1–17. [Google Scholar] [CrossRef]
- Oostingh, G.J.; Casals, E.; Italiani, P.; Colognato, R.; Stritzinger, R.; Ponti, J.; Pfaller, T.; Kohl, Y.; Ooms, D.; Favilli, F.; et al. Problems and Challenges in the Development and Validation of Human Cell-Based Assays to Determine Nanoparticle-Induced Immunomodulatory Effects. Part. Fibre Toxicol. 2011, 8, 8. [Google Scholar] [CrossRef]
- Jones, K.; Kim, D.W.; Park, J.S.; Khang, C.H. Live-Cell Fluorescence Imaging to Investigate the Dynamics of Plant Cell Death during Infection by the Rice Blast Fungus Magnaporthe Oryzae. BMC Plant Biol. 2016, 16, 69. [Google Scholar] [CrossRef]
- Huang, C.-N.; Cornejo, M.J.; Bush, D.S.; Jones, R.L. Estimating Viability of Plant Protoplasts Using Double and Single Staining. Protoplasma 1986, 135, 80–87. [Google Scholar] [CrossRef]
- Ciacci, C.; Canonico, B.; Bilaniĉovă, D.; Fabbri, R.; Cortese, K.; Gallo, G.; Marcomini, A.; Pojana, G.; Canesi, L. Immunomodulation by Different Types of N-Oxides in the Hemocytes of the Marine Bivalve Mytilus Galloprovincialis. PLoS ONE 2012, 7, e36937. [Google Scholar] [CrossRef]
- Moyen, N.E.; Bump, P.A.; Somero, G.N.; Denny, M.W. Establishing Typical Values for Hemocyte Mortality in Individual California Mussels, Mytilus Californianus. Fish Shellfish Immunol. 2020, 100, 70–79. [Google Scholar] [CrossRef]
- de Araújo, R.F., Jr.; de Araújo, A.A.; Pessoa, J.B.; Freire Neto, F.P.; da Silva, G.R.; Leitão Oliveira, A.L.; de Carvalho, T.G.; Silva, H.F.; Eugênio, M.; Sant’Anna, C.; et al. Anti-Inflammatory, Analgesic and Anti-Tumor Properties of Gold Nanoparticles. Pharmacol. Rep. 2017, 69, 12. [Google Scholar] [CrossRef]
- Ikegawa, H.; Yamamoto, Y.; Matsumoto, H. Cell Death Caused by a Combination of Aluminum and Iron in Cultured Tobacco Cells. Physiol. Plant. 1998, 104, 474–478. [Google Scholar] [CrossRef]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of Toxicity of Ag Nanoparticles in Comparison to Bulk and Ionic Ag on Mussel Hemocytes and Gill Cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef]
- Fernández-Bautista, N.; Domínguez-Núñez, J.; Moreno, M.M.; Berrocal-Lobo, M. Plant Tissue Trypan Blue Staining During Phytopathogen Infection. Bio-Protocol 2016, 6. [Google Scholar] [CrossRef]
- Gupta, S.; Kushwah, T.; Yadav, S. Earthworm Coelomocytes as Nanoscavenger of ZnO NPs. Nanoscale Res. Lett 2014, 9, 259. [Google Scholar] [CrossRef]
- Parisi, M.G. Effects of Organic Mercury on Mytilus Galloprovincialis Hemocyte Function and Morphology. J. Comp. Physiol. B 2021, 191, 143–158. [Google Scholar] [CrossRef]
- Murano, C.; Bergami, E.; Liberatori, G.; Palumbo, A.; Corsi, I. Interplay Between Nanoplastics and the Immune System of the Mediterranean Sea Urchin Paracentrotus Lividus. Front. Mar. Sci. 2021, 8, 647394. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Watanabe, M.; Setoguchi, D.; Uehara, K.; Ohtsuka, W.; Watanabe, Y. Apoptosis-like Cell Death of Brassica Napus Leaf Protoplasts. New Phytol. 2002, 156, 417–426. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, X.; Li, H.; Cui, J.; Liu, C.; Chen, X.; Zhang, W. Induction of Caspase-3-like Activity in Rice Following Release of Cytochrome-f from the Chloroplast and Subsequent Interaction with the Ubiquitin-Proteasome System. Sci. Rep. 2015, 4, 5989. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for Immunomodulation and Apoptotic Processes Induced by Cationic Polystyrene Nanoparticles in the Hemocytes of the Marine Bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Kumar, G.; Degheidy, H.; Casey, B.J.; Goering, P.L. Flow Cytometry Evaluation of in Vitro Cellular Necrosis and Apoptosis Induced by Silver Nanoparticles. Food Chem. Toxicol. 2015, 85, 45–51. [Google Scholar] [CrossRef]
