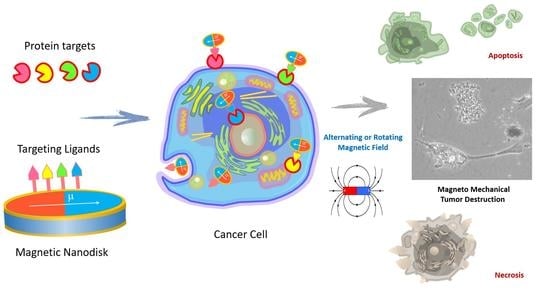
Magnetic Nanodiscs—A New Promising Tool for Microsurgery of Malignant Neoplasms
Abstract
1. Introduction
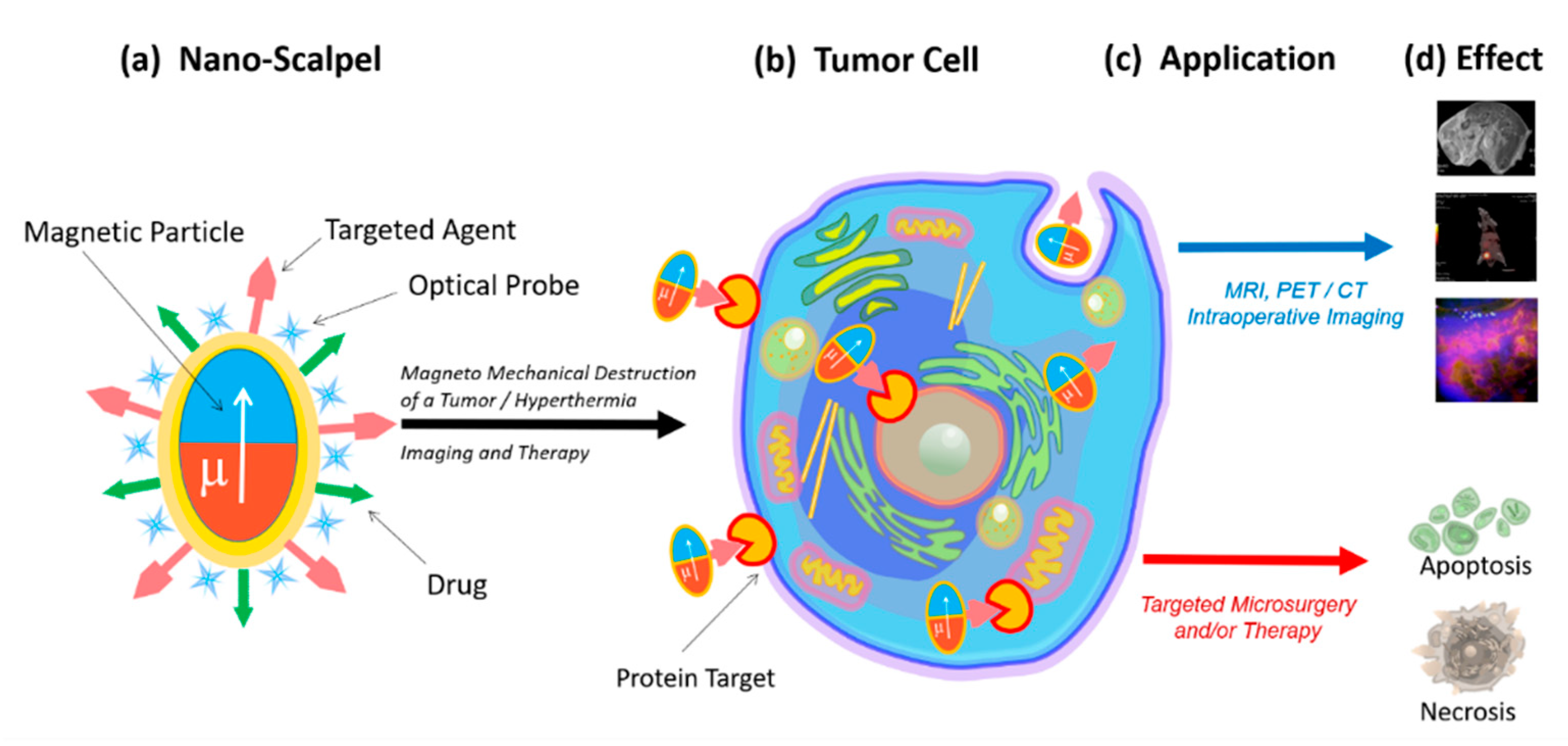
2. Nanoscalpel
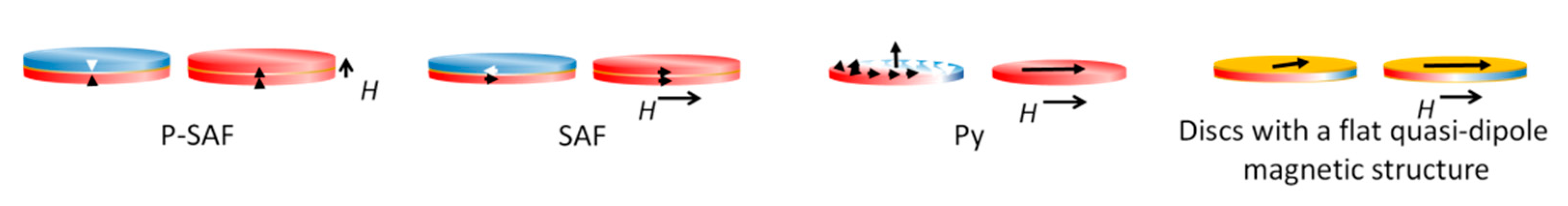
3. Properties of Magnetic Nano- and Microdiscs
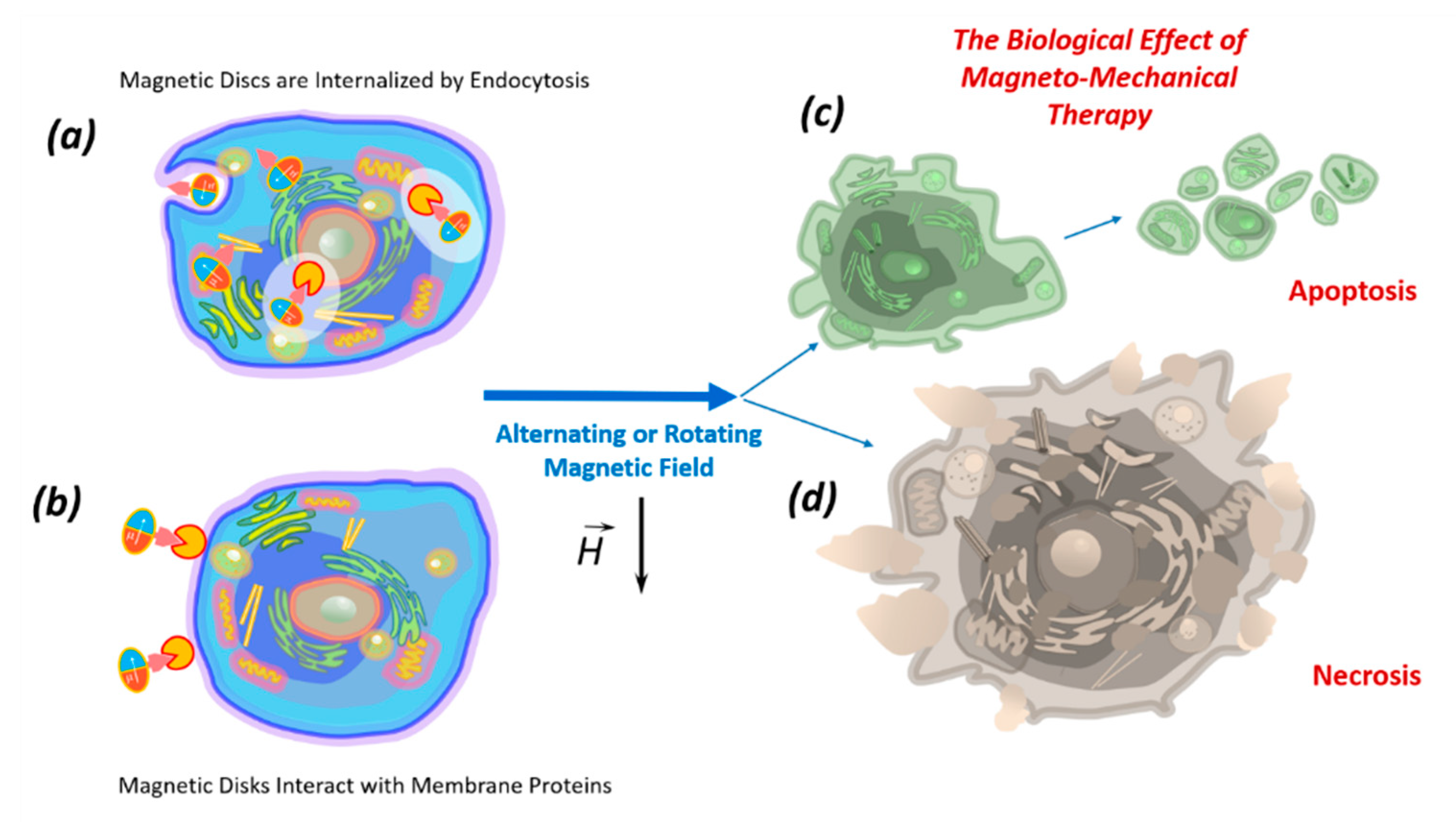
4. Biological Effects of Discs on Tumor Cells in an Alternating or Rotating Magnetic Field
5. The Mechanism of Tumor Cell Death Exposed to Discs under the Influence of a Magnetic Field
6. Internalization of Magnetic Discs
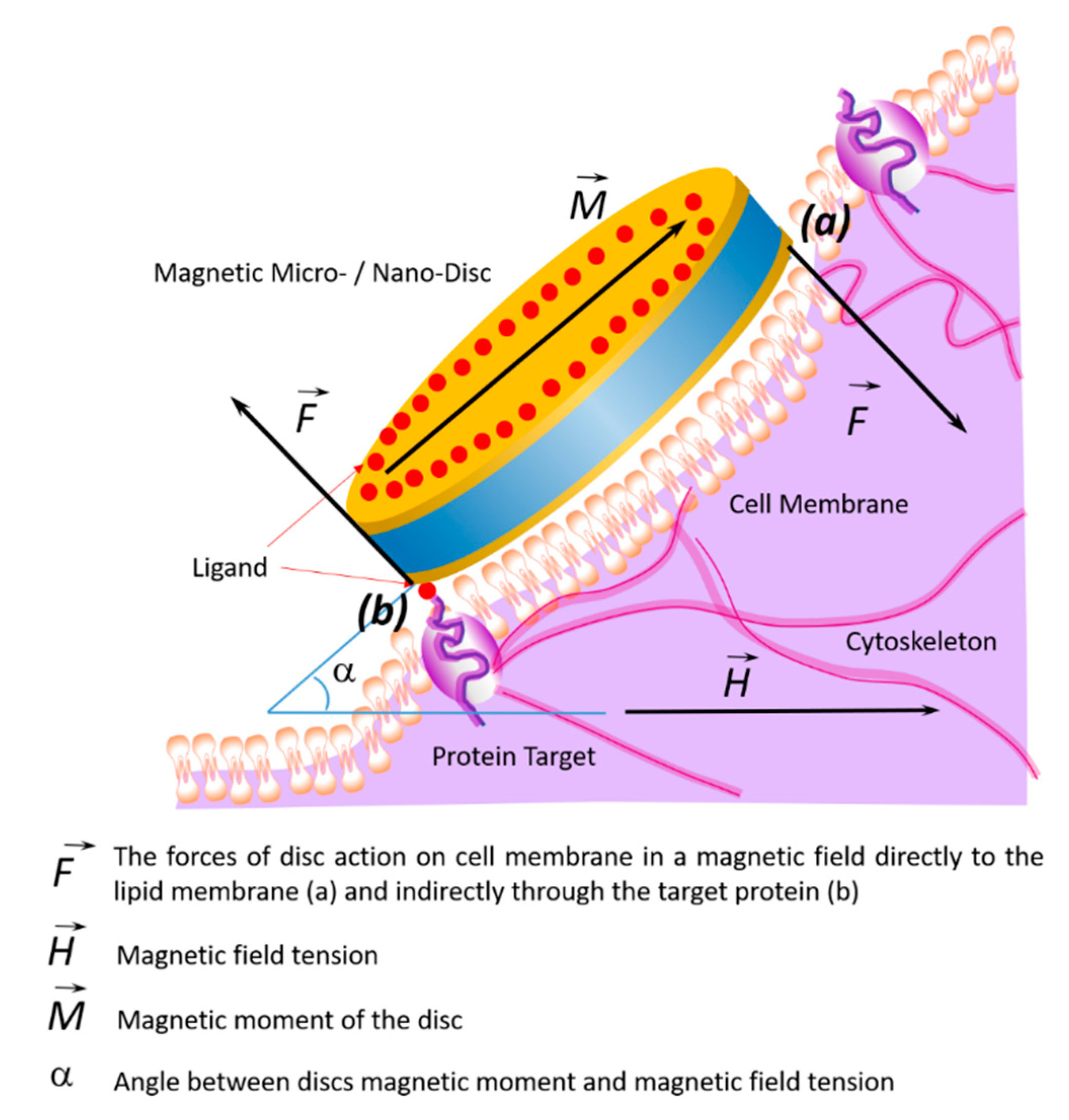
7. Impact of Discs on the Cell Membrane
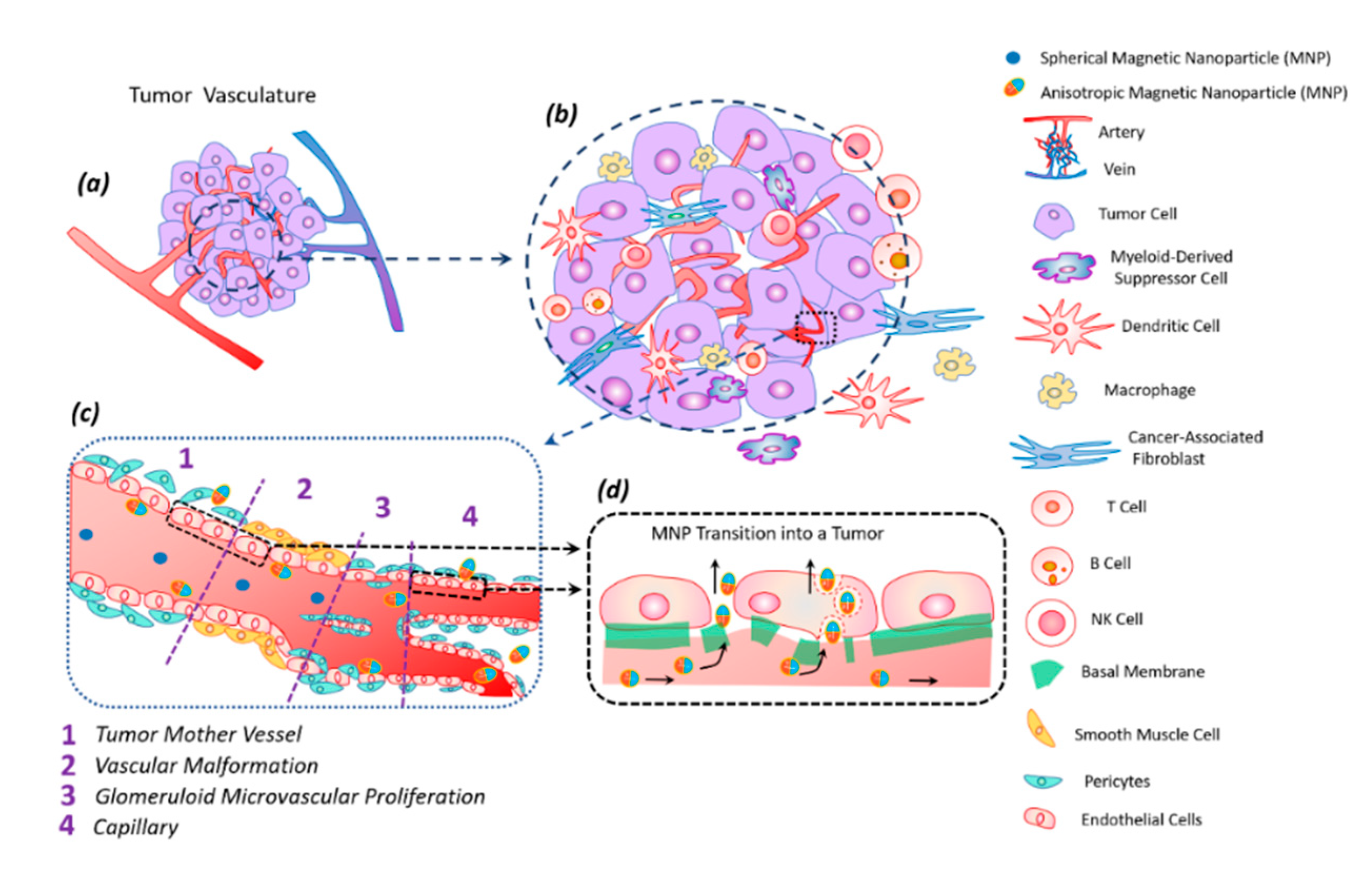
8. Biological Effect of Magnetic Discs In Vivo
9. Transfer of Magnetic Discs along with the Bloodstream
10. Modification and Functionalization of Discs
11. Toxicity of Magnetic Discs
12. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Barnett, G.C.; West, C.M.L.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.P.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Pichot, S.L.; Bentouati, S.; Ahmad, S.S.; Sotiropoulos, M.; Jena, R.; Cowburn, R. Versatile magnetic microdiscs for the radio enhancement and mechanical disruption of glioblastoma cancer cells. RSC Adv. 2020, 10, 8161–8171. [Google Scholar] [CrossRef]
- Torchilin, V. Handbook of Materials for Nanomedicine: Metal Based and Other; Jenny Stanford Pub: Danvers, MA, USA, 2020; ISBN 978-981-4800-93-8. [Google Scholar]
- Cheng, Y.; Muroski, M.E.; Petit, D.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.; et al. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J. Control Release 2016, 223, 75–84. [Google Scholar] [CrossRef]
- Wo, F.; Xu, R.; Shao, Y.; Zhang, Z.; Chu, M.; Shi, D.; Liu, S. A Multimodal System with Synergistic Effects of Magneto-Mechanical, Photothermal, Photodynamic and Chemo Therapies of Cancer in Graphene-Quantum Dot-Coated Hollow Magnetic Nanospheres. Theranostics 2016, 6, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Banderas, A.I.; Aires, A.; Teran, F.J.; Perez, J.E.; Cadenas, J.F.; Alsharif, N.; Ravasi, T.; Cortajarena, A.L.; Kosel, J. Functionalized magnetic nanowires for chemical and magneto-mechanical induction of cancer cell death. Sci. Rep. 2016, 6, 35786. [Google Scholar] [CrossRef]
