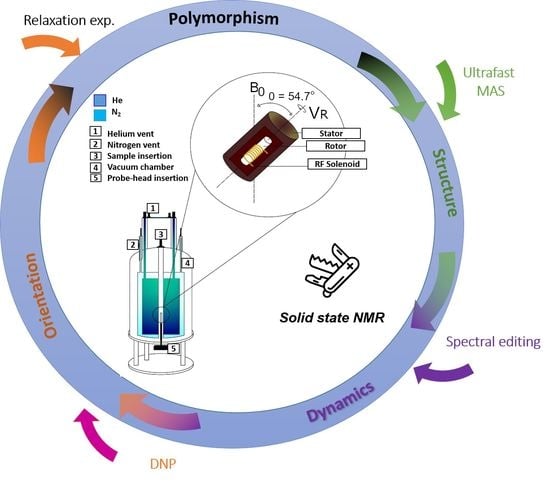
Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review
Abstract
1. Introduction
- The alignment of the nuclear spins along the applied external magnetic field.
- The perturbation of this alignment using a weak oscillating magnetic field.
- The detection of the NMR signal (voltage induced in a detection coil).
2. Chemical Connectivity
2.1. Inorganic Films
2.2. Organic Films
3. Recent Advancement in NMR Strategies and Hardware Design
3.1. Hardware Advancements (Probe and Coil Design)
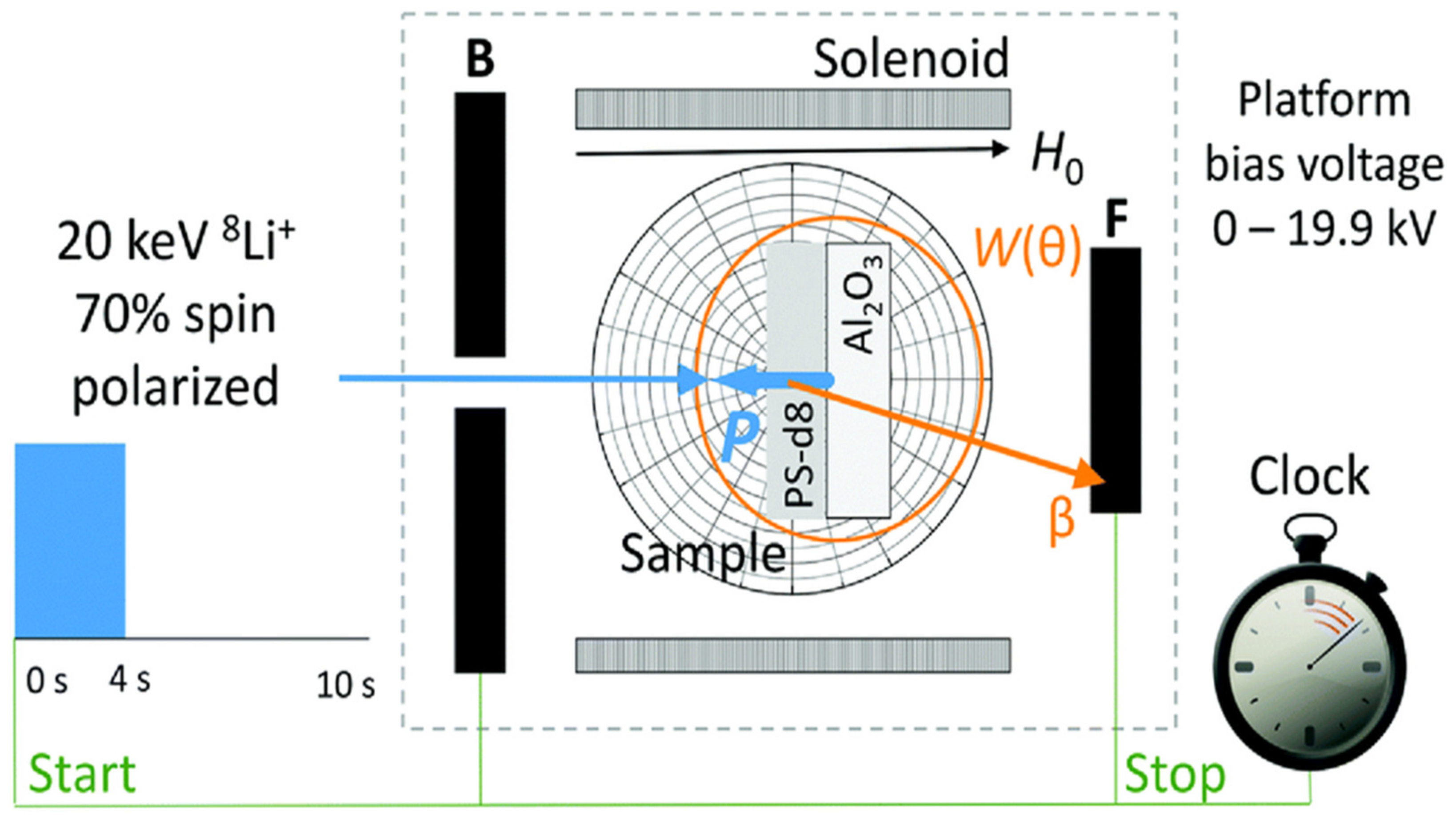
3.2. Sensitivity Detection (MRFM, β-Magnet)
3.3. Polarization Enhancement (Natural Abundance DNP versus Thin Films)
4. High-Tech Opportunities beyond Conventional Methods
4.1. Ultra-Fast MAS Spinning for 1H, 19F and Heavy Spin-½ Nuclei
4.2. Ultra-High Magnetic Fields for Quadrupole Nuclei
4.3. Isotopic Enrichment of NMR Active Nuclei vs. Paramagnetic Doping for Sensitivity Boosting
4.4. Advanced Hyperpolarization Techniques
4.5. NMR Techniques beyond Spectroscopy (NMR Diffusometry, Fast Field Cycle NMR, Zero Field NMR, Magnetic Resonance Imaging)
5. Summary, Concluding Remarks and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Kindlund, H.; Sangiovanni, D.G.; Petrov, I.; Greene, J.E.; Hultman, L. A review of the intrinsic ductility and toughness of hard transition-metal nitride alloy thin films. Thin Solid Film. 2019, 688, 137479. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Han, R.; Ren, J.; Li, L.; Han, N.; Xing, F.; Zhu, J. A review on the mechanical properties for thin film and block structure characterized by using nanoscratch test. Nano-Technol. Rev. 2019, 8, 628–644. [Google Scholar] [CrossRef]
- Jansson, U.; Lewin, E. Carbon-containing multi-component thin films. Thin Solid Film. 2019, 688, 137411. [Google Scholar] [CrossRef]
- Zhao, C.; Li, L.-Y.; Guo, M.-M.; Zheng, J. Functional polymer thin films designed for anti-fouling materials and biosensors. Chem. Pap. 2012, 66, 323–339. [Google Scholar] [CrossRef]
- Spivack, M.A. Mechanical Properties of Very Thin Polymer Films. Rev. Sci. Instrum. 1972, 43, 985–990. [Google Scholar] [CrossRef]
- Hu, C.; Guo, K.; Li, Y.; Gu, Z.; Quan, J.; Zhang, S.; Zheng, W. Optical coatings of durability based on transition metal nitrides. Thin Solid Film. 2019, 688, 137339. [Google Scholar] [CrossRef]
- Moon, D.-B.; Lee, J.; Roh, E.; Lee, N.-E. Three-dimensional out-of-plane geometric engineering of thin films for stretchable electronics: A brief review. Thin Solid Film. 2019, 688, 137435. [Google Scholar] [CrossRef]
- Serrano, A.; de la Fuente, O.R.; García, M.A. Extended and localized surface plasmons in annealed Au films on glass substrates. J. Appl. Phys. 2010, 108, 074303. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Chen, K.; Zhu, B.; Zhao, J.; Jiang, T. Planar surface plasmonic waveguide devices based on symmetric corrugated thin film structures. Opt. Express 2014, 22, 20107. [Google Scholar] [CrossRef]
- Foley, J.J., IV; Harutyunyan, H.; Rosenmann, D.; Divan, R.; Wiederrecht, G.P.; Gray, S.K. When are Surface Plasmon Polaritons Excited in the Kretschmann-Raether Configuration? Sci. Rep. 2015, 5, 9929. [Google Scholar] [CrossRef]
- Branco, R.; Sousa, T.; Piedade, A.P.; Morais, P.V. Immobilization of Ochrobactrum tritici As5 on PTFE thin films for arsenite biofiltration. Chemosphere 2016, 146, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Khlyustova, A.; Cheng, Y.; Yang, R. Vapor-deposited functional polymer thin films in biological applications. J. Mater. Chem. B 2020, 8, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yao, Y.; Wu, J.; Niu, X.; Rogach, A.L.; Wang, Z. Effects of Plasmonic Metal Core -Dielectric Shell Nanoparticles on the Broadband Light Absorption Enhancement in Thin Film Solar Cells. Sci. Rep. 2017, 7, 7696. [Google Scholar] [CrossRef]
- Terry, M.L.; Straub, A.; Inns, D.; Song, D.; Aberle, A.G. Large open-circuit voltage improvement by rapid thermal annealing of evaporated solid-phase-crystallized thin-film silicon so-lar cells on glass. Appl. Phys. Lett. 2005, 86, 172108. [Google Scholar] [CrossRef]
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285. [Google Scholar] [CrossRef]
- Mukanova, A.; Jetybayeva, A.; Myung, S.-T.; Kim, S.-S.; Bakenov, Z. A mini-review on the development of Si-based thin film anodes for Li-ion batteries. Mater. Today Energy 2018, 9, 49–66. [Google Scholar] [CrossRef]
- Yang, G.; Abraham, C.; Ma, Y.; Lee, M.; Helfrick, E.; Oh, D.; Lee, D. Advances in Materials De-sign for All-Solid-state Batteries: From Bulk to Thin Films. Appl. Sci. 2020, 10, 4727. [Google Scholar] [CrossRef]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D Thin-Film Li-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1900805. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-wahab, M.S.; Hammad, A.H. Advance Deposition Techniques for Thin Film and Coating. In Modern Technologies for Creating the Thin-Film Systems and Coatings; Nikitenkov, N.N., Ed.; Intech: London, UK, 2017; pp. 137–149. [Google Scholar] [CrossRef]
- Campbell, D.S. Preparation Methods for Thin Films. In Physics of Nonmetallic Thin Films; Dupuy, C.H.S., Cachard, A., Eds.; Springer US: Boston, MA, USA, 1976; pp. 9–48. [Google Scholar] [CrossRef]
- Mehran, Q.M.; Fazal, M.A.; Bushroa, A.R.; Rubaiee, S. A Critical Review on Physical Vapor Deposition Coatings Applied on Different Engine Components. Crit. Rev. Solid State Mater. Sci. 2018, 43, 158–175. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chal-cogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Hannachi, A.; Segura, H. Maghraoui-Meherzi, Growth of manganese sulfide (α-MnS) thin films by thermal vacuum evaporation: Structural, morphological and optical properties. Mater. Chem. Phys. 2016, 181, 326–332. [Google Scholar] [CrossRef]
- Ananth Kumar, R.T.; Das, C.; Chithra Lekha, P.; Asokan, S.; Sanjeeviraja, C.; Pathinettam Padiyan, D. Enhancement in threshold voltage with thickness in memory switch fabricated using GeSe1.5S0.5 thin films. J. Alloy. Compd. 2014, 615, 629–635. [Google Scholar] [CrossRef]
- Malligavathy, M.; Ananth Kumar, R.T.; Das, C.; Asokan, S.; Pathinettam Padiyan, D. Growth and characteristics of amorphous Sb2Se3 thin films of various thicknesses for memory switching applications. J. Non-Cryst. Solids 2015, 429, 93–97. [Google Scholar] [CrossRef]