- Irizar, A. Establishment of Toxicity Thresholds in Subpopulations of Coelomocytes (Amoebocytes vs. Eleocytes) of Eisenia Fetida Exposed in Vitro to a Variety of Metals: Implications for Biomarker Measurements. Ecotoxicology 2015, 24, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Auguste, M.; Canesi, L. Shift in Immune Parameters After Repeated Exposure to Nanoplastics in the Marine Bivalve Mytilus. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.L.; Gomes, T.; Cardoso, C.; Letendre, J.; Pinheiro, J.P.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Immunocytotoxicity, Cytogenotoxicity and Genotoxicity of Cadmium-Based Quantum Dots in the Marine Mussel Mytilus Galloprovincialis. Mar. Environ. Res. 2014, 101, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tomic, S.; Ðokic, J.; Vasilijic, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljevic, P.; Rupnik, M.S.; Friedrich, B. Size-Dependent Effects of Gold Nanoparticles Uptake on Maturation and Antitumor Functions of Human Dendritic Cells In Vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. IJMS 2019, 20, 1003. [Google Scholar] [CrossRef]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.N.; Bruce, N.C. Investigating the Toxicity, Uptake, Nanoparticle Formation and Genetic Response of Plants to Gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef]
- Hayashi, Y. Earthworms and Humans in Vitro: Characterizing Evolutionarily Conserved Stress and Immune Responses to Silver Nanoparticles. Environ. Sci. Technol. 2012, 46, 4166–4173. [Google Scholar] [CrossRef]
- Auguste, M.; Mayall, C.; Barbero, F.; Hočevar, M.; Alberti, S.; Grassi, G.; Puntes, V.F.; Drobne, D.; Canesi, L. Functional and Morphological Changes Induced in Mytilus Hemocytes by Selected Nanoparticles. Nanomaterials 2021, 11, 470. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Balbi, T.; Salis, A.; Damonte, G.; Cortese, K.; Caratto, V.; Monopoli, M.P.; Dawson, K.; et al. Interactions of Cationic Polystyrene Nanoparticles with Marine Bivalve Hemocytes in a Physiological Environment: Role of Soluble Hemolymph Proteins. Environ. Res. 2016, 150, 73–81. [Google Scholar] [CrossRef]
- Katsumiti, A.; Arostegui, I.; Oron, M.; Gilliland, D.; Valsami-Jones, E.; Cajaraville, M.P. Cytotoxicity of Au, ZnO and SiO2 NPs Using in Vitro Assays with Mussel Hemocytes and Gill Cells: Relevance of Size, Shape and Additives. Nanotoxicology 2015, 10, 185–193. [Google Scholar] [CrossRef]
- Swartzwelter, B.J.; Barbero, F.; Verde, A.; Mangini, M.; Pirozzi, M.; Luca, A.C.D.; Puntes, V.F.; Leite, L.C.C.; Italiani, P.; Boraschi, D. Gold Nanoparticles Modulate BCG-Induced Innate Immune Memory in Human Monocytes by Shifting the Memory Response towards Tolerance. Nanomaterials 2019, 9, 1354. [Google Scholar] [CrossRef]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and Distribution of Ultrasmall Anatase TiO2 Alizarin Red S Nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef]