- Kim, D.-H.; Rozhkova, E.A.; Ulasov, I.V.; Bader, S.D.; Rajh, T.; Lesniak, M.S.; Novosad, V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2009, 9, 165–171. [Google Scholar] [CrossRef]
- Cheng, D.; Li, X.; Zhang, G.; Shi, H. Morphological effect of oscillating magnetic nanoparticles in killing tumor cells. Nanoscale Res. Lett. 2014, 9, 195. [Google Scholar] [CrossRef]
- Zhang, E.; Kircher, M.F.; Koch, M.; Eliasson, L.; Goldberg, S.N.; Renström, E. Dynamic Magnetic Fields Remote-Control Apoptosis via Nanoparticle Rotation. ACS Nano 2014, 8, 3192–3201. [Google Scholar] [CrossRef]
- Domenech, M.; Marrero-Berrios, I.; Torres-Lugo, M.; Rinaldi, C. Lysosomal Membrane Permeabilization by Targeted Magnetic Nanoparticles in Alternating Magnetic Fields. ACS Nano 2013, 7, 5091–5101. [Google Scholar] [CrossRef] [PubMed]
- Muroski, M.E.; Morshed, R.A.; Cheng, Y.; Vemulkar, T.; Mansell, R.; Han, Y.; Zhang, L.; Aboody, K.S.; Cowburn, R.P.; Lesniak, M.S. Controlled Payload Release by Magnetic Field Triggered Neural Stem Cell Destruction for Malignant Glioma Treatment. PLoS ONE 2016, 11, e0145129. [Google Scholar] [CrossRef]
- Contreras, M.; Sougrat, R.; Zaher, A.; Ravasi, T.; Kosel, J. Non-chemotoxic induction of cancer cell death using magnetic nanowires. Int. J. Nanomed. 2015, 10, 2141–2153. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.-H.; Yoo, D.; Kim, J.-W.; Park, S.W.; Shin, J.-S.; Cheon, J. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 2012, 11, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Leulmi, S.; Chauchet, X.; Morcrette, M.; Ortiz, G.; Joisten, H.; Sabon, P.; Livache, T.; Hou, Y.; Carrière, M.; Lequien, S.; et al. Triggering the apoptosis of targeted human renal cancer cells by the vibration of anisotropic magnetic particles attached to the cell membrane. Nanoscale 2015, 7, 15904–15914. [Google Scholar] [CrossRef]
- Kim, P.D.; Zamay, S.S.; Zamay, T.N.; Prokopenko, V.S.; Kolovskaya, O.S.; Zamay, G.S.; Princ, V.Y.; Seleznev, V.A.; Komonov, A.I.; Spivak, E.A.; et al. The antitumor effect of magnetic nanodisks and DNA aptamer conjugates. Dokl. Biochem. Biophys. 2016, 466, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Vergés, M.A.