Novel Strategy for the Development of Antibacterial TiO2 Thin Film onto Polymer Substrate at Room Temperature
Abstract
1. Introduction
2. Methodology
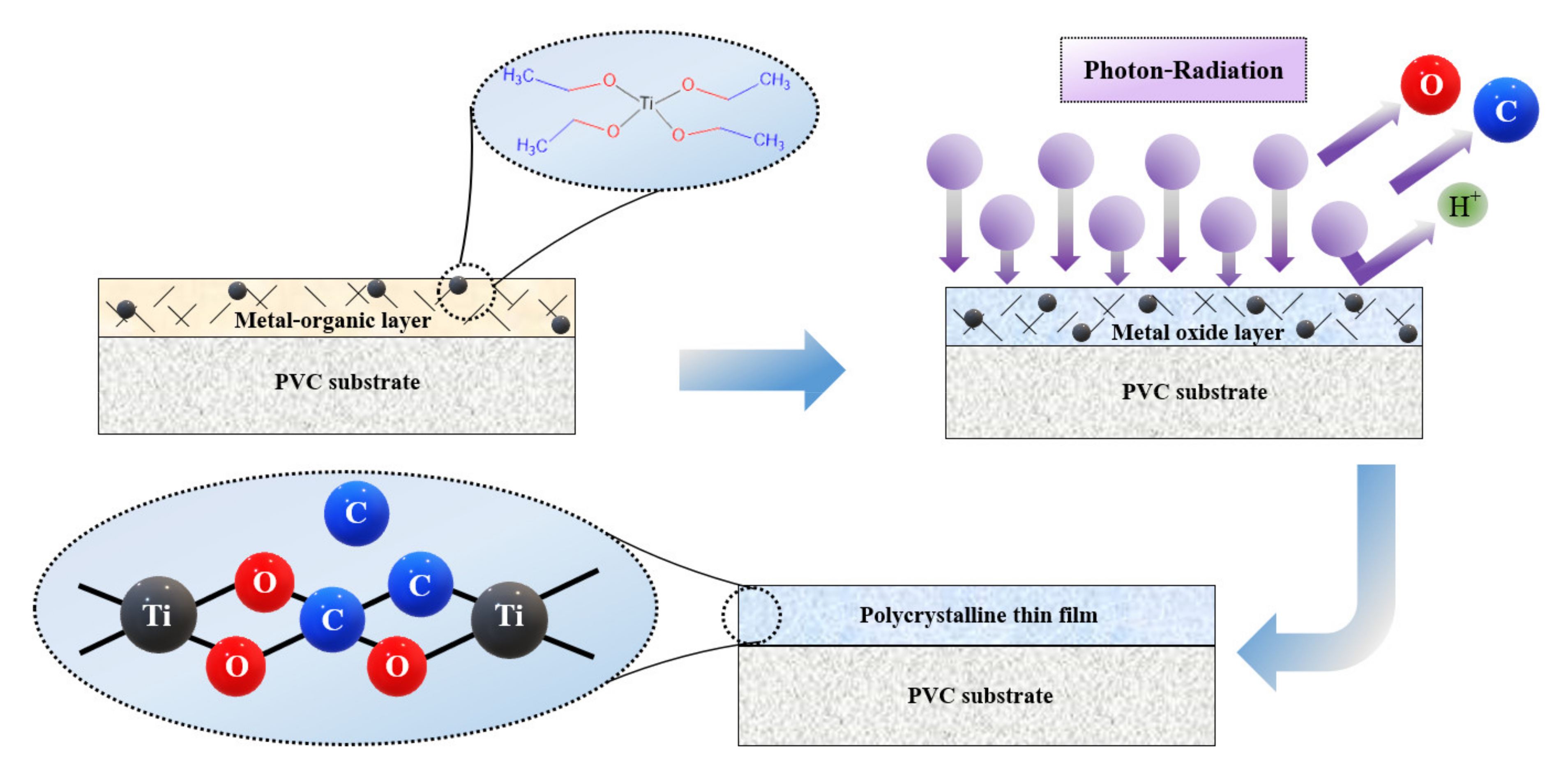
2.1. TiO2 Thin Film Coating
2.2. Thin Film Characterizations
2.3. Bacteria Viability Assay
3. Results and Discussion
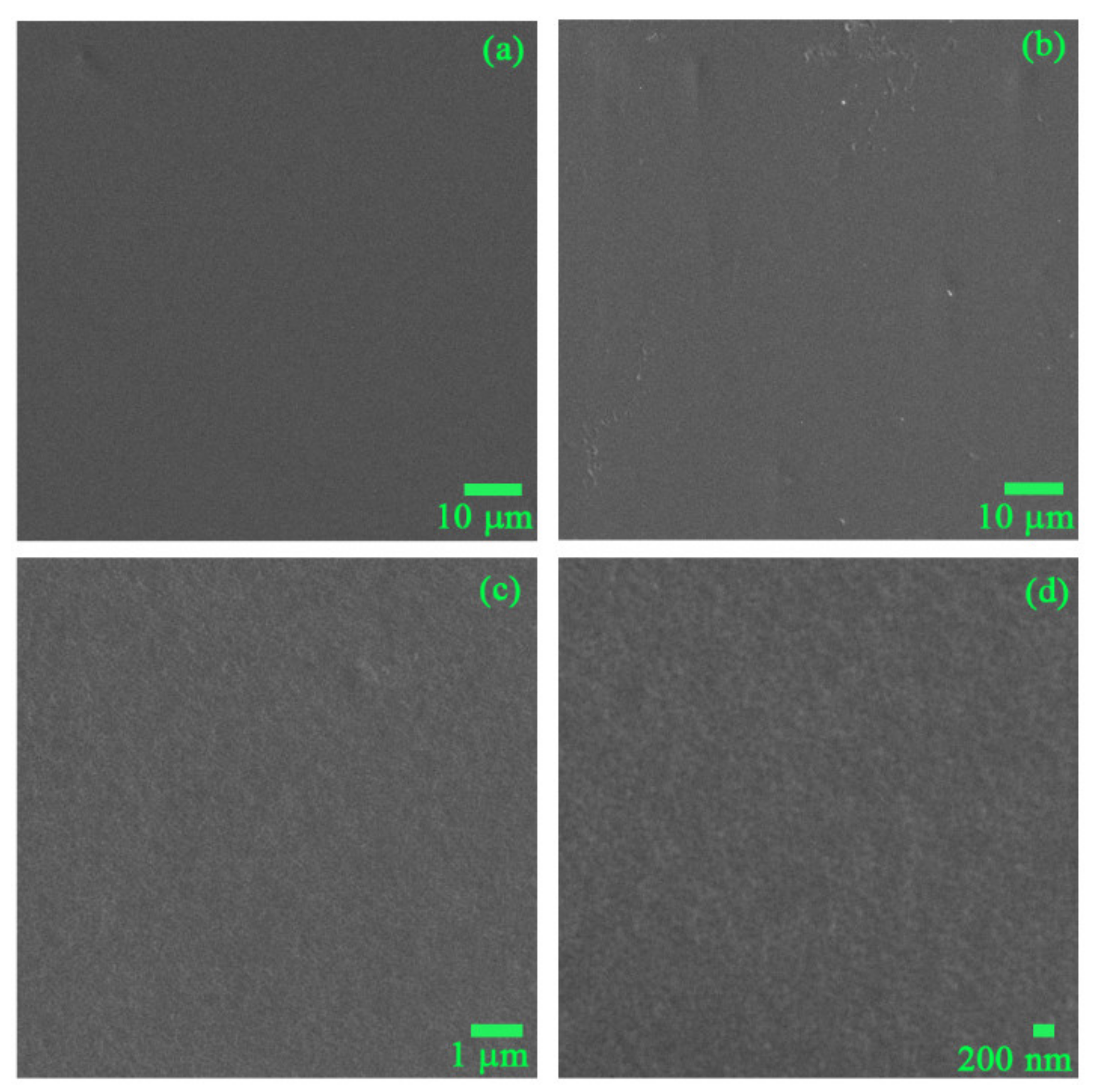
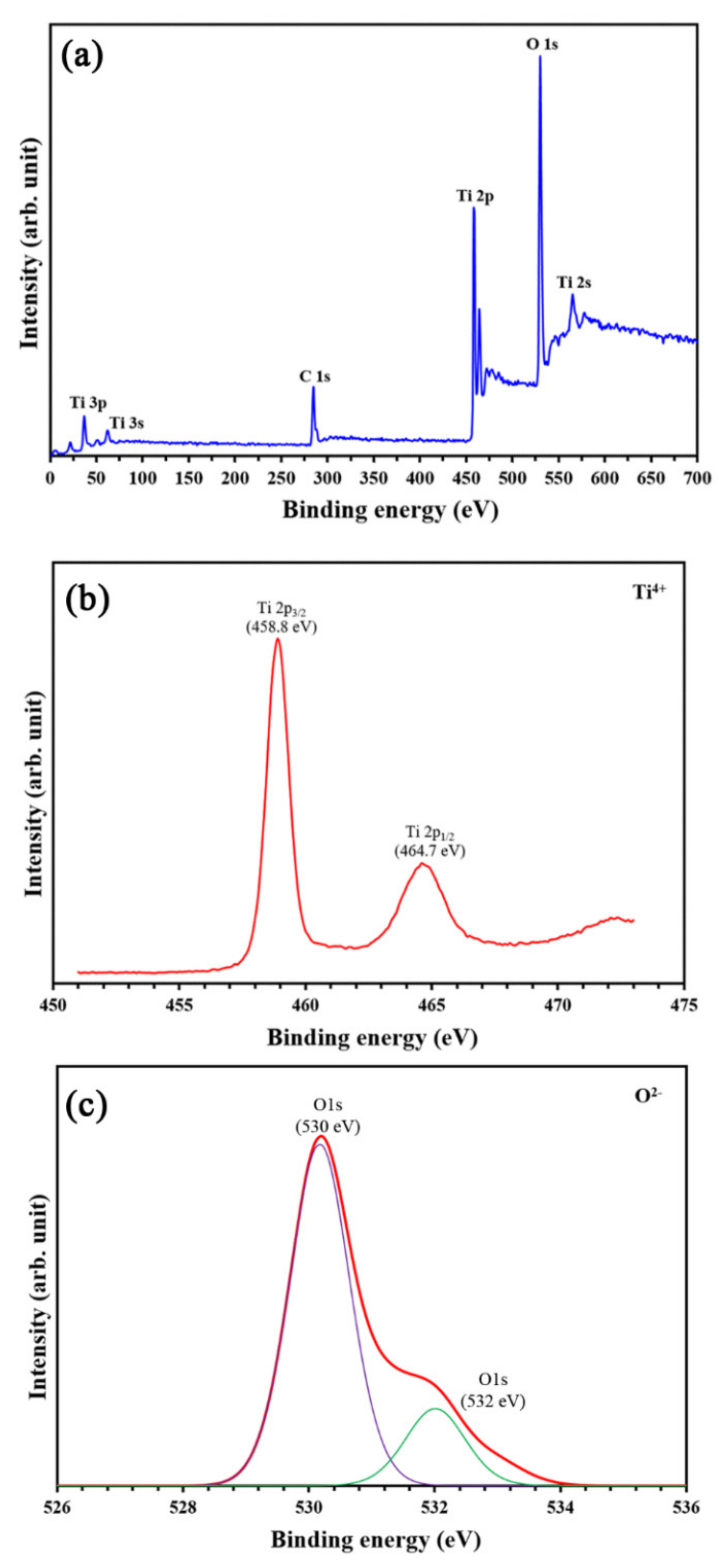
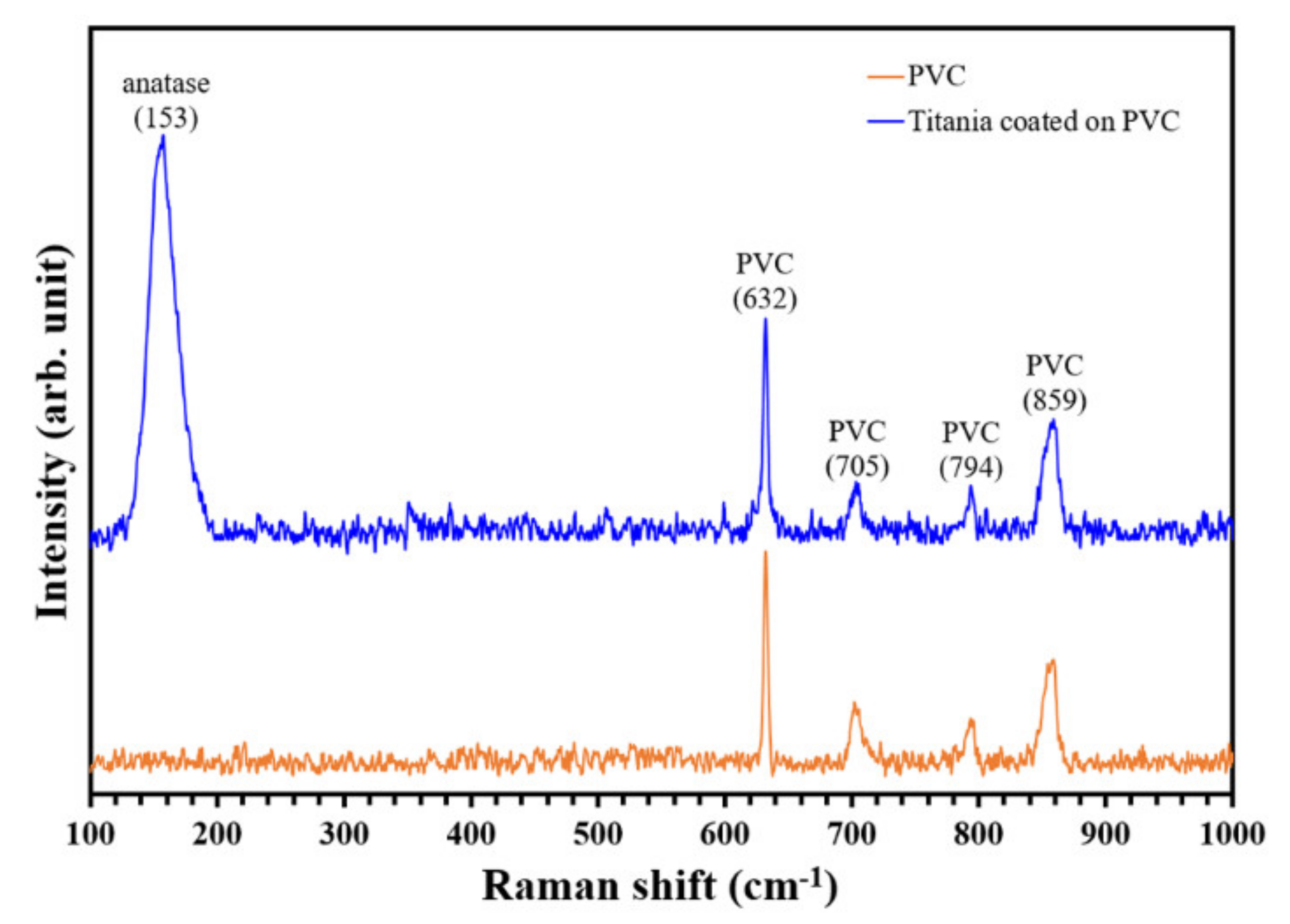
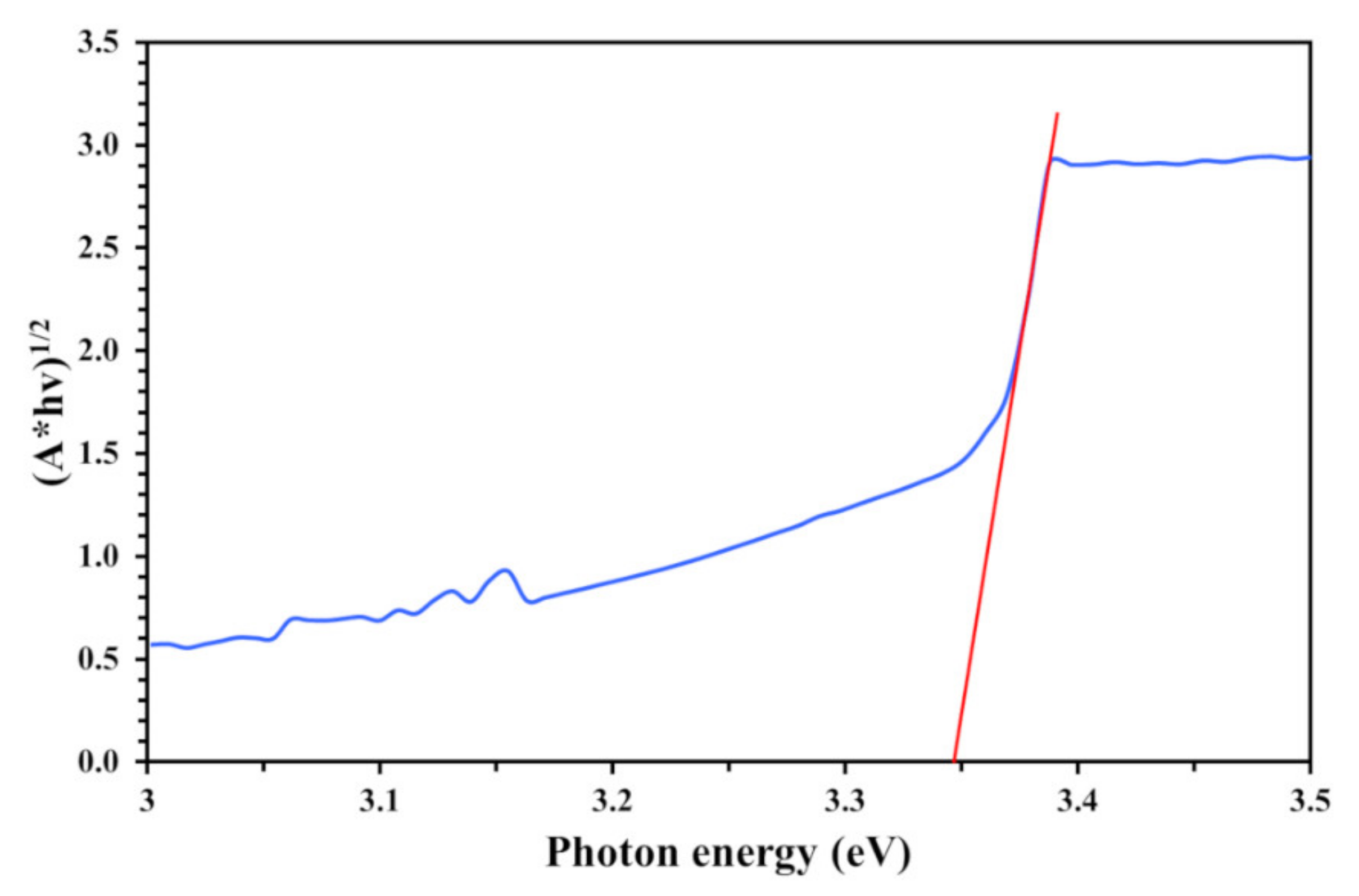
3.1. Physicochemical Characteristics of PVC and TiO2-Coated PVC
3.2. Bacteria Viability Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Shen, Z.-P.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, H.; Chen, C.; Yang, S.; Chen, L.; Alsaedi, A.; Hayat, T. Enhanced removal of uranium(VI) from aqueous solution by a novel Mg-MOF-74-derived porous MgO/carbon adsorbent. J. Colloid Interface Sci. 2019, 537, A1–A10. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Levchuk, I.; Homola, T.; Moreno-Andrés, J.; Rueda-Márquez, J.J.; Dzik, P.; Moríñigo, M.Á.; Sillanpää, M.; Manzano, M.A.; Vahala, R. Solar photocatalytic disinfection using ink-jet printed composite TiO2/SiO2 thin films on flexible substrate: Applicability to drinking and marine water. Sol. Energy 2019, 191, 518–529. [Google Scholar] [CrossRef]