- Timmers, A.C.J.; Tirlapur, U.K.; Schel, J.H.N. Vacuolar Accumulation of Acridine Orange and Neutral Red in Zygotic and Somatic Embryos of Carrot (Daucus Carota L.). Protoplasma 1995, 188, 236–244. [Google Scholar] [CrossRef]
- Weeks, J.M.; Svendsen, C. Neutral Red Retention by Lysosomes from Earthworm (Lumbricus rubellus) Coelomocytes: A Simple Biomarker of Exposure to Soil Copper. Environ. Toxicol. Chem. 1996, 15, 1801–1805. [Google Scholar] [CrossRef]
- Long, J. Internalization, Cytotoxicity, Oxidative Stress and Inflammation of Multi-Walled Carbon Nanotubes in Human Endothelial Cells: Influence of Pre-Incubation with Bovine Serum Albumin. RSC Adv. 2018, 8, 9253–9260. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Czymmek, K.; Tallóczy, Z.; Levine, B.; Dinesh-Kumar, S.P. Autophagy Regulates Programmed Cell Death during the Plant Innate Immune Response. Cell 2005, 121, 567–577. [Google Scholar] [CrossRef]
- Patel, S.; Dinesh-Kumar, S.P. Arabidopsis ATG6 Is Required to Limit the Pathogen-Associated Cell Death Response. Autophagy 2008, 4, 20–27. [Google Scholar] [CrossRef]
- Auguste, M. Effects of Nanosilver on Mytilus Galloprovincialis Hemocytes and Early Embryo Development. Aquat. Toxicol. 2018, 203, 107–116. [Google Scholar] [CrossRef]
- Borges, J.; Porto-Neto, L.; Mangiaterra, M.; Jensch-Junior, B.; da Silva, J. Phagocytosis in Vitro and in Vivo in the Antarctic Sea Urchin Sterechinus Neumayeri at 0 °C. Polar Biol. 2002, 25, 891–897. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Thwala, M.; Musee, N.; Sikhwivhilu, L.; Wepener, V. The Oxidative Toxicity of Ag and ZnO Nanoparticles towards the Aquatic Plant Spirodela Punctuta and the Role of Testing Media Parameters. Environ. Sci. Processes Impacts 2013, 15, 1830. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver Nanoparticle-Mediated Enhancement in Growth and Antioxidant Status of Brassica Juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Romero, A.; Rey-Campos, M.; Pereiro, P.; Rosani, U.; Novoa, B.; Figueras, A. Stimulation of Mytilus Galloprovincialis Hemocytes With Different Immune Challenges Induces Differential Transcriptomic, MiRNomic, and Functional Responses. Front. Immunol. 2020, 11, 606102. [Google Scholar] [CrossRef]
- Magesky, A.; de Oliveira Ribeiro, C.A.; Beaulieu, L.; Pelletier, É. Silver Nanoparticles and Dissolved Silver Activate Contrasting Immune Responses and Stress-Induced Heat Shock Protein Expression in Sea Urchin: Nanosilver and Dissolved Ag Effects in Sea Urchins. Environ. Toxicol. Chem. 2017, 36, 1872–1886. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Yelin, D. High Levels of Reactive Oxygen Species in Gold Nanoparticle-Targeted Cancer Cells Following Femtosecond Pulse Irradiation. Sci. Rep. 2013, 3, srep02146. [Google Scholar] [CrossRef]
- Shi, J. Inflammatory Caspases Are Innate Immune Receptors for Intracellular LPS. Nat. Cell Biol. 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO Nanoparticles Increase Photosynthetic Pigments and Decrease Lipid Peroxidation in Soil Grown Cilantro (Coriandrum Sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- Capolupo, M.; Valbonesi, P.; Fabbri, E. A Comparative Assessment of the Chronic Effects of Micro- and Nano-Plastics on the Physiology of the Mediterranean Mussel Mytilus Galloprovincialis. Nanomaterials 2021, 11, 649. [Google Scholar] [CrossRef]