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P. Uniform and water stable magnetite nanoparticles with diameters around the monodomain-multidomain limit. J. Phys. D Appl. Phys. 2008, 41, 134003. [Google Scholar] [CrossRef]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Yu, M.K.; Jeong, Y.Y.; Park, J.; Park, S.; Kim, J.W.; Min, J.J.; Kim, K.; Jon, S. Drug-Loaded Superparamagnetic Iron Oxide Nanoparticles for Combined Cancer Imaging and Therapy In Vivo. Angew. Chem. Int. Ed. 2008, 47, 5362–5365. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Anti-Cancer Drug Loaded Iron–Gold Core–Shell Nanoparticles (Fe@Au) for Magnetic Drug Targeting. J. Nanosci. Nanotechnol. 2010, 10, 5527–5539. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Chen, X. Production, modification and bio-applications of magnetic nanoparticles gestated by magnetotactic bacteria. Nano Res. 2009, 2, 261–278. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Hamilton, A.M.; Aidoudi-Ahmed, S.; Sharma, S.; Kotamraju, V.R.; Foster, P.J.; Sugahara, K.N.; Ruoslahti, E.; Rutt, B.K. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J. Mol. Med. 2015, 93, 991–1001. [Google Scholar] [CrossRef]
- Montenegro, J.-M.; Grazu, V.; Sukhanova, A.; Agarwal, S.; de la Fuente, J.M.; Nabiev, I.; Greiner, A.; Parak, W.J. Controlled antibody/(bio-) conjugation of inorganic nanoparticles for targeted delivery. Adv. Drug Deliv. Rev. 2013, 65, 677–688. [Google Scholar] [CrossRef]
- Zamay, G.S.; Zamay, T.N.; Lukyanenko, K.A.; Kichkailo, A.S. Aptamers Increase Biocompatibility and Reduce the Toxicity of Magnetic Nanoparticles Used in Biomedicine. Biomedicines 2020, 8, 59. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Sokolsky-Papkov, M.; Pothayee, N.; Efremova, M.V.; Gulin, D.A.; Pothayee, N.; Kuznetsov, A.A.; Majouga, A.G.; Riffle, J.S.; Golovin, Y.I.; et al. Changing the Enzyme Reaction Rate in Magnetic Nanosuspensions by a Non-Heating Magnetic Field. Angew. Chem. 2012, 124, 12182–12185. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, Z.; Yin, J.; Wang, X. Tumor therapy by fast moving magnetic nanoparticle under low-frequency alternating magnetic field. J. Innov. Opt. Health Sci. 2015, 8, 1550008. [Google Scholar] [CrossRef]
- Nabavinia, M.; Beltran-Huarac, J. Recent Progress in Iron Oxide Nanoparticles as Therapeutic Magnetic Agents for Cancer Treatment and Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 8172–8187. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef]