- Abdel Rafea, M.; Farid, H. Phase change and optical band gap behavior of Se0.8S0.2 chalcogenide glass films. Mater. Chem. Phys. 2009, 113, 868–872. [Google Scholar] [CrossRef]
- Sangeetha, B.G.; Joseph, C.M.; Suresh, K. Preparation and characterization of Ge1Sb2Te4 thin films for phase change memory applications. Microelectron. Eng. 2014, 127, 77–80. [Google Scholar] [CrossRef]
- Salomé, P.M.P.; Rodriguez-Alvarez, H.; Sadewasser, S. Incorporation of alkali metals in chalcogenide solar cells. Sol. Energy Mater. Sol. Cells 2015, 143, 9–20. [Google Scholar] [CrossRef]
- Merkel, J.J.; Sontheimer, T.; Rech, B.; Becker, C. Directional growth and crystallization of silicon thin films prepared by electron-beam evaporation on oblique and textured surfaces. J. Cryst. Growth 2013, 367, 126–130. [Google Scholar] [CrossRef]
- Yang, B.; Duan, H.; Zhou, C.; Gao, Y.; Yang, J. Ordered nanocolumn-array organic semiconductor thin films with controllable molecular orientation. Appl. Surf. Sci. 2013, 286, 104–108. [Google Scholar] [CrossRef]
- Schulz, U.; Terry, S.G.; Levi, C.G. Microstructure and texture of EB-PVD TBCs grown under different rotation modes. Mater. Sci. Eng. A 2003, 360, 319–329. [Google Scholar] [CrossRef]
- Ashfold, M.N.R.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2004, 33, 23–31. [Google Scholar] [CrossRef]
- Lynds, L.; Weinberger, B.R.; Potrepka, D.M.; Peterson, G.G.; Lindsay, M.P. High temperature superconducting thin films: The physics of pulsed laser ablation. Phys. C Supercond. 1989, 159, 61–69. [Google Scholar] [CrossRef]
- Angusmacleod, H. Recent developments in deposition techniques for optical thin films and coatings. In Optical Thin Films and Coatings; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–25. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Morosanu, C.; Dumitru, V.; Cimpoiasu, E.; Nenu, C. Comparison Between DC and RF Magnetron Sputtered Aluminum Nitride Films. In Diamond Based Composites; Prelas, M.A., Benedictus, A., Lin, L.-T.S., Po-povici, G., Gielisse, P., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 127–132. [Google Scholar] [CrossRef]
- Dumitru, V.; Morosanu, C.; Sandu, V.; Stoica, A. Optical and structural differences between RF and DC AlxNy magnetron sputtered films. Thin Solid Film. 2000, 359, 17–20. [Google Scholar] [CrossRef]
- Mersagh Dezfuli, S.; Sabzi, M. Deposition of self-healing thin films by the sol–gel method: A review of layer-deposition mechanisms and activation of self-healing mechanisms. Appl. Phys. A 2019, 125, 557. [Google Scholar] [CrossRef]
- Nagyal, L.; Gupta, S.S.; Singh, R.; Kumar, A.; Chaudhary, P. Sol-Gel Deposition of Thin Films. In Digital Encyclopedia of Applied Physics; Wiley-VCH Verlag GmbH & Co. KGaA, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Brinker, C.J.; Hurd, A.J.; Schunk, P.R.; Frye, G.C.; Ashley, C.S. Review of sol-gel thin film formation. J. Non-Cryst. Solids 1992, 147–148, 424–436. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C.; Henry, M.; Doeuff, S. The chemistry of the sol-gel process. Solid State Ion. 1989, 32–33, 633–638. [Google Scholar] [CrossRef]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Chen, N.; Kim, D.H.; Kovacik, P.; Sojoudi, H.; Wang, M.; Gleason, K.K. Polymer Thin Films and Surface Modification by Chemical Vapor Deposition: Recent Progress. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 373–393. [Google Scholar] [CrossRef]
- Danglad-Flores, J.; Eickelmann, S.; Riegler, H. Deposition of polymer films by spin casting: A quantitative analysis. Chem. Eng. Sci. 2018, 179, 257–264. [Google Scholar] [CrossRef]
- Taylor, J.F. Spin coating: An overview. Met. Finish. 2001, 99, 16–21. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; McBride, S.A.; Warsinger, D.M.; Dizge, N.; Hasan, S.W.; Arafat, H.A. Thin film deposition techniques for polymeric membranes–A review. J. Membr. Sci. 2020, 610, 118258. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol. Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Setiawan, M.; Palaniappan, A.; Yam, G.H.F.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Functionalization of the Polymeric Surface with Bioceramic Nanoparticles via a Novel, Nonthermal Dip Coating Method. ACS Appl. Mater. Interfaces 2016, 8, 35565–35577. [Google Scholar] [CrossRef] [PubMed]
- Guire, M.R.D.; Bauermann, L.P.; Parikh, H.; Bill, J. Chemical Bath Deposition. In Chemical Solution Deposition of Functional Ox-ide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer: Vienna, Austria, 2013; pp. 319–339. [Google Scholar] [CrossRef]
- Contreras-Rascón, J.I.; Díaz-Reyes, J.; Luna-Suárez, S.; Carrillo-Torres, R.C.; Sánchez-Zeferino, R. Characterisation of chemical bath deposition PbS nanofilms using polyethylene-imine, triethanolamine and ammonium nitrate as complexing agents. Thin Solid Film. 2019, 692, 137609. [Google Scholar] [CrossRef]
- Mooney, J.B.; Radding, S.B. Spray Pyrolysis Processing. Annu. Rev. Mater. Sci. 1982, 12, 81–101. [Google Scholar] [CrossRef]
- Falcony, C.; Aguilar-Frutis, M.; García-Hipólito, M. Spray Pyrolysis Technique; High-K Die-lectric Films and Luminescent Materials: A Review. Micromachines 2018, 9, 414. [Google Scholar] [CrossRef]
- Baer, D.R.; Thevuthasan, S. Characterization of Thin Films and Coatings. In Handbook of Deposition Technologies for Films and Coatings; Elsevier: Amsterdam, The Netherlands, 2010; pp. 749–864. [Google Scholar] [CrossRef]
- Pourjamal, S.; Mäntynen, H.; Jaanson, P.; Rosu, D.M.; Hertwig, A.; Manoocheri, F.; Ikonen, E. Characterization of thin-film thickness. Metrologia 2014, 51, S302–S308. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, G.; Narita, A.; Orosco, M.; Feng, X.; Müllen, K.; Zhou, C. Deposition, Characterization, and Thin-Film-Based Chemical Sensing of Ultra-long Chemically Synthesized Graphene Nanoribbons. J. Am. Chem. Soc. 2014, 136, 7555–7558. [Google Scholar] [CrossRef]
- Reif, B.; Ashbrook, S.E.; Emsley, L.; Hong, M. Solid-state NMR spectroscopy. Nat. Rev. Methods Primers 2021, 1, 2. [Google Scholar] [CrossRef]
- Laws, D.D.; Bitter, H.-M.L.; Jerschow, A. Solid-state NMR spectroscopic methods in chemistry. Angew. Chem. 2002, 34, 3096–3129. [Google Scholar] [CrossRef]
- De Almeida, N.E.; Paul, D.K.; Karan, K.; Goward, G.R. 1H Solid-State NMR Study of Nanothin Nafion Films. J. Phys. Chem. C 2015, 119, 1280–1285. [Google Scholar] [CrossRef]