- Paciorek, P. Products of Lipid Peroxidation as a Factor in the Toxic Effect of Silver Nanoparticles. Materials 2020, 13, 2460. [Google Scholar] [CrossRef]
- Chen, W.; Provart, N.J.; Glazebrook, J.; Katagiri, F.; Chang, H.-S.; Eulgem, T.; Mauch, F.; Luan, S.; Zou, G.; Whitham, S.A.; et al. Expression Profile Matrix of Arabidopsis Transcription Factor Genes Suggests Their Putative Functions in Response to Environmental Stresses. Plant Cell 2002, 14, 559–574. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef]
- Homa, J.; Zorska, A.; Wesolowski, D.; Chadzinska, M. Dermal Exposure to Immunostimulants Induces Changes in Activity and Proliferation of Coelomocytes of Eisenia Andrei. J. Comp. Physiol. B 2013, 183, 313–322. [Google Scholar] [CrossRef]
- Ma, J.S.; Kim, W.J.; Kim, J.J.; Kim, T.J.; Ye, S.K.; Song, M.D.; Kang, H.; Kim, D.W.; Moon, W.K.; Lee, K.H. Gold Nanoparticles Attenuate LPS-Induced NO Production through the Inhibition of NF-ΚB and IFN-β/STAT1 Pathways in RAW264.7 Cells. Nitric Oxide 2010, 23, 214–219. [Google Scholar] [CrossRef]
- Sakthivel, M.; Karthikeyan, N.; Palani, P. Detection and analysis of lysozyme activity in some tuberous plants and calotropis procera’s latex. J. Phytol. 2010, 2, 65–72. [Google Scholar]
- Fiołka, M.J.; Zagaja, M.P.; Hułas-Stasiak, M.; Wielbo, J. Activity and Immunodetection of Lysozyme in Earthworm Dendrobaena Veneta (Annelida). J. Invertebr. Pathol. 2012, 109, 83–90. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Balbi, T.; Pallavicini, A.; Vezzulli, L.; Canesi, L. Impact of Nanoplastics on Hemolymph Immune Parameters and Microbiota Composition in Mytilus Galloprovincialis. Mar. Environ. Res. 2020, 159, 105017. [Google Scholar] [CrossRef]
- Shimizu, M.; Kohno, S.; Kagawa, H.; Ichise, N. Lytic Activity and Biochemical Properties of Lysozyme in the Coelomic Fluid of the Sea UrchinStrongylocentrotus Intermedius. J. Invertebr. Pathol. 1999, 73, 214–222. [Google Scholar] [CrossRef]
- Pagliara, P.; Stabili, L. Zinc Effect on the Sea Urchin Paracentrotus Lividus Immunological Competence. Chemosphere 2012, 89, 563–568. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From Bacterial Killing to Immune Modulation: Recent Insights into the Functions of Lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical Insights into Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Procházková, P.; Silerova, M.; Stijlemans, B.; Dieu, M.; Halada, P.; Joskova, R.; Beschin, A.; De Baetselier, P.; Bilej, M. Evidence for Proteins Involved in Prophenoloxidase Cascade Eisenia Fetida Earthworms. J. Comp. Physiol. B 2006, 176, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. Identification and Characterization of Proteins with Phenoloxidase-like Activities in the Sea Urchin Strongylocentrotus Nudus. Fish Shellfish. Immunol. 2015, 47, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guleria, P.; Kumar, V.; Yadav, S.K. Gold Nanoparticle Exposure Induces Growth and Yield Enhancement in Arabidopsis Thaliana. Sci. Total Environ. 2013, 462–468. [Google Scholar] [CrossRef]
- Bergami, E.; Krupinski Emerenciano, A.; González-Aravena, M.; Cárdenas, C.A.; Hernández, P.; Silva, J.R.M.C.; Corsi, I. Polystyrene Nanoparticles Affect the Innate Immune System of the Antarctic Sea Urchin Sterechinus Neumayeri. Polar Biol. 2019, 42, 743–757. [Google Scholar] [CrossRef]