- Long, M.; Wang, J.; Zhuang, H.; Zhang, Y.; Wu, H.; Zhang, J. Performance and mechanism of standard nano-TiO2 (P-25) in photocatalytic disinfection of foodborne microorganisms—Salmonella typhimurium and Listeria monocytogenes. Food Control 2014, 39, 68–74. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Al-Jawad, S.M.H.; Taha, A.A.; Salim, M.M. Synthesis and characterization of pure and Fe doped TiO2 thin films for antimicrobial activity. Optik 2017, 142, 42–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, C.; Zhao, Y.; Zhou, S.; Hu, X.; Chen, P. Low Ag-Doped Titanium Dioxide Nanosheet Films with Outstanding Antimicrobial Property. Environ. Sci. Technol. 2010, 44, 8270–8275. [Google Scholar] [CrossRef]
- Rehan, M.; Nada, A.A.; Khattab, T.A.; Abdelwahed, N.A.M.; El-Kheir, A.A.A. Development of multifunctional polyacrylonitrile/silver nanocomposite films: Antimicrobial activity, catalytic activity, electrical conductivity, UV protection and SERS-active sensor. J. Mater. Res. Technol. 2020, 9, 9380–9394. [Google Scholar] [CrossRef]
- Khattab, T.A.; El-Naggar, M.E.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Facile development of photochromic cellulose acetate transparent nanocomposite film immobilized with lanthanide-doped pigment: Ultraviolet blocking, superhydrophobic, and antimicrobial activity. Luminescence 2021, 36, 543–555. [Google Scholar] [CrossRef]
- Cho, D.; Min, H.; Kim, J.; Cha, G.-S.; Kim, G.-S.; Kim, B.; Ohk, S. Photocatalytic characteristics of TiO2 thin films deposited by PECVD. J. Ind. Eng. Chem. 2007, 13, 434–437. [Google Scholar]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.-T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. J. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Navío, J.A.; Fernández-Hevia, D.; Doña-Rodríguez, J.M. Enhancement of stability and photoactivity of TiO2 coatings on annular glass reactors to remove emerging pollutants from waters. Chem. Eng. J. 2015, 279, 488–497. [Google Scholar] [CrossRef]