- Pfeffermann, M.; Dong, R.; Graf, R.; Zajaczkowski, W.; Gorelik, T.; Pisula, W.; Narita, A.; Müllen, K.; Feng, X. Free-Standing Monolayer Two-Dimensional Supramolecular Organic Framework with Good Internal Order. J. Am. Chem. Soc. 2015, 137, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Blum, F.D. NMR Studies of Organic Thin films. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1994; pp. 277–321. [Google Scholar] [CrossRef]
- Jagadeesh, B.; Demco, D.E.; Blümich, B. Surface induced order and dynamic heterogeneity in ultra-thin polymer films: A 1H multiple-quantum NMR study. Chem. Phys. Lett. 2004, 393, 416–420. [Google Scholar] [CrossRef]
- Wu, X.; Yang, S.; Samuelson, L.A.; Cholli, A.L.; Kumar, J. Conformation of Azobenzene-Modified Poly(α-L-Glutamate) (AZOPLGA) in Thin Films: Solid State NMR Studies. J. Macromol. Sci. Part A 2004, 41, 1359–1368. [Google Scholar] [CrossRef]
- Alam, T.M.; Friedmann, T.A.; Schultz, P.A.; Sebastiani, D. Low temperature annealing in tetrahedral amorphous carbon thin films observed by 13C NMR spectroscopy. Phys. Rev. B 2003, 67, 245309. [Google Scholar] [CrossRef]
- Dalvi, L.C.; Laine, C.; Virtanen, T.; Liitiä, T.; Tenhunen, T.-M.; Orelma, H.; Tammelin, T.; Tamminen, T. Study of xylan and cellulose interactions monitored with solid-state NMR and QCM-D. Holzforschung 2020, 74, 643–653. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Chen, C.-Y.; Chen, W.-C. Synthesis and characterization of organic–inorganic hybrid thin films from poly(acrylic) and monodispersed colloidal silica. Polymer 2003, 44, 593–601. [Google Scholar] [CrossRef]
- Rivillon, S.; Auroy, P.; Deloche, B. Chain Segment Order in Polymer Thin Films on a Non-adsorbing Surface: A NMR Study. Phys. Rev. Lett. 2000, 84, 499–502. [Google Scholar] [CrossRef]
- Nieuwendaal, R.C.; DeLongchamp, D.M.; Richter, L.J.; Snyder, C.R.; Jones, R.L.; Engmann, S.; Herzing, A.; Heeney, M.; Fei, Z.; Sieval, A.B.; et al. Characterization of Interfacial Structure in Polymer-Fullerene Bulk Heterojunctions via 13C {2H} Rotational Echo Double Resonance NMR. Phys. Rev. Lett. 2018, 121, 026101. [Google Scholar] [CrossRef]
- Marple, M.A.T.; Wynn, T.A.; Cheng, D.; Shimizu, R.; Mason, H.E.; Meng, Y.S. Local Structure of Glassy Lithium Phosphorus Oxynitride Thin Films: A Combined Experimental and Ab Initio Approach. Angew. Chem. 2020, 59, 22185–22193. [Google Scholar] [CrossRef] [PubMed]
- Stallworth, P.E.; Vereda, F.; Greenbaum, S.G.; Haas, T.E.; Zerigian, P.; Goldner, R.B. Solid-State NMR Studies of Lithium Phosphorus Oxynitride Films Prepared by Nitrogen Ion Beam-Assisted Deposition. J. Electrochem. Soc. 2005, 152, A516. [Google Scholar] [CrossRef]
- MacFarlane, W.A.; Morris, G.D.; Beals, T.R.; Chow, K.H.; Baartman, R.A.; Daviel, S.; Dunsiger, S.R.; Hatakeyama, A.; Kreitzman, S.R.; Levy, C.D.P.; et al. 8Li β-NMR in thin metal films. Physica B 2003, 326, 213–216. [Google Scholar] [CrossRef]
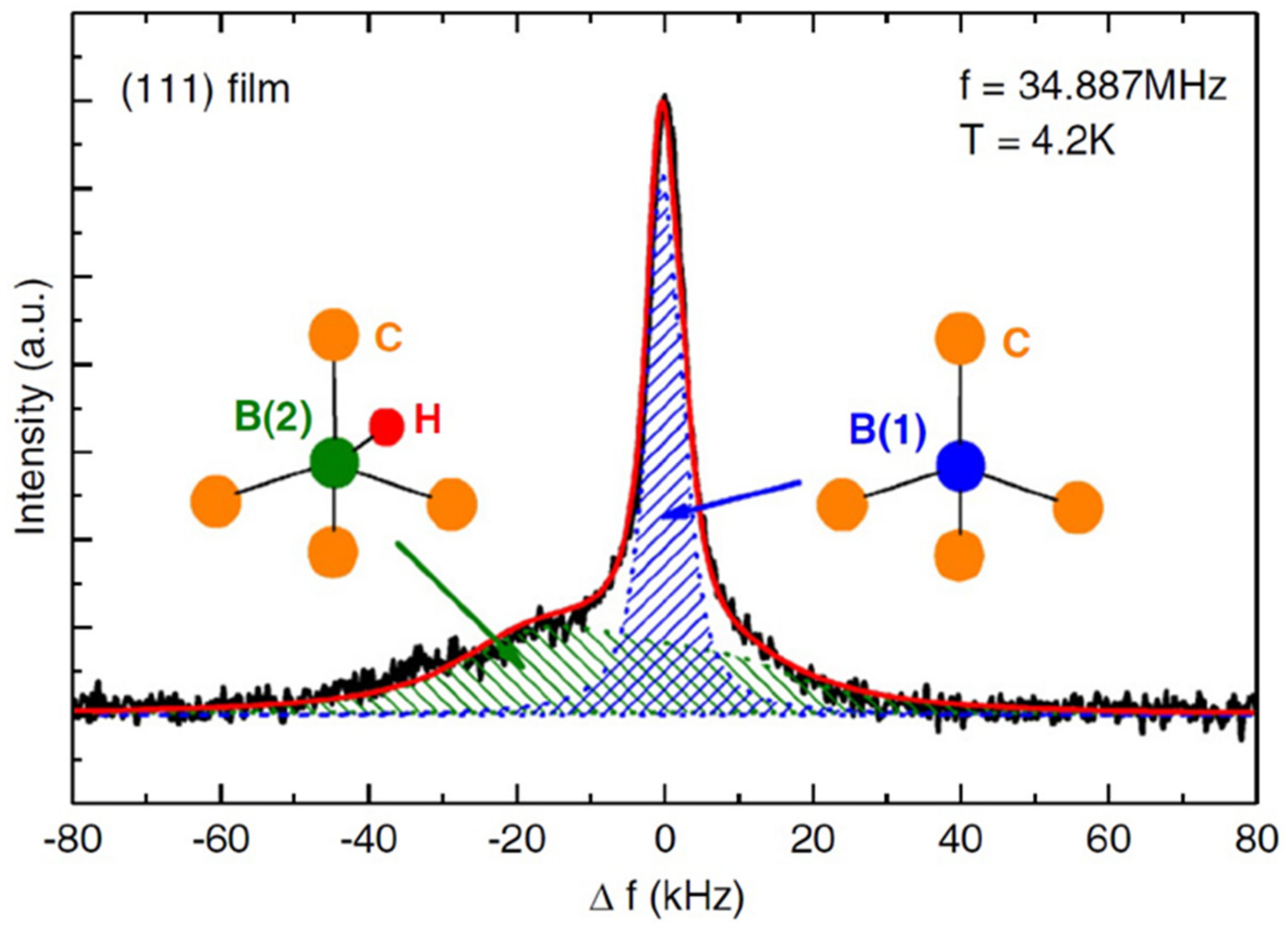
- Mukuda, H.; Tsuchida, T.; Harada, A.; Kitaoka, Y.; Takenouchi, T.; Takano, Y.; Nagao, M.; Sakaguchi, I.; Kawarada, H. 11B-NMR study in boron-doped diamond films. Sci. Technol. Adv. Mater. 2006, 7, S37–S40. [Google Scholar] [CrossRef]
- Yesinowski, J.P. 69,71Ga and 14N high-field NMR of gallium nitride films: 69,71Ga and 14N high-field NMR of gallium nitride films. Phys. Stat. Sol. 2005, 2, 2399–2402. [Google Scholar] [CrossRef]
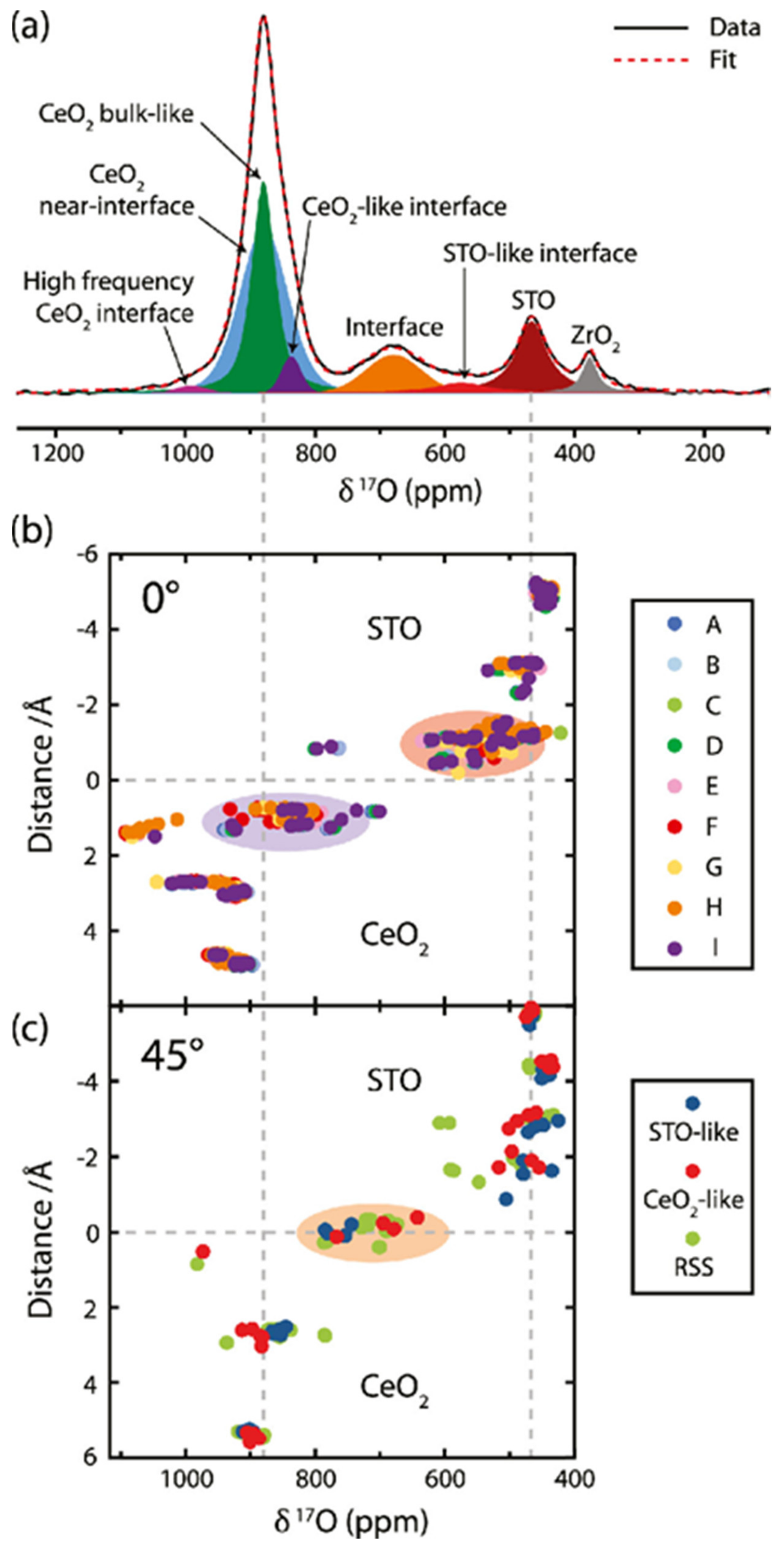
- Hope, M.A.; Zhang, B.; Zhu, B.; Halat, D.M.; MacManus-Driscoll, J.L.; Grey, C.P. Revealing the Structure and Oxygen Transport at Interfaces in Complex Oxide Heterostructures via 17O NMR Spectroscopy. Chem. Mater. 2020, 32, 7921–7931. [Google Scholar] [CrossRef]
- Won, S.; Saun, S.-B.; Lee, S.; Lee, S.; Kim, K.; Han, Y. NMR Spectroscopy for Thin Films by Magnetic Resonance Force Microscopy. Sci. Rep. 2013, 3, 3189. [Google Scholar] [CrossRef] [PubMed]
- Aimi, K.; Ando, S. Solid-state 19F MAS and 1H→19F CP/MAS NMR study of the phase-transition behavior of vinylidene fluoride–trifluoroethylene copolymers: 2. semi-crystalline films of VDF 75% copolymer. Polym. J. 2012, 44, 786–794. [Google Scholar] [CrossRef][Green Version]
- Koseki, Y.; Aimi, K.; Ando, S. Crystalline structure and molecular mobility of PVDF chains in PVDF/PMMA blend films analyzed by solid-state 19F MAS NMR spectroscopy. Polym. J. 2012, 44, 757–763. [Google Scholar] [CrossRef]
- Cui, J.; Kast, M.G.; Hammann, B.A.; Afriyie, Y.; Woods, K.N.; Plassmeyer, P.N.; Perkins, C.K.; Ma, Z.L.; Keszler, D.A.; Page, C.J.; et al. Aluminum Oxide Thin Films from Aqueous Solutions: Insights from Solid-State NMR and Dielectric Response. Chem. Mater. 2018, 30, 7456–7463. [Google Scholar] [CrossRef]
- Mazaj, M.; Costacurta, S.; Zabukovec Logar, N.; Mali, G.; Novak Tušar, N.; Innocenzi, P.; Malfatti, L.; Thibault-Starzyk, F.; Amenitsch, H.; Kaučič, V.; et al. Mesoporous Aluminophosphate Thin Films with Cubic Pore Arrangement. Langmuir 2008, 24, 6220–6225. [Google Scholar] [CrossRef] [PubMed]
- Sarou-Kanian, V.; Gleizes, A.N.; Florian, P.; Samélor, D.; Massiot, D.; Vahlas, C. Temperature-Dependent 4-, 5- and 6-Fold Coordination of Aluminum in MOCVD-Grown Amorphous Alumina Films: A Very High Field 27 Al-NMR study. J. Phys. Chem. C 2013, 117, 21965–21971. [Google Scholar] [CrossRef]
- Kim, N.; Bassiri, R.; Fejer, M.M.; Stebbins, J.F. The structure of ion beam sputtered amorphous alumina films and effects of Zn doping: High-resolution 27Al NMR. J. Non-Cryst. Solids 2014, 405, 1–6. [Google Scholar] [CrossRef]
- Landskron, K. Periodic Mesoporous Organosilicas Containing Interconnected [Si(CH2)]3 Rings. Science 2003, 302, 266–269. [Google Scholar] [CrossRef]
- Hatton, B.D.; Landskron, K.; Whitnall, W.; Perovic, D.D.; Ozin, G.A. Spin-Coated Periodic Mesoporous Organosilica Thin Films? Towards a New Generation of Low-Dielectric-Constant Materials. Adv. Funct. Mater. 2005, 15, 823–829. [Google Scholar] [CrossRef]
- Pallister, P.J.; Barry, S.T. Surface chemistry of group 11 atomic layer deposition precursors on silica using solid-state nuclear magnetic resonance spectroscopy. J. Chem. Phys. 2017, 146, 052812. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.A.; Tavares, M.I.B.; Neto, R.C.P.; Ferreira, A.G. The Use of Solid-State NMR to Evaluate EVA/Silica FILMs. J. Nano R. 2012, 18–19, 219–226. [Google Scholar] [CrossRef]