- Nair, B.G.; Nagaoka, Y.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Aptamer conjugated magnetic nanoparticles as nanosurgeons. Nanotechnology 2010, 21, 455102. [Google Scholar] [CrossRef]
- Belyanina, I.V.; Zamay, T.N.; Zamay, G.S.; Zamay, S.S.; Kolovskaya, O.S.; Ivanchenko, T.I.; Denisenko, V.V.; Kirichenko, A.K.; Glazyrin, Y.E.; Garanzha, I.V.; et al. In Vivo Cancer Cells Elimination Guided by Aptamer-Functionalized Gold-Coated Magnetic Nanoparticles and Controlled with Low Frequency Alternating Magnetic Field. Theranostics 2017, 7, 3326–3337. [Google Scholar] [CrossRef]
- Gandhi, H.; Sharma, A.K.; Mahant, S.; Kapoor, D.N. Recent advancements in brain tumor targeting using magnetic nanoparticles. Ther. Deliv. 2020, 11, 97–112. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2019, 27, 19214–19225. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Vegerhof, A.; Motei, M.; Rudinzky, A.; Malka, D.; Popovtzer, R.; Zalevsky, Z. Thermal therapy with magnetic nanoparticles for cell destruction. Biomed. Opt. Express 2016, 7, 4581–4594. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.K.; Wydra, R.J.; Stocke, N.A.; Anderson, K.W.; Hilt, J.Z. Magnetic nanoparticles and nanocomposites for remote controlled therapies. J. Control Release 2015, 219, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.P.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, srep01652. [Google Scholar] [CrossRef]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Goiriena-Goikoetxea, M.; Muñoz, D.; Orue, I.; Fernández-Gubieda, M.L.; Bokor, J.; Muela, A.; García-Arribas, A. Disk-shaped magnetic particles for cancer therapy. Appl. Phys. Rev. 2020, 7, 011306. [Google Scholar] [CrossRef]
- Goiriena-Goikoetxea, M.; García-Arribas, A.; Rouco, M.; Svalov, A.V.; Barandiaran, J.M. High-yield fabrication of 60 nm Permalloy nanodiscs in well-defined magnetic vortex state for biomedical applications. Nanotechnology 2016, 27, 175302. [Google Scholar] [CrossRef]
- Zamay, T.N.; Zamay, G.S.; Belyanina, I.V.; Zamay, S.S.; Denisenko, V.V.; Kolovskaya, O.S.; Ivanchenko, T.I.; Grigorieva, V.L.; Garanzha, I.V.; Veprintsev, D.V.; et al. Noninvasive Microsurgery Using Aptamer-Functionalized Magnetic Microdisks for Tumor Cell Eradication. Nucleic Acid Ther. 2017, 27, 105–114. [Google Scholar] [CrossRef]