- Mincarelli, L. Evaluation of Gene Expression of Different Molecular Biomarkers of Stress Response as an Effect of Copper Exposure on the Earthworm EIsenia Andrei. Ecotoxicology 2019, 28, 938–948. [Google Scholar] [CrossRef]
- Chan, S.L.; Mukasa, T.; Santelli, E.; Low, L.Y.; Pascual, J. The Crystal Structure of a TIR Domain from Arabidopsis Thaliana Reveals a Conserved Helical Region Unique to Plants. Protein Sci. 2009. [Google Scholar] [CrossRef]
- Vasilichin, V.A.; Tsymbal, S.A.; Fakhardo, A.F.; Anastasova, E.I.; Marchenko, A.S.; Shtil, A.A.; Vinogradov, V.V.; Koshel, E.I. Effects of Metal Oxide Nanoparticles on Toll-Like Receptor MRNAs in Human Monocytes. Nanomaterials 2020, 10, 127. [Google Scholar] [CrossRef]
- Iizasa, S.; Iizasa, E.; Matsuzaki, S.; Tanaka, H.; Kodama, Y.; Watanabe, K.; Nagano, Y. Arabidopsis LBP/BPI Related-1 and -2 Bind to LPS Directly and Regulate PR1 Expression. Sci. Rep. 2016, 6, 27527. [Google Scholar] [CrossRef]
- OSPAR. Background Document and Technical Annexes for Biological Effects Monitoring. 2013. Available online: https://mcc.jrc.ec.europa.eu/documents/OSPAR/OSPAR_CoordinatedEnvironmentalMonitoringProgramme_CEMP.pdf (accessed on 8 June 2021).
- Conte, C.; Dal Poggetto, G.; Swartzwelter, B.; Esposito, D.; Ungaro, F.; Laurienzo, P.; Boraschi, D.; Quaglia, F. Surface Exposure of PEG and Amines on Biodegradable Nanoparticles as a Strategy to Tune Their Interaction with Protein-Rich Biological Media. Nanomaterials 2019, 9, 1354. [Google Scholar] [CrossRef]
- Gautam, A. Immunotoxicity of Copper Nanoparticle and Copper Sulfate in a Common Indian Earthworm. Ecotoxicol. Environ. Saf. 2018, 148, 620–631. [Google Scholar] [CrossRef]
- Dvořák, J.; Mančíková, V.; Pižl, V.; Elhottová, D.; Šilerová, M.; Roubalová, R.; Škanta, F.; Procházková, P.; Bilej, M. Microbial Environment Affects Innate Immunity in Two Closely Related Earthworm Species Eisenia Andrei and Eisenia Fetida. PLoS ONE 2013, 8, e79257. [Google Scholar]
- Dvořák, J. Sensing Microorganisms in the Gut Triggers the Immune Response in Eisenia Andrei Earthworms. Dev. Comp. Immunol. 2016, 57, 67–74. [Google Scholar] [CrossRef]
- Bhattacharya, K. Fundamentals of Qualitative Research A Practical Guide; Routledge: London, UK, 2017. [Google Scholar]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Buffet, P.-E.; Richard, M.; Caupos, F.; Vergnoux, A.; Perrein-Ettajani, H.; Luna-Acosta, A.; Akcha, F.; Amiard, J.-C.; Amiard-Triquet, C.; Guibbolini, M.; et al. A Mesocosm Study of Fate and Effects of CuO Nanoparticles on Endobenthic Species (Scrobicularia Plana, Hediste Diversicolor). Environ. Sci. Technol. 2013, 130110104824003. [Google Scholar] [CrossRef]
- van Straalen, N.M.; Feder, M.E. Ecological and Evolutionary Functional Genomics—How Can It Contribute to the Risk Assessment of Chemicals? Environ. Sci. Technol. 2012, 46, 3–9. [Google Scholar] [CrossRef]
- Maleck, K.; Levine, A.; Eulgem, T.; Morgan, A.; Schmid, J.; Lawton, K.A.; Dietrich, R.A. The Transcriptome of Arabidopsis Thaliana during Systemic Acquired Resistance. Nat. Genet. 2000, 26, 8. [Google Scholar] [CrossRef]
- Détrée, C.; Gallardo-Escárate, C. Single and Repetitive Microplastics Exposures Induce Immune System Modulation and Homeostasis Alteration in the Edible Mussel Mytilus Galloprovincialis. Fish Shellfish Immunol. 2018, 83, 52–60. [Google Scholar] [CrossRef]