- Järvinen, J.; Ahokas, J.; Sheludyakov, S.; Vainio, O.; Lehtonen, L.; Vasiliev, S.; Zvezdov, D.; Fujii, Y.; Mitsudo, S.; Mizusaki, T.; et al. Efficient dynamic nu-clear polarization of phosphorus in silicon in strong magnetic fields and at low temperatures. Phys. Rev. B 2014, 90, 214401. [Google Scholar] [CrossRef]
- Stuart, B.W.; Grant, C.A.; Stan, G.E.; Popa, A.C.; Titman, J.J.; Grant, D.M. Gallium incorporation into phosphate-based glasses: Bulk and thin film properties. J. Mech. Behav. Biomed. Mater. 2018, 82, 371–382. [Google Scholar] [CrossRef]
- Riedi, P.C.; Thomson, T.; Tomka, G.J. Chapter 2 NMR of thin magnetic films and superlattices. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 1999; pp. 97–258. [Google Scholar] [CrossRef]
- Bibes, M.; Balcells, L.; Valencia, S.; Fontcuberta, J.; Wojcik, M.; Jedryka, E.; Nadolski, S. Nanoscale Multiphase Separation at La2/3Ca1/3MnO3/SrTiO3 Interfaces. Phys. Rev. Lett. 2001, 87, 067210. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.L.; Veillet, P.; Sakakima, H.; Krishnan, R. NMR studies of compositionally modulated Co/Mn thin films. J. Phys. F Met. Phys. 1986, 16, 93–97. [Google Scholar] [CrossRef]
- Wieldraaijer, H.; de Jonge, W.J.M.; Kohlhepp, J.T. 59Co NMR observation of monolayer re-solved hyperfine fields in ultrathin epitaxial FCT-Co(001) films. J. Magn. Magn. Mater. 2005, 286, 390–393. [Google Scholar] [CrossRef]
- Wojcik, M.; Jay, J.P.; Panissod, P.; Jedryka, E.; Dekoster, J.; Langouche, G. New phases and chemical short range order in co-deposited CoFe thin films with bcc structure: An NMR study. Z. Phys. B Condens. Matter. 1997, 103, 5–12. [Google Scholar] [CrossRef]
- Thomson, T.; Riedi, P.C.; Platt, C.L.; Berkowitz, A.E. NMR studies of sputtered CoFe alloy thin films. IEEE Trans. Magn 1998, 34, 1045–1047. [Google Scholar] [CrossRef]
- Hammann, B.A.; Ma, Z.L.; Wentz, K.M.; Kamunde-Devonish, M.K.; Johnson, D.W.; Hayes, S.E. Structural study by solid-state 71 Ga NMR of thin film transistor precursors. Dalton Trans. 2015, 44, 17652–17659. [Google Scholar] [CrossRef]
- Degen, C.; Tomaselli, M.; Meier, B.H.; Voncken, M.M.A.J.; Kentgens, A.P.M. NMR investigation of atomic ordering in AlxGa1−x as thin films. Phys. Rev. B 2004, 69, 193303. [Google Scholar] [CrossRef]
- Chandran, C.V.; Schreuders, H.; Dam, B.; Janssen, J.W.G.; Bart, J.; Kentgens, A.P.M.; van Bentum, P.J.M. Solid-State NMR Studies of the Photochromic Effects of Thin Films of Oxygen-Containing Yttrium Hydride. J. Phys. Chem. C 2014, 118, 22935–22942. [Google Scholar] [CrossRef]
- Raftery, D.; Long, H.; Reven, L.; Tang, P.; Pines, A. NMR of optically pumped xenon thin films. Chem. Phys. Lett. 1992, 191, 385–390. [Google Scholar] [CrossRef]
- Nossov, A.; Haddad, E.; Guenneau, F.; Mignon, C.; Gédéon, A.; Grosso, D.; Babonneau, F.; Bonhomme, C.; Sanchez, C. The first direct probing of porosity on supported mesoporous silica thin films through hyperpolarised 129Xe NMR. Chem. Commun. 2002, 2476–2477. [Google Scholar] [CrossRef]
- Hanrahan, M.P.; Men, L.; Rosales, B.A.; Vela, J.; Rossini, A.J. Sensitivity-Enhanced 207Pb Solid-State NMR Spectroscopy for the Rapid, Non-Destructive Characterization of Organolead Halide Perovskites. Chem. Mater. 2018, 30, 7005–7015. [Google Scholar] [CrossRef]
- Alam, T.M.; Friedmann, T.A.; Jurewicz, A.J.G. Solid State 13C MAS NMR Investigations of Amorphous Carbon Thin Films. In Thin Films: Preparation, Characterization, Applications; Soriaga, M.P., Stickney, J., Bottomley, L.A., Kim, Y.-G., Eds.; Springer: Boston, MA, USA, 2002; pp. 277–289. [Google Scholar] [CrossRef]
- Yuan, K.; Hu, T.; Xu, Y.; Graf, R.; Brunklaus, G.; Forster, M.; Chen, Y.; Scherf, U. Engineering the Morphology of Carbon Materials: 2D Porous Carbon Nanosheets for High-Performance Supercapacitors. ChemElectroChem 2016, 3, 822–828. [Google Scholar] [CrossRef]
- Yuan, K.; Hu, T.; Xu, Y.; Graf, R.; Shi, L.; Forster, M.; Pichler, T.; Riedl, T.; Chen, Y.; Scherf, U. Nitrogen-doped porous carbon/graphene nanosheets derived from two-dimensional conjugated microporous polymer sandwiches with promising capacitive performance. Mater. Chem. Front. 2017, 1, 278–285. [Google Scholar] [CrossRef]
- Merwin, L.H.; Johnson, C.E.; Weimer, W.A. 13C NMR investigation of CVD diamond: Correlation of NMR and Raman spectral linewidths. J. Mater. Res. 1994, 9, 631–635. [Google Scholar] [CrossRef]
- Keeler, E.G.; Michaelis, V.K.; Colvin, M.T.; Hung, I.; Gor’kov, P.L.; Cross, T.A.; Gan, Z.; Griffin, R.G. 17O MAS NMR Correlation Spectroscopy at High Magnetic Fields. J. Am. Chem. Soc. 2017, 139, 17953–17963. [Google Scholar] [CrossRef]
- Ashbrook, S.E.; Smith, M.E. Solid state 17O NMR—an introduction to the background principles and applications to inorganic materials. Chem. Soc. Rev. 2006, 35, 718–735. [Google Scholar] [CrossRef]
- Grover, R.; Srivastava, R.; Rana, O.; Mehta, D.S.; Kamalasanan, M.N. New Organic Thin-Film Encapsulation for Organic Light Emitting Diodes. JEAS 2011, 1, 23–28. [Google Scholar] [CrossRef][Green Version]
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Chandar Shekar, B.; Lee, J.; Rhee, S.-W. Organic thin film transistors: Materials, processes and devices. Korean J. Chem. Eng. 2004, 21, 267–285. [Google Scholar] [CrossRef]
- Suzuki, K.; Kubo, S.; Aussenac, F.; Engelke, F.; Fukushima, T.; Kaji, H. Analysis of Molecular Orientation in Organic Semiconducting Thin Films Using Static Dynamic Nuclear Polarization Enhanced Solid-State NMR Spectroscopy. Angew. Chem. 2017, 56, 14842–14846. [Google Scholar] [CrossRef]
- Chaudhari, R.; Griffin, J.M.; Broch, K.; Lesage, A.; Lemaur, V.; Dudenko, D.; Olivier, Y.; Sirringhaus, H.; Emsley, L.; Grey, C.P. Donor–acceptor stacking arrangements in bulk and thin-film high-mobility conjugated polymers characterized using molecular modelling and MAS and surface-enhanced solid-state NMR spectroscopy. Chem. Sci. 2017, 8, 3126–3136. [Google Scholar] [CrossRef]
- Park, S.-H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Chisca, S.; Marchesi, T.; Falca, G.; Musteata, V.-E.; Huang, T.; Abou-Hamad, E.; Nunes, S.P. Organic solvent and thermal resistant polytriazole membranes with enhanced mechanical properties cast from solutions in non-toxic solvents. J. Membr. Sci. 2020, 597, 117634. [Google Scholar] [CrossRef]