- Hu, W.; Wilson, R.J.; Earhart, C.M.; Koh, A.L.; Sinclair, R.; Wang, S.X. Synthetic antiferromagnetic nanoparticles with tunable susceptibilities. J. Appl. Phys. 2009, 105, 07B508. [Google Scholar] [CrossRef]
- Vemulkar, T.; Mansell, R.; Petit, D.C.M.C.; Cowburn, R.P.; Lesniak, M.S. Highly tunable perpendicularly magnetized synthetic antiferromagnets for biotechnology applications. Appl. Phys. Lett. 2015, 107, 012403. [Google Scholar] [CrossRef]
- Guslienko, K.Y.; Novosad, V.; Otani, Y.; Shima, H.; Fukamichi, K. Field evolution of magnetic vortex state in ferromagnetic disks. Appl. Phys. Lett. 2001, 78, 3848–3850. [Google Scholar] [CrossRef]
- Wong, D.W.; Gan, W.L.; Liu, N.; Lew, W.S. Magneto-actuated cell apoptosis by biaxial pulsed magnetic field. Sci. Rep. 2017, 7, 10919. [Google Scholar] [CrossRef]
- Joisten, H.; Courcier, T.; Balint, P.; Sabon, P.; Faure-Vincent, J.; Auffret, S.; Dieny, B. Self-polarization phenomenon and control of dispersion of synthetic antiferromagnetic nanoparticles for biological applications. Appl. Phys. Lett. 2010, 97, 253112. [Google Scholar] [CrossRef]
- Scholz, W.; Guslienko, K.; Novosad, V.; Suess, D.; Schrefl, T.; Chantrell, R.; Fidler, J. Transition from single-domain to vortex state in soft magnetic cylindrical nanodots. J. Magn. Magn. Mater. 2003, 266, 155–163. [Google Scholar] [CrossRef]
- Koh, A.L.; Hu, W.; Wilson, R.J.; Earhart, C.M.; Wang, S.X.; Sinclair, R. Structural and Magnetic Characterizations of High Moment Synthetic Antiferromagnetic Nanoparticles Fabricated Using Self-Assembled Stamps. J. Appl. Phys. 2010, 107, 09B522. [Google Scholar] [CrossRef]
- Mansell, R.; Vemulkar, T.; Petit, D.C.M.C.; Cheng, Y.; Murphy, J.; Lesniak, M.S.; Cowburn, R.P. Magnetic particles with perpendicular anisotropy for mechanical cancer cell destruction. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Illi, B.; Scopece, A.; Nanni, S.; Farsetti, A.; Morgante, L.; Biglioli, P.; Capogrossi, M.C.; Gaetano, C. Epigenetic Histone Modification and Cardiovascular Lineage Programming in Mouse Embryonic Stem Cells Exposed to Laminar Shear Stress. Circ. Res. 2005, 96, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, S.; McCloskey, K.E. Can Shear Stress Direct Stem Cell Fate? Biotechnol. Prog. 2009, 25, 10–19. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Goiriena-Goikoetxea, M. Magnetic Vortex Nanodiscs for Cancer Cell Destruction. Ph.D. Thesis, University of the Basque Country, Leioa, Spain, 2017. [Google Scholar]