- Felice, B.D.; Parolini, M. Can Proteomics Be Considered as a Valuable Tool to Assess the Toxicity of Nanoparticles in Marine Bivalves? J. Mar. Sci. Eng. 2020, 8, 1033. [Google Scholar] [CrossRef]
- Duroudier, N. Changes in Protein Expression in Mussels Mytilus Galloprovincialis Dietarily Exposed to PVP/PEI Coated Silver Nanoparticles at Different Seasons. Aquat. Toxicol. 2019, 210, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y. Impacts of Size and Shape of Silver Nanoparticles on Arabidopsis Plant Growth and Gene Expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef] [PubMed]
| Name | Innate Immune Cell Types | Whole Organism Level Defense | Cellular Response | Humoral/Extracellular Factors | Recognition & Activation |
|---|---|---|---|---|---|
| Plant Arabidopsis thaliana | All cells |
Cell wall Waxy epidermal cuticle | MAMP-triggered immunity Effector triggered Immunity Hypersensitive response | ROS production Hormones (ethylene, JA, SA) Antimicrobial secreted peptides | PRRs: RLKs RLPs NLRs |
| Earthworm Eisenia fetida | Amoebocytes (granular and hyaline) Eleocytes | Skin Mucus Expulsion by dorsal pore Autotomy | Phagocytosis Agglutination-encapsulation ProPO cascade → melanization | AMPs (lumbricin I) Bacteriolytic enzyme (lysozyme) Hemolytic, proteolytic and cytotoxic proteins (fetidin and lysenins) ROS production | PRRs: CCF (lectinlike domain) TLR LBP/BPI |
| Terrestrial isopod Porcelio scaber | Hemocyte Granular and hyaline | Cuticle | Phagocytosis Encapsulation ProPO cascade → melanization | AMPs ROS/NO production | PRRs: TLR |
| Marine mussel Mytilus galloprovincialis | Hemocyte Granular and hyaline | Shell barrier Mucus layer Pseudo- feces | Phagocytosis Encapsulation ProPO | AMPs (mytilin, myticin, mytimicin), Defensins Complement system (C1qDC) Bacteriolytic enzyme-Lysozyme ROS /NO production | PRRs: lectins PGRPS TLR C1qDC FRED |
| Sea urchin Paracentrotus lividus | Macrophage-like phagocytes, amoebocytes (colorless, red); vibratile cells | Test Gut barrier Faeces | Phagocytosis Encapsulation | ROS production, AMPs (strongylocins, centrocins, paracentrin 1), lysozyme | PRRs: TLRs NLRs SRCR domain-containing proteins |
| Human | Monocytes Macrophages DCs Granulocytes 1 | Epithelial and mucosal tissue | Phagocytosis Inflammation Granulocyte recruitment Antigen presentation | Complement antibodies, AMPs NETs, ROS/NO | PRRs:TLRs, NLRs, Scavenger Receptors, RLRs, CLRs, |
| Plants | Earthworms | Isopods | Mussels | Sea Urchins | Human | |
|---|---|---|---|---|---|---|
| 1. Whole cell | ||||||
| Cell viability | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| LDH or ATP release | [180] | [181] | [153,182] | |||
| Fluorescent probes (FDA or PI) | [183,184] | [138,139] | [185,186] | [178] | [187] | |
| Metabolic activity (MTT or CTB) | [188] | [181] | [189] | [153] | ||
| Blue tryptan | [190] | [191] | [173] | [192] | [193] | [194] |
| (Pre)-apoptosis (Annexin-V, DAPI, PI) | [195,196] | [138,139] | [141] | [197] | [121] | [198] |
| Cell subpopulation or polarization | [139,199] | [141,173] | [200,201] | [179] | [153,202] | |
| NP internalization | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| TEM/SEM | [203,204] | [181,205] | [141] | [189,206,207,208] | [90,151,178] | [153,209] |