- Begni, F.; Paul, G.; Lasseuguette, E.; Mangano, E.; Bisio, C.; Ferrari, M.-C.; Gatti, G. Synthetic Saponite Clays as Additives for Reducing Aging Effects in PIM1 Membranes. ACS Appl. Polym. Mater. 2020, 2, 3481. [Google Scholar] [CrossRef]
- Ren, D.; Jin, Y.-T.; Liu, T.-Y.; Wang, X. Phenanthroline-Based Polyarylate Porous Membranes with Rapid Water Transport for Metal Cation Separation. ACS Appl. Mater. Interfaces 2020, 12, 7605–7616. [Google Scholar] [CrossRef] [PubMed]
- Andrew, E.R.; Bradbury, A.; Eades, R.G. Nuclear Magnetic Resonance Spectra from a Crystal rotated at High Speed. Nature 1958, 182, 1659. [Google Scholar] [CrossRef]
- Andrew, E.R.; Bradbury, A.; Eades, R.G. Removal of Dipolar Broadening of Nuclear Magnetic resonance Spectra of Solids by Specimen Rotation. Nature 1959, 183, 1802–1803. [Google Scholar] [CrossRef]
- Alam, T.M.; Fan, H. Investigation of Templated Mesoporous Silicate Thin Films Using High Speed, Solid-State 1H MAS and Double Quantum NMR Spectroscopy. Macromol. Chem. Phys. 2003, 204, 2023–2030. [Google Scholar] [CrossRef]
- Steinbeck, C.A.; Ernst, M.; Meier, B.H.; Chmelka, B.F. Anisotropic Optical Properties and Structures of Block Copolymer/Silica Thin Films Containing Aligned Porphyrin J–Aggregates. J. Phys. Chem. C 2008, 112, 2565–2573. [Google Scholar] [CrossRef]
- Lu, D.; Baek, D.J.; Hong, S.S.; Kourkoutis, L.F.; Hikita, Y.; Hwang, H.Y. Synthesis of freestanding single-crystal perovskite films and heterostructures by etching of sacrificial water-soluble layers. Nat. Mater. 2016, 15, 1255–1260. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward Clean and Crackless Transfer of Graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef]
- VanderHart, L.; Prabhu, V.M.; Lavery, K.A.; Dennis, C.L.; Rao, A.B.; Lin, E.K. Thin-film solid-state proton NMR measurements using a synthetic mica substrate: Polymer blends. J. Magn. Reson. 2009, 201, 100–110. [Google Scholar] [CrossRef]
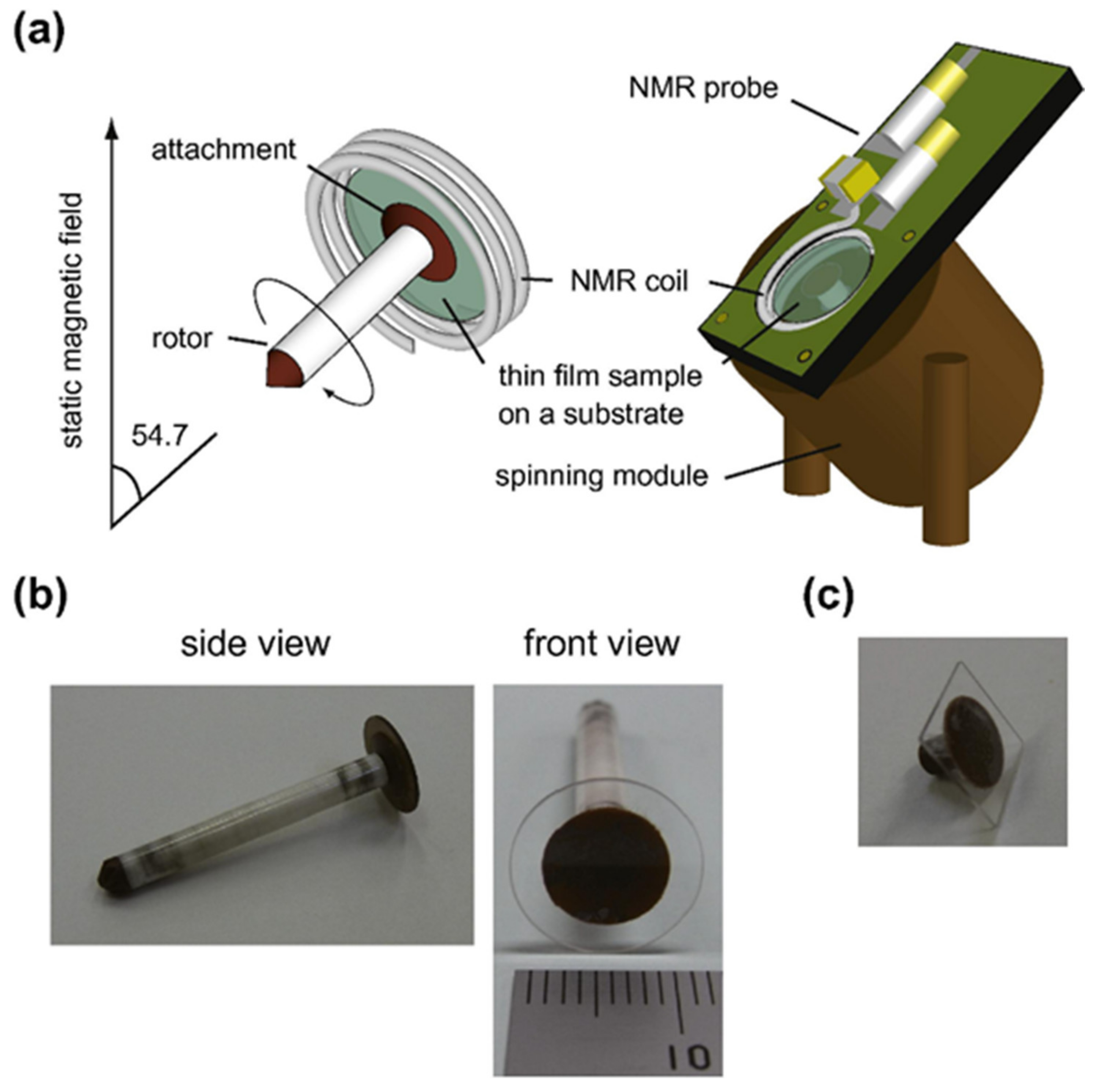
- Inukai, M.; Noda, Y.; Takeda, K. Nondestructive high-resolution solid-state NMR of rotating thin films at the magic-angle. J. Magn. Reson. 2011, 213, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Inukai, M.; Takeda, K. Studies on multiple-quantum magic-angle-spinning NMR of half-integer quadrupolar nuclei under strong rf pulses with a microcoil. Concepts Magn. Reson. B 2008, 33, 115–123. [Google Scholar] [CrossRef]
- Janssen, H.; Brinkmann, A.; Kentgens, A.P.M. Microcoil High-Resolution Magic Angle Spinning NMR Spectroscopy. J. Am. Chem. Soc. 2006, 128, 8722–8723. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Asakura, T. Development of MicroMAS NMR Probehead for Mass-limited Solid-state Samples. Chem. Lett. 2006, 35, 426–427. [Google Scholar] [CrossRef]
- Wensink, H.; Benito-Lopez, F.; Hermes, D.C.; Verboom, W.; Gardeniers, H.J.G.E.; Reinhoudt, D.N.; van den Berg, A. Measuring reaction kinetics in a lab on a chip by microcoil NMR. Lab. Chip 2005, 5, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Massin, C.; Boero, G.; Vincent, F.; Abenhaim, J.; Besse, P.-A.; Popovic, R.S. High-Q factor RF planar microcoils for micro-scale NMR spectroscopy. Sens. Actuators A Phys. 2002, 97–98, 280–288. [Google Scholar] [CrossRef]
- Dechow, J.; Forchel, A.; Lanz, T.; Haase, A. Fabrication of NMR—Microsensors for nano-liter sample volumes. Microelectron. Eng. 2000, 53, 517–519. [Google Scholar] [CrossRef]
- Van Bentum, P.J.M.; Janssen, J.W.G.; Kentgens, A.P.M. Towards nuclear magnetic resonance μ-spectroscopy and μ-imaging. Analyst 2004, 129, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Maguire, Y.; Chuang, I.L.; Zhang, S.; Gershenfeld, N. Ultra-small-sample molecular structure detection using microslot waveguide nuclear spin resonance. Proc. Natl. Acad. Sci. USA 2007, 104, 9198–9203. [Google Scholar] [CrossRef]
- Van Bentum, P.J.M.; Janssen, J.W.G.; Kentgens, A.P.M.; Bart, J.; Gardeniers, J.G.E. Stripline probes for nuclear magnetic resonance. J. Magn. Reson. 2007, 189, 104–113. [Google Scholar] [CrossRef]
- Bart, J.; Janssen, J.W.G.; van Bentum, P.J.M.; Kentgens, A.P.M.; Gardeniers, J.G.E. Optimization of stripline-based microfluidic chips for high-resolution NMR. J. Magn. Reson. 2009, 201, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Oosthoek de Vries, A.J.; Bart, J.; Tiggelaar, R.M.; Janssen, J.W.G.; van Bentum, P.J.M.; Gardeniers, H.J.G.E.; Kentgens, A.P.M. Continuous Flow 1H and 13C NMR Spectroscopy in Micro-fluidic Stripline NMR Chips. Anal. Chem. 2017, 89, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Sidles, J.A. Noninductive detection of single-proton magnetic resonance. Appl. Phys. Lett. 1991, 58, 2854–2856. [Google Scholar] [CrossRef]
- Sidles, J.A.; Garbini, J.L.; Bruland, K.J.; Rugar, D.; Züger, O.; Hoen, S.; Yannoni, C.S. Magnetic resonance force microscopy. Rev. Mod. Phys. 1995, 67, 249–265. [Google Scholar] [CrossRef]
- Rugar, D.; Budakian, R.; Mamin, H.J.; Chui, B.W. Single spin detection by magnetic resonance force microscopy. Nature 2004, 430, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Rugar, D.; Yannoni, C.S.; Sidles, J.A. Mechanical detection of magnetic resonance. Nature 1992, 360, 563. [Google Scholar] [CrossRef]
- Rugar, D.; Zuger, O.; Hoen, S.; Yannoni, C.S.; Vieth, H.-M.; Kendrick, R.D. Force Detection of Nuclear Magnetic Resonance. Science 1994, 264, 1560–1563. [Google Scholar] [CrossRef]
- Mamin, H.J.; Oosterkamp, T.H.; Poggio, M.; Degen, C.L.; Rettner, C.T.; Rugar, D. Isotope-Selective Detection and Imaging of Organic Nanolayers. Nano Lett. 2009, 9, 3020–3024. [Google Scholar] [CrossRef]
- Leskowitz, G.M.; Madsen, L.A.; Weitekamp, D.P. Force-detected magnetic resonance without field gradients. Solid State Nucl. Magn. Reson. 1998, 11, 73–86. [Google Scholar] [CrossRef]
- Degen, C.L.; Poggio, M.; Mamin, H.J.; Rettner, C.T.; Rugar, D. Nanoscale magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2009, 106, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, R.; Wittlin, A.; Hilbers, C.W.; van Kempen, H.; Kentgens, A.P.M. Spatially Resolved Spectroscopy and Structurally Encoded Imaging by Magnetic Resonance Force Microscopy of Quadrupolar Spin Systems. J. Am. Chem. Soc. 2002, 124, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Nestle, N.; Schaff, A.; Veeman, W.S. Mechanically detected NMR, an evaluation of the applicability for chemical investigations. Prog. Nucl. Magn. Reson. Spectrosc. 2001, 38, 1–35. [Google Scholar] [CrossRef]