- Hope, J.M.; Greenlee, J.D.; King, M.R. Mechanosensitive Ion Channels. Cancer J. 2018, 24, 84–92. [Google Scholar] [CrossRef]
- Martinac, B. Mechanosensitive ion channels: Molecules of mechanotransduction. J. Cell Sci. 2004, 117, 2449–2460. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Carboni, E.; Tschudi, K.; Nam, J.; Lu, X.; Ma, A.W.K. Particle Margination and Its Implications on Intravenous Anticancer Drug Delivery. AAPS Pharm. Sci. Tech. 2014, 15, 762–771. [Google Scholar] [CrossRef]
- Ye, H.; Shen, Z.; Yu, L.; Wei, M.; Li, Y. Manipulating Nanoparticle Transport within Blood Flow through External Forces: An Exemplar of Mechanics in Nanomedicine. Proc. R. Soc. Math. Phys. Eng. Sci. 2018, 474, 20170845. [Google Scholar] [CrossRef]
- Su, C.; Li, J.; Zhang, L.; Wang, H.; Wang, F.; Tao, Y.; Wang, Y.; Guo, Q.; Li, J.; Liu, Y.; et al. The Biological Functions and Clinical Applications of Integrins in Cancers. Front. Pharmacol. 2020, 11, 579068. [Google Scholar] [CrossRef]
- Lian, L.; Li, X.-L.; Xu, M.-D.; Li, X.-M.; Wu, M.-Y.; Zhang, Y.; Tao, M.; Li, W.; Shen, X.-M.; Zhou, C.; et al. VEGFR2 Promotes Tumorigenesis and Metastasis in a Pro-Angiogenic-Independent Way in Gastric Cancer. BMC Cancer 2019, 19, 183. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, R. Calcium Phosphate Nanoparticles in Biomineralization and Biomaterials. J. Mater. Chem. 2008, 18, 3775. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles to Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hsiao, J.-K.; Tai, M.-F.; Chen, S.-T.; Cheng, H.-Y.; Wang, J.-L.; Liu, H.-M. Direct Labeling of hMSC with SPIO: The Long-Term Influence on Toxicity, Chondrogenic Differentiation Capacity, and Intracellular Distribution. Mol. Imaging Biol. 2010, 13, 443–451. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Häfeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahmed, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef]
- Heymer, A.; Haddad, D.; Weber, M.; Gbureck, U.; Jakob, P.M.; Eulert, J.; Nöth, U. Iron oxide labelling of human mesenchymal stem cells in collagen hydrogels for articular cartilage repair. Biomaterial 2008, 29, 1473–1483. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron Oxide Magnetic Nanoparticles: Characterization and Toxicity Evaluation byIn VitroandIn VivoAssays. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Chao, X.; Shi, F.; Zhao, Y.-Y.; Li, K.; Peng, M.-L.; Chen, C.; Cui, Y.-L. Cytotoxicity of Fe3O4/Au composite nanoparticles loaded with doxorubicin combined with magnetic field. Die Pharm. 2010, 65, 500–504. [Google Scholar]