- Levchuk, I.; Guillard, C.; Dappozze, F.; Parola, S.; Leonard, D.; Sillanpää, M. Photocatalytic activity of TiO2 films immobilized on aluminum foam by atomic layer deposition technique. J. Photochem. Photobiol. A Chem. 2016, 328, 16–23. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Wang, Q.; Wang, X.; Jiang, W.; Zhang, Z.; Yang, Y. A novel double-cylindrical-shell photoreactor immobilized with monolayer TiO2-coated silica gel beads for photocatalytic degradation of Rhodamine B and Methyl Orange in aqueous solution. Sep. Purif. Technol. 2014, 123, 130–138. [Google Scholar] [CrossRef]
- Fabiyi, M.E.; Skelton, R.L. Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J. Photochem. Photobiol. A Chem. 2000, 132, 121–128. [Google Scholar] [CrossRef]
- Damodar, R.A.; Swaminathan, T. Performance evaluation of a continuous flow immobilized rotating tube photocatalytic reactor (IRTPR) immobilized with TiO2 catalyst for azo dye degradation. Chem. Eng. J. 2008, 144, 59–66. [Google Scholar] [CrossRef]
- Bang, H.-S.; Bang, H.-S.; Lee, Y.-K. The functional TiO2-Biodegradable plastic composite material produced by HVOF spraying Process. J. Nanosci. Nanotechnol. 2007, 7, 3830–3833. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Palmero, P.; Montanaro, L. UV-cured polysiloxane epoxy coatings containing titanium dioxide as photosensitive semiconductor. Macromol. Mater. Eng. 2009, 294, 323–329. [Google Scholar] [CrossRef]