- Jancso, A.; Correia, J.G.; Gottberg, A.; Schell, J.; Stachura, M.; Szunyogh, D.; Pallada, S.; Lupascu, D.C.; Kowalska, M.; Hemmingsen, L. TDPAC and β-NMR applications in chemistry and biochemistry. J. Phys. G Nucl. Part. Phys. 2017, 44, 064003. [Google Scholar] [CrossRef]
- Neugart, R.; Balabanski, D.L.; Blaum, K.; Borremans, D.; Himpe, P.; Kowalska, M.; Lievens, P.; Mallion, S.; Neyens, G.; Vermeulen, N.; et al. Precision Measurement of 11Li Moments: Influence of Halo Neutrons on the 9Li Core. Phys. Rev. Lett 2008, 101, 132502. [Google Scholar] [CrossRef]
- Keim, M.; Georg, U.; Klein, A.; Neugart, R.; Neuroth, M.; Wilbert, S.; Lievens, P.; Vermeeren, L.; Brown, B.A. ISOLDE Collaboration. Measurement of the electric quadrupole moments of 26–29Na. Eur. Phys. J. A 2000, 8, 31–40. [Google Scholar] [CrossRef]
- McKenzie, I.; Chai, Y.; Cortie, D.L.; Forrest, J.A.; Fujimoto, D.; Karner, V.L.; Kiefl, R.F.; Levy, C.D.P.; MacFarlane, W.A.; McFadden, R.M.L.; et al. Direct measurements of the temperature, depth and processing dependence of phenyl ring dynamics in polystyrene thin films by β-detected NMR. Soft Matter 2018, 14, 7324–7334. [Google Scholar] [CrossRef]
- Brewer, W.D. Recent developments in radiative detection of nuclear magnetic resonance. Hyperfine Interact 1982, 12, 173–210. [Google Scholar] [CrossRef]
- Winnefeld, H.; Czanta, M.; Fahsold, G.; Jänsch, H.J.; Kirchner, G.; Mannstadt, W.; Paggel, J.J.; Platzer, R.; Schillinger, R.; Veith, R.; et al. Electron localization in (7 × 7) re-constructed and hydrogen-covered Si(111) surfaces as seen by NMR on adsorbed Li. Phys. Rev. B 2002, 65, 195319. [Google Scholar] [CrossRef]
- Koumoulis, D.; Morris, G.D.; He, L.; Kou, X.; King, D.; Wang, D.; Hossain, M.D.; Wang, K.L.; Fiete, G.A.; Kanatzidis, M.G.; et al. Nanoscale β-nuclear magnetic resonance depth imaging of topological insulators. Proc. Natl. Acad. Sci. USA 2015, 112, E3645–E3650. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.I.; Morris, G.D.; Salman, Z.; Chow, K.H.; Dunlop, T.; Jung, J.; Fan, I.; MacFarlane, W.A.; Kiefl, R.F.; Parolin, T.J.; et al. Development of the 8Li cross-relaxation technique: Applications in semiconductors and other condensed matter systems. Physica B Condensed Matter 2007, 401–402, 662–665. [Google Scholar] [CrossRef]
- Chow, K.H.; Salman, Z.; Kiefl, R.F.; MacFarlane, W.A.; Levy, C.D.P.; Amaudruz, P.; Baartman, R.; Chakhalian, J.; Daviel, S.; Hirayama, Y.; et al. The new β-NMR facility at TRI-UMF and applications in semiconductors. Physica B Condensed Matter 2003, 340–342, 1151–1154. [Google Scholar] [CrossRef]
- Cortie, D.L.; Buck, T.; Dehn, M.H.; Karner, V.L.; Kiefl, R.F.; Levy, C.D.P.; McFadden, R.M.L.; Morris, G.D.; McKenzie, I.; Pearson, M.R.; et al. β-NMR Investigation of the Depth-Dependent Magnetic Properties of an Antiferromagnetic Surface. Phys. Rev. Lett. 2016, 116, 106103. [Google Scholar] [CrossRef]
- Morris, G.D.; MacFarlane, W.A.; Chow, K.H.; Salman, Z.; Arseneau, D.J.; Daviel, S.; Hatakeyama, A.; Kreitzman, S.R.; Levy, C.D.P.; Poutissou, R.; et al. Depth-Controlled β-NMR of 8Li in a Thin Silver Film. Phys. Rev. Lett. 2004, 93, 157601. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.; Daley, C.R.; Kiefl, R.F.; Levy, C.D.P.; MacFarlane, W.A.; Morris, G.D.; Pearson, M.R.; Wang, D.; Forrest, J.A. Enhanced high-frequency molecular dynamics in the near-surface region of polystyrene thin films observed with β-NMR. Soft Matter 2015, 11, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.; Harada, M.; Kiefl, R.F.; Levy, C.D.P.; MacFarlane, W.A.; Morris, G.D.; Ogata, S.-I.; Pearson, M.R.; Sugiyama, J. β-NMR Measurements of Lithium Ion Transport in Thin Films of Pure and Lithium-Salt-Doped Poly(ethylene oxide). J. Am. Chem. Soc. 2014, 136, 7833–7836. [Google Scholar] [CrossRef]
- Rossini, A.J.; Zagdoun, A.; Lelli, M.; Lesage, A.; Copéret, C.; Emsley, L. Dynamic Nuclear Polarization Surface Enhanced NMR Spectroscopy. Acc. Chem. Res. 2013, 46, 1942–1951. [Google Scholar] [CrossRef]
- Elena, B.; Lesage, A.; Steuernagel, S.; Böckmann, A.; Emsley, L. Proton to Carbon-13 INEPT in Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2005, 127, 17296–17302. [Google Scholar] [CrossRef]
- Agarwal, V.; Reif, B. Residual methyl protonation in perdeuterated proteins for multi-dimensional correlation experiments in MAS solid-state NMR spectroscopy. J. Magn. Reson. 2008, 194, 16–24. [Google Scholar] [CrossRef]
- Baldus, M.; Meier, B.H. Total Correlation Spectroscopy in the Solid State. The Use of Scalar Couplings to Determine the Through-Bond Connectivity. J. Magn. Reson. Ser. A 1996, 121, 65–69. [Google Scholar] [CrossRef]
- Phyo, P.; Hong, M. Fast MAS 1H–13C correlation NMR for structural investigations of plant cell walls. J. Biomol. NMR 2019, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Mance, D.; Sinnige, T.; Kaplan, M.; Narasimhan, S.; Daniëls, M.; Houben, K.; Baldus, M.; Weingarth, M. An Efficient Labelling Approach to Harness Backbone and Side-Chain Protons in 1H-Detected Solid-State NMR Spectroscopy. Angew. Chem. 2015, 54, 15799–15803. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; Sinnige, T.; Schellenberger, P.; de Keyzer, J.; Siebert, C.A.; Driessen, A.J.M.; Baldus, M.; Grünewald, K. Combined 1H-Detected Solid-State NMR Spectroscopy and Electron Cryotomography to Study Membrane Proteins across Resolutions in Native Environments. Structure 2018, 26, 161–170.e3. [Google Scholar] [CrossRef]
- Roos, M.; Mandala, V.S.; Hong, M. Determination of Long-Range Distances by Fast Magic-Angle-Spinning Radiofrequency-Driven 19F–19F Dipolar Recoupling NMR. J. Phys. Chem. B 2018, 122, 9302–9313. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, M.; Fritz, M.P.; Quinn, C.M.; Byeon, I.-J.L.; Byeon, C.-H.; Struppe, J.; Maas, W.; Gronenborn, A.M.; Polenova, T. Fast Magic-Angle Spinning 19F NMR Spectroscopy of HIV-1 Capsid Protein Assemblies. Angew. Chem. 2018, 57, 16375–16379. [Google Scholar] [CrossRef]
- Shcherbakov, A.A.; Mandala, V.S.; Hong, M. High-Sensitivity Detection of Nanometer 1H–19F Distances for Protein Structure Determination by 1H-Detected Fast MAS NMR. J. Phys. Chem. B 2019, 123, 4387–4391. [Google Scholar] [CrossRef]