- Salado, J.; Insausti, M.; Lezama, L.; Gil De Muro, I.; Moros, M.; Pelaz, B.; Grazú, V.; De La Fuente, J.M.; Rojo, T. Functionalized Fe3O4@Au superparamagnetic nanoparticles:in vitrobioactivity. Nanotechnology 2012, 23, 315102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; An, Y.-L.; Lin, M.; Gao, Z.; Zhang, J. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
| Size | Disc Type | Composition | Magnetic Field Characteristics | Biological Effect In Vitro | Reference |
|---|---|---|---|---|---|
| 2 μm | P-SAF | CoFeB connected by Pt/Ru/Pt spacers | Rotating magnetic field, 10 kOe Duration 1 min Torque 18 nN | Destruction of 62% of U87 cells | [53] |
| 2 μm | Py | Ni80Fe20 | Rotating magnetic field, 10 kOe Duration 1 min Torque 75 nN | Destruction of 12% U87 cells | [53] |
| 150/200/350 nm | Py | Ni80Fe20 | Alternating magnetic field, 20 HzDuration 2 h | Destruction of 83.4/83.2/82.5% of HeLa cells | [49] |
| 2 μm | P-SAF | (Ta/Pt/CoFeB/Pt/Ru/ PT/CoFeB)10 | Rotating magnetic field, 1 T Duration 20 min | Destruction of 70% of U87 cells | [7] |
| 1.3 μm | Py | Ni80Fe20 | Rotating magnetic field, ∼20–30 mT, 20 Hz Duration 1 h | Destruction of 70% of renal cancer cells | [17] |
| 1 μm thickness 60 nm | Py | Ni80Fe20 | Rotating magnetic field, 9 mT 20 Hz Duration 10 min | Destruction of 90% of human glioma tumor cell line No. 10 cells (N10) | [10] |
| 2 μm | Py | Ni80Fe20 | Rotating magnetic field 1 T, 20 Hz, Duration 30 min | Destruction of 60% of U87 cells. In vivo survival is 3 times higher, and the tumor is 3 times smaller | [7] |
| 0.14 μm | Py | Ni80Fe20 | Rotating magnetic field 10 mT, 20 Hz Duration 30 min | Destruction of 60% of cells | [57] |
| 2 μm | Py | Ni80Fe20 | Rotating magnetic field 10 mT, 20 Hz Duration 30 min | Destruction of 12% of cells | [57] |
| 1 μm | Discs with a flat quasi-dipole magnetic structure | Au/Ni/Au | Rotating magnetic field, 50 Hz 5 mT Duration 20 min In vitro In vivo | Destruction of 80% of Ehrlich ascites adenocarcinoma cell | [18] |