| Organelles | ✔ | ✔ | ✖ | ✔ | ✔ | ✔ |
| Neural red uptake/ release | [210,211] | [212] | [185,189] | [150,179] | [213] | |
| Lysosome acidification | [214,215] | [200] | [179] | |||
| Other organelles integrity (Trans-Golgi apparatus, Mitochondria) | [216] | [178,179] | ||||
| 2. Phagocytic activity | ||||||
| Phagocytosis | ✖ | ✔ | ✖ | ✔ | ✔ | ✔ |
| Phagocytic activity (index, rate) | [139,181] | [174,216] | [150,217] | [218] | ||
| 3. Cytotoxic factors | ||||||
| Oxygen and nitrogen radicals | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| ROS production | [219,220] | [139,205] | [146,208,221] | [151,222] | [223,224] | |
| Lipid peroxidase activity | [220,225] | [138,139] | [226] | [227] | ||
| RNS (including NO) production | [228,229] | [230] | [173] | [185] | [231] | |
| Hydrolytic enzymes | ✔ | ✔ | ✖ | ✔ | ✔ | ✔ |
| Lysozyme | [232] | [233] | [234] | [235,236] | [237] | |
| Other species specific enzymes | lysenin [119] | |||||
| 4. Humoral factors | ||||||
| Cytokines | ✖ | ✖ | ✖ | ✖ | ✔ | ✔ |
| IL, TNF, IF secretion | [151] | [94,238,239] | ||||
| Melanization | ✖ | ✔ | ✔ | ✔ | ✔ | ✖ |
| Phenoloxidase activation | [230,240] | [44] | [83] | [241] | ||
| 5. Gene expression | ||||||
| Oxidative stress genes | ✔ | ✔ | ✖ | ✔ | ✔ | ✖ |
| Antioxidant defense and detoxification genes (e.g., CAT, SOD) | [242] | [140,195] | [176,200] | [243] | ||
| Circulating protein genes | ✖ | ✔ | ✖ | ✔ | ✖ | ✔ |
| Signal transduction protein, enzymes, AMPs (general and species-specific) | Lysenin/Fetidin [141,170,192] CCF [181,244] | mytilin, myticin, EPp [176,200] | [231] | |||
| Receptor protein genes | ✔ | ✔ | ✖ | ✔ | ✔ | ✔ |
| TLR | [245] | [244] | [177] | [151,179] | [246] | |
| LBP/BPI (LPS-binding protein/bacterial permeability-increasing protein) | [247] | [64] | [243] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swartzwelter, B.J.; Mayall, C.; Alijagic, A.; Barbero, F.; Ferrari, E.; Hernadi, S.; Michelini, S.; Navarro Pacheco, N.I.; Prinelli, A.; Swart, E.; et al. Cross-Species Comparisons of Nanoparticle Interactions with Innate Immune Systems: A Methodological Review. Nanomaterials 2021, 11, 1528. https://doi.org/10.3390/nano11061528
Swartzwelter BJ, Mayall C, Alijagic A, Barbero F, Ferrari E, Hernadi S, Michelini S, Navarro Pacheco NI, Prinelli A, Swart E, et al. Cross-Species Comparisons of Nanoparticle Interactions with Innate Immune Systems: A Methodological Review. Nanomaterials. 2021; 11(6):1528. https://doi.org/10.3390/nano11061528
Chicago/Turabian StyleSwartzwelter, Benjamin J., Craig Mayall, Andi Alijagic, Francesco Barbero, Eleonora Ferrari, Szabolcs Hernadi, Sara Michelini, Natividad Isabel Navarro Pacheco, Alessandra Prinelli, Elmer Swart, and et al. 2021. "Cross-Species Comparisons of Nanoparticle Interactions with Innate Immune Systems: A Methodological Review" Nanomaterials 11, no. 6: 1528. https://doi.org/10.3390/nano11061528
APA StyleSwartzwelter, B. J., Mayall, C., Alijagic, A., Barbero, F., Ferrari, E., Hernadi, S., Michelini, S., Navarro Pacheco, N. I., Prinelli, A., Swart, E., & Auguste, M. (2021). Cross-Species Comparisons of Nanoparticle Interactions with Innate Immune Systems: A Methodological Review. Nanomaterials, 11(6), 1528. https://doi.org/10.3390/nano11061528