- Fry, R.A.; Tsomaia, N.; Pantano, C.G.; Mueller, K.T. 19F MAS NMR Quantification of Accessible Hydroxyl Sites on Fiberglass Surfaces. J. Am. Chem. Soc. 2003, 125, 2378–2379. [Google Scholar] [CrossRef]
- Venkatesh, A.; Hanrahan, M.P.; Rossini, A.J. Proton detection of MAS solid-state NMR spectra of half-integer quadrupolar nuclei. Solid State Nucl. Magn. Reson. 2017, 84, 171–181. [Google Scholar] [CrossRef]
- Pöppler, A.-C.; Demers, J.-P.; Malon, M.; Singh, A.P.; Roesky, H.W.; Nishiyama, Y.; Lange, A. Ultrafast Magic-Angle Spinning: Benefits for the Acquisition of Ultrawide-Line NMR Spectra of Heavy Spin-1/2 Nuclei. ChemPhysChem 2016, 17, 812–816. [Google Scholar] [CrossRef]
- Iwasa, Y.; Bascunan, J.; Hahn, S.; Voccio, J.; Kim, Y.; Lecrevisse, T.; Song, J.; Kajikawa, K. A High-Resolution 1.3-GHz/54-mm LTS/HTS NMR Magnet. IEEE Trans. Appl. Supercond 2015, 25, 1–5. [Google Scholar] [CrossRef]
- Gan, Z.; Hung, I.; Wang, X.; Paulino, J.; Wu, G.; Litvak, I.M.; Gor’kov, P.L.; Brey, W.W.; Lendi, P.; Schiano, J.L.; et al. NMR spectroscopy up to 35.2 T using a series-connected hybrid magnet. J. Magn. Reson. 2017, 284, 125–136. [Google Scholar] [CrossRef]
- Bryce, D.L. New frontiers for solid-state NMR across the periodic table: A snapshot of mod-ern techniques and instrumentation. Dalton Trans. 2019, 48, 8014–8020. [Google Scholar] [CrossRef]
- Gan, Z.; Gor’kov, P.; Cross, T.A.; Samoson, A.; Massiot, D. Seeking Higher Resolution and Sensitivity for NMR of Quadrupolar Nuclei at Ultrahigh Magnetic Fields. J. Am. Chem. Soc. 2002, 124, 5634–5635. [Google Scholar] [CrossRef]
- Madsen, R.S.K.; Qiao, A.; Sen, J.; Hung, I.; Chen, K.; Gan, Z.; Sen, S.; Yue, Y. Ultrahigh-field 67Zn NMR reveals short-range disorder in zeolitic imidazolate framework glasses. Science 2020, 367, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, S.E.; Sneddon, S. New Methods and Applications in Solid-State NMR Spectroscopy of Quadrupolar Nuclei. J. Am. Chem. Soc. 2014, 136, 15440–15456. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Quinn, C.M.; Struppe, J.; Sergeyev, I.V.; Zhang, C.; Guo, C.; Runge, B.; Theint, T.; Dao, H.H.; Jaroniec, C.P.; et al. Sensitivity boosts by the CPMAS CryoProbe for challenging biological assemblies. J. Magn. Reson. 2020, 311, 106680. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, J.F. Anionic speciation in sodium and potassium silicate glasses near the metasilicate ([Na,K]2SiO3) composition: 29Si, 17O, and 23Na MAS NMR. J. Non-Cryst. Solids X 2020, 6, 100049. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Q.; Xu, J.; Wu, Q.; Wang, C.; Zhao, X.; Meng, X.; Xiao, F.; Deng, F. Direct observation of tin sites and their reversible interconversion in zeolites by solid-state NMR spectroscopy. Commun. Chem. 2018, 1, 22. [Google Scholar] [CrossRef]
- Widdifield, C.M. Applications of Solid-State 43Ca Nuclear Magnetic Resonance: Superconductors, Glasses, Biomaterials, and NMR Crystallography. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 227–363. [Google Scholar] [CrossRef]
- Laurencin, D.; Smith, M.E. Development of 43Ca solid state NMR spectroscopy as a probe of local structure in inorganic and molecular materials. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 68, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Bryce, D.L. Calcium binding environments probed by 43Ca NMR spectroscopy. Dalton Trans. 2010, 39, 8593–8602. [Google Scholar] [CrossRef]
- Leroy, C.; Bryce, D.L. Recent advances in solid-state nuclear magnetic resonance spectroscopy of exotic nuclei. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 160–199. [Google Scholar] [CrossRef] [PubMed]
- Natusch, D.F.S. Carbon-13 intensity problem. Elimination of the Overhauser effect with an added paramagnetic species. J. Am. Chem. Soc. 1971, 93, 2566–2567. [Google Scholar] [CrossRef]
- Gansow, O.A.; Burke, A.R.; Vernon, W.D. Temperature-dependent carbon-13 nuclear magnetic resonance spectra of the h5-cyclopentadienyliron dicarbonyl dimer, an application of a shiftless relaxation reagent. J. Am. Chem. Soc. 1972, 94, 2550–2552. [Google Scholar] [CrossRef]
- Long, H.W.; Tycko, R. Biopolymer Conformational Distributions from Solid-State NMR: α-Helix and 310-Helix Contents of a Helical Peptide. J. Am. Chem. Soc. 1998, 120, 7039–7048. [Google Scholar] [CrossRef]
- Meinhold, R.H.; MacKenzie, K.J.D. Effect of lanthanides on the relaxation rates of 89Y and 29Si in yttrium silicon oxynitride phases. Solid State Nucl. Magn. Reson. 1995, 5, 151–161. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Fukushima, T.; Fukuchi, M.; Fujimura, S.; Kaji, H. Sensitivity boosting in solid-state NMR of thin organic semiconductors by a paramagnetic dopant of copper phthalo-cyanine. Chem. Phys. Lett. 2013, 556, 195–199. [Google Scholar] [CrossRef]
- Wang, Z.; Hanrahan, M.P.; Kobayashi, T.; Perras, F.A.; Chen, Y.; Engelke, F.; Reiter, C.; Purea, A.; Rossini, A.J.; Pruski, M. Combining fast magic angle spinning dynamic nuclear polarization with indirect detection to further enhance the sensitivity of solid-state NMR spectroscopy. Solid State Nucl. Magn. Reson. 2020, 109, 101685. [Google Scholar] [CrossRef] [PubMed]
- Berruyer, P.; Björgvinsdóttir, S.; Bertarello, A.; Stevanato, G.; Rao, Y.; Karthikeyan, G.; Casano, G.; Ouari, O.; Lelli, M.; Reiter, C.; et al. Dynamic Nuclear Polarization Enhancement of 200 at 21.15 T Enabled by 65 kHz Magic Angle Spinning. J. Phys. Chem. Lett. 2020, 11, 8386–8391. [Google Scholar] [CrossRef]
- Hoehne, F.; Dreher, L.; Franke, D.P.; Stutzmann, M.; Vlasenko, L.S.; Itoh, K.M.; Brandt, M.S. Submillisecond Hyperpolarization of Nuclear Spins in Silicon. Phys. Rev. Lett. 2015, 114, 117602. [Google Scholar] [CrossRef]
- Can, T.V.; Walish, J.J.; Swager, T.M.; Griffin, R.G. Time domain DNP with the NOVEL sequence. J. Chem. Phys. 2015, 143, 054201. [Google Scholar] [CrossRef]
- Griffin, R.G. Clear signals from surfaces. Nature 2010, 468, 381–382. [Google Scholar] [CrossRef]
- W.-Liao, C.; Ghaffari, B.; Gordon, C.P.; Xu, J.; Copéret, C. Dynamic Nuclear Polarization Surface Enhanced NMR spectroscopy (DNP SENS): Principles, protocols, and practice. Curr. Opin. Colloid Interface Sci. 2018, 33, 63–71. [Google Scholar] [CrossRef]
- Lesage, A.; Lelli, M.; Gajan, D.; Caporini, M.A.; Vitzthum, V.; Miéville, P.; Alauzun, J.; Rous-sey, A.; Thieuleux, C.; Mehdi, A.; et al. Surface Enhanced NMR Spectroscopy by Dynamic Nuclear Polarization. J. Am. Chem. Soc. 2010, 132, 15459–15461. [Google Scholar] [CrossRef]