- Koziej, D.; Fischer, F.; Kränzlin, N.; Caseri, W.R.; Niederberger, M. Nonaqueous TiO2 nanoparticle synthesis: A Versatile Basis for the fabrication of Self-supporting, Transparent, and UV-Absorbing composite films. ACS Appl. Mater. Interfaces 2009, 1, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of Antimicrobial Packaging Film Made from Poly (Lactic Acid) Incorporating Titanium Dioxide and Silver Nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Scuderi, V.; Romano, L.; Napolitani, E.; Sanz, R.; Carles, R.; Privitera, V. C ion-implanted TiO2 thin film for photocatalytic applications. J. Appl. Phys. 2015, 117, 105308. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells. Sci. Rep. 2016, 6, 30196. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, Y.-H.; Sun, J.-S.; Huang, Y.-C.; Lu, C.-H.; Chang, W.H.-S.; Wang, C.-C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuinthiang, P.; Trinh, D.T.T.; Channei, D.; Ratananikom, K.; Sirilak, S.; Khanitchaidecha, W.; Nakaruk, A. Novel Strategy for the Development of Antibacterial TiO2 Thin Film onto Polymer Substrate at Room Temperature. Nanomaterials 2021, 11, 1493. https://doi.org/10.3390/nano11061493
Phuinthiang P, Trinh DTT, Channei D, Ratananikom K, Sirilak S, Khanitchaidecha W, Nakaruk A. Novel Strategy for the Development of Antibacterial TiO2 Thin Film onto Polymer Substrate at Room Temperature. Nanomaterials. 2021; 11(6):1493. https://doi.org/10.3390/nano11061493
Chicago/Turabian StylePhuinthiang, Patcharaporn, Dang Trung Tri Trinh, Duangdao Channei, Khakhanang Ratananikom, Sirikasem Sirilak, Wilawan Khanitchaidecha, and Auppatham Nakaruk. 2021. "Novel Strategy for the Development of Antibacterial TiO2 Thin Film onto Polymer Substrate at Room Temperature" Nanomaterials 11, no. 6: 1493. https://doi.org/10.3390/nano11061493
APA StylePhuinthiang, P., Trinh, D. T. T., Channei, D., Ratananikom, K., Sirilak, S., Khanitchaidecha, W., & Nakaruk, A. (2021). Novel Strategy for the Development of Antibacterial TiO2 Thin Film onto Polymer Substrate at Room Temperature. Nanomaterials, 11(6), 1493. https://doi.org/10.3390/nano11061493