| 1 μm | Discs with a flat quasi-dipole magnetic structure | Au/Ni/Au | Alternating magnetic field, 50 Hz, 5 mT Duration 20 min | Destruction of 90% of Ehrlich ascites adenocarcinoma cell | [45] |
| Disc Type | Recognizing Agent | Disc Binding to Recognizing Agent | Cell Type; Destruction Rate | Reference |
|---|---|---|---|---|
| The 60-nm-thick, ~1-μm-diameter 20:80% iron–nickel (permalloy) discs, coated with a 5-nm-thick layer of gold on each side | Antibodies anti-IL13α2R | S–Au bond | Human glioma N10 cell line; 90% (in vitro) | [10] |
| The 60-nm-thick, ~1-μm-diameter 20:80% iron–nickel (permalloy) discs | Antibody antihCA9 | S–Au bond | Renal SCRC-59 renal cancer line; 90% (in vitro) | [17] |
| Discs with a flat quasi-dipole magnetic structure Au/Ni/Au | Aptamer | S–Au bond | Ehrlich ascites adenocarcinoma cell line; 80% (in vitro, in vivo) | [18] |
| Discs with a flat quasi-dipole magnetic structure Au/Ni/Au | Aptamer | S–Au bond | Ehrlich ascites adenocarcinoma cell line; 90% (in vitro, in vivo) | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamay, T.N.; Prokopenko, V.S.; Zamay, S.S.; Lukyanenko, K.A.; Kolovskaya, O.S.; Orlov, V.A.; Zamay, G.S.; Galeev, R.G.; Narodov, A.A.; Kichkailo, A.S. Magnetic Nanodiscs—A New Promising Tool for Microsurgery of Malignant Neoplasms. Nanomaterials 2021, 11, 1459. https://doi.org/10.3390/nano11061459
Zamay TN, Prokopenko VS, Zamay SS, Lukyanenko KA, Kolovskaya OS, Orlov VA, Zamay GS, Galeev RG, Narodov AA, Kichkailo AS. Magnetic Nanodiscs—A New Promising Tool for Microsurgery of Malignant Neoplasms. Nanomaterials. 2021; 11(6):1459. https://doi.org/10.3390/nano11061459
Chicago/Turabian StyleZamay, Tatiana N., Vladimir S. Prokopenko, Sergey S. Zamay, Kirill A. Lukyanenko, Olga S. Kolovskaya, Vitaly A. Orlov, Galina S. Zamay, Rinat G. Galeev, Andrey A. Narodov, and Anna S. Kichkailo. 2021. "Magnetic Nanodiscs—A New Promising Tool for Microsurgery of Malignant Neoplasms" Nanomaterials 11, no. 6: 1459. https://doi.org/10.3390/nano11061459
APA StyleZamay, T. N., Prokopenko, V. S., Zamay, S. S., Lukyanenko, K. A., Kolovskaya, O. S., Orlov, V. A., Zamay, G. S., Galeev, R. G., Narodov, A. A., & Kichkailo, A. S. (2021). Magnetic Nanodiscs—A New Promising Tool for Microsurgery of Malignant Neoplasms. Nanomaterials, 11(6), 1459. https://doi.org/10.3390/nano11061459