- Lee, S. Sensitive detection of NMR for thin films. Solid State Nucl. Magn. Reson. 2015, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kärger, J.; Avramovska, M.; Freude, D.; Haase, J.; Hwang, S.; Valiullin, R. Pulsed field gradient NMR diffusion measurement in nanoporous materials. Adsorption 2021, 27, 453–484. [Google Scholar] [CrossRef]
- Nieuwendaal, R.C.; Ro, H.W.; Germack, D.S.; Kline, R.J.; Toney, M.F.; Chan, C.K.; Agrawal, A.; Gundlach, D.; VanderHart, D.L.; Delongchamp, D.M. Measuring Domain Sizes and Compositional Heterogeneities in P3HT-PCBM Bulk Heterojunction Thin Films with 1H Spin Diffusion NMR Spectroscopy. Adv. Funct. Mater 2012, 22, 1255–1266. [Google Scholar] [CrossRef]
- Sel, O.; To Thi Kim, L.; Debiemme-Chouvy, C.; Gabrielli, C.; Laberty-Robert, C.; Perrot, H. Determination of the Diffusion Coefficient of Protons in Nafion Thin Films by ac Electrogravimetry. Langmuir 2013, 29, 13655–13660. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Mariette, F. Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Zhang, J.; MacGregor, R.P.; Balcom, B.J. Liquid crystal diffusion in thin films investigated by PFG magnetic resonance and magnetic resonance imaging. Chem. Phys. Lett. 2008, 461, 106–110. [Google Scholar] [CrossRef]
- Mens, R.; Adriaensens, P.; Lutsen, L.; Swinnen, A.; Bertho, S.; Ruttens, B.; D’Haen, J.; Manca, J.; Cleij, T.; Vanderzande, D.; et al. NMR study of the nanomorphology in thin films of polymer blends used in organic PV devices: MDMO-PPV/PCBM. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 138–145. [Google Scholar] [CrossRef]
- Koller, H.; Weiß, M. Solid State NMR of Porous Materials. In Solid State NMR; Chan, J.C.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 189–227. [Google Scholar] [CrossRef]
- Borisov, A.S.; Hazendonk, P.; Hayes, P.G. Solid-State Nuclear Magnetic Resonance Spectroscopy: A Review of Modern Techniques and Applications for Inorganic Polymers. J. Inorg. Organomet. Polym. 2010, 20, 183–212. [Google Scholar] [CrossRef]
- Knitsch, R.; Brinkkötter, M.; Wiegand, T.; Kehr, G.; Erker, G.; Hansen, M.R.; Eckert, H. Solid-State NMR Techniques for the Structural Characterization of Cyclic Aggregates Based on Borane–Phosphane Frustrated Lewis Pairs. Molecules 2020, 25, 1400. [Google Scholar] [CrossRef] [PubMed]
- Van der Wel, P.C.A. New applications of solid-state NMR in structural biology. Emerg. Top. Life Sci. 2018, 2, 57–67. [Google Scholar] [CrossRef]
- Rodin, V.V. NMR techniques in studying water in biotechnological systems. Biophys. Rev. 2020, 12, 683–701. [Google Scholar] [CrossRef] [PubMed]
- El Hariri El Nokab, M.; van der Wel, P.C.A. Use of solid-state NMR spectroscopy for investigating polysaccharide-based hydrogels: A review. Carbohydr. Polym. 2020, 240, 116276. [Google Scholar] [CrossRef] [PubMed]
- Krushelnitsky, A.; Reichert, D.; Saalwächter, K. Solid-State NMR Approaches to Internal Dynamics of Proteins: From Picoseconds to Microseconds and Seconds. Acc. Chem. Res. 2013, 46, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Molugu, T.R.; Lee, S.; Brown, M.F. Concepts and Methods of Solid-State NMR Spectroscopy Applied to Biomembranes. Chem. Rev. 2017, 117, 12087–12132. [Google Scholar] [CrossRef]
- Zhao, W.; Fernando, L.D.; Kirui, A.; Deligey, F.; Wang, T. Solid-state NMR of plant and fungal cell walls: A critical review. Solid State Nucl. Magn. Reson. 2020, 107, 101660. [Google Scholar] [CrossRef]
- Simpson, A.J.; Simpson, M.J.; Soong, R. Environmental Nuclear Magnetic Resonance Spectroscopy: An Overview and a Primer. Anal. Chem. 2018, 90, 628–639. [Google Scholar] [CrossRef]
- Brunner, E.; Rauche, M. Solid-state NMR spectroscopy: An advancing tool to analyse the structure and properties of metal–organic frameworks. Chem. Sci. 2020, 11, 4297–4304. [Google Scholar] [CrossRef]
- Piveteau, L.; Morad, V.; Kovalenko, M.V. Solid-State NMR and NQR Spectroscopy of Lead-Halide Perovskite Materials. J. Am. Chem. Soc. 2020, 142, 19413–19437. [Google Scholar] [CrossRef] [PubMed]
- Seifrid, M.; Reddy, G.N.M.; Chmelka, B.F.; Bazan, G.C. Insight into the structures and dynamics of organic semiconductors through solid-state NMR spectroscopy. Nat. Rev. Mater. 2020, 5, 910–930. [Google Scholar] [CrossRef]
- Marchetti, A.; Chen, J.; Pang, Z.; Li, S.; Ling, D.; Deng, F.; Kong, X. Understanding Surface and Interfacial Chemistry in Functional Nanomaterials via Solid-State NMR. Adv. Mater. 2017, 29, 1605895. [Google Scholar] [CrossRef] [PubMed]




| NMR Active Nuclei | Chemical Connectivity | Solid State NMR Technique | References |
|---|---|---|---|
| 1H | Organic/Inorganic | 1D, 2D MAS and multiple quantum | [61,62,63,64] |
| 2H | Organic | 1D, 2D MAS | [69,70] |
| 7,8Li | Inorganic | 1D MAS and β-NMR | [71,72,73] |
| 11B | Inorganic | 1D MAS | [74] |
| 13C | Organic | 1D, 2D MAS | [65,66,67,68] |
| 14,15N | Inorganic | High-field NMR | [72,75] |
| 17O | Inorganic | fast MAS, isotopic enrichment | [76] |
| 19F | Organic/Inorganic | 1D MAS, MRFM | [77,78,79] |
| 27Al | Inorganic | 1D, 2D MAS, high-field NMR | [80,81,82,83] |
| 29Si | Organic/Inorganic | 1D MAS | [84,85,86,87] |
| 31P | Organic/Inorganic | 1D, 2D MAS, DNP | [71,72,86,88,89] |
| 55Mn | Inorganic | NMR relaxometry | [90,91,92] |
| 59Co | Inorganic | NMR relaxometry | [93,94,95] |
| 69,71Ga | Inorganic | High-field NMR | [75,96] |
| 75As | Inorganic | 1D MAS | [97] |
| 89Y | Inorganic | 1D MAS | [98] |
| 129Xe | Inorganic | Hyper-polarization | [99,100] |
| 207Pd | Inorganic | Fast MAS, DNP | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hariri El Nokab, M.; Sebakhy, K.O. Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomaterials 2021, 11, 1494. https://doi.org/10.3390/nano11061494
El Hariri El Nokab M, Sebakhy KO. Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomaterials. 2021; 11(6):1494. https://doi.org/10.3390/nano11061494
Chicago/Turabian StyleEl Hariri El Nokab, Mustapha, and Khaled O. Sebakhy. 2021. "Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review" Nanomaterials 11, no. 6: 1494. https://doi.org/10.3390/nano11061494
APA StyleEl Hariri El Nokab, M., & Sebakhy, K. O. (2021). Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomaterials, 11(6), 1494. https://doi.org/10.3390/nano11061494