Oxido- and Dioxido-Vanadium(V) Complexes Supported on Carbon Materials: Reusable Catalysts for the Oxidation of Cyclohexane
Abstract
1. Introduction
2. Materials and Methods
2.1. General Materials and Procedures
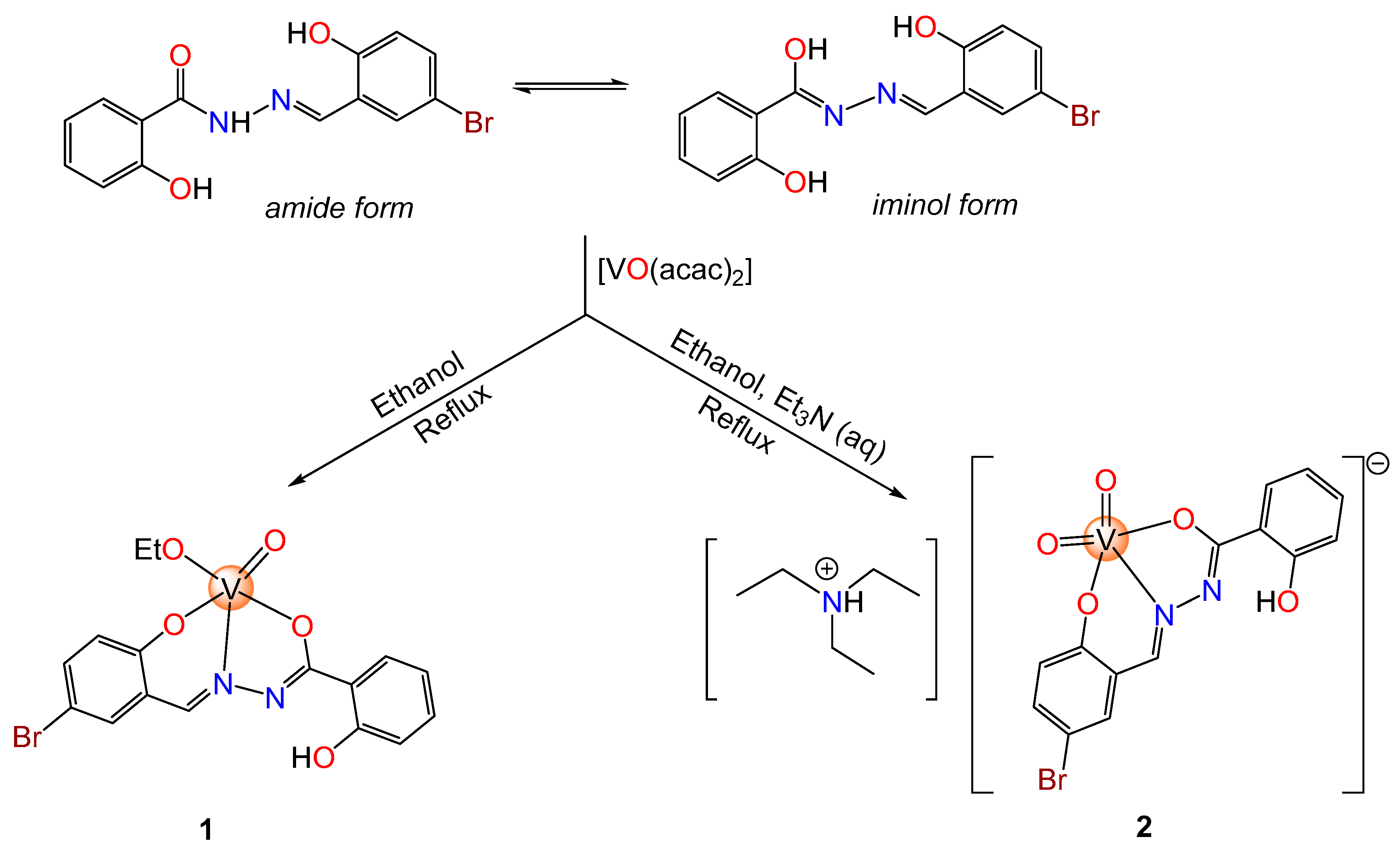
2.1.1. Synthesis of the Pro-Ligand H2L
2.1.2. Synthesis of the Oxidovanadium(V) Compound [VO(OEt)L] (1)
2.1.3. Synthesis of the Dioxidovanadium(V) Compound [Et3NH][VO2L] (2)
2.2. X-ray Measurements
2.3. Carbon Materials Production and Treatments
2.4. Carbon Materials Characterization
2.5. Heterogenization Protocol
2.6. Characterization of Heterogenized Complexes
2.7. Catalytic Studies
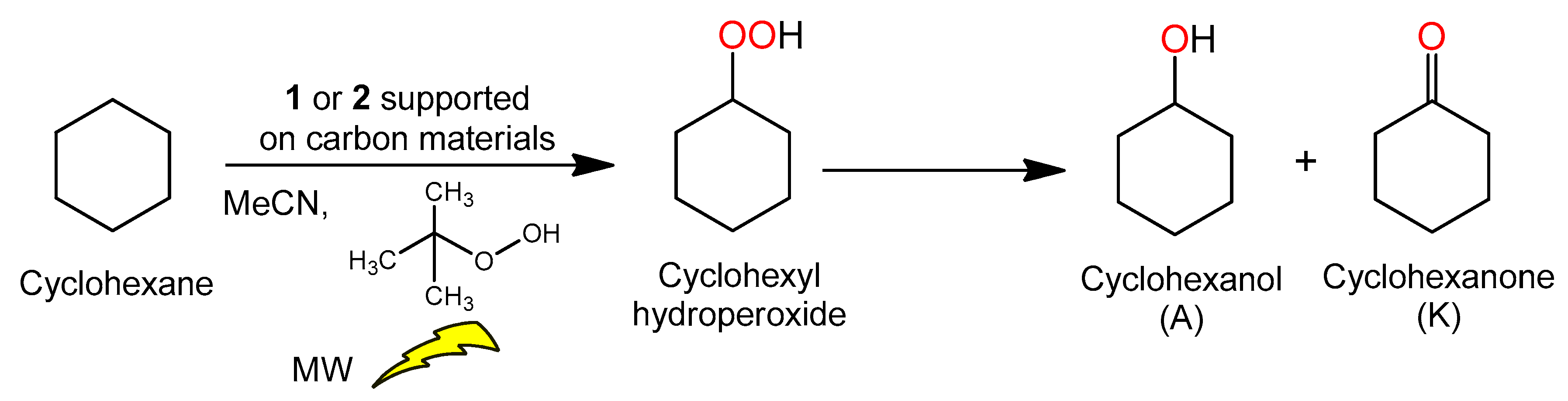
General Procedure for the Peroxidative Oxidation of Cyclohexane
3. Results and Discussion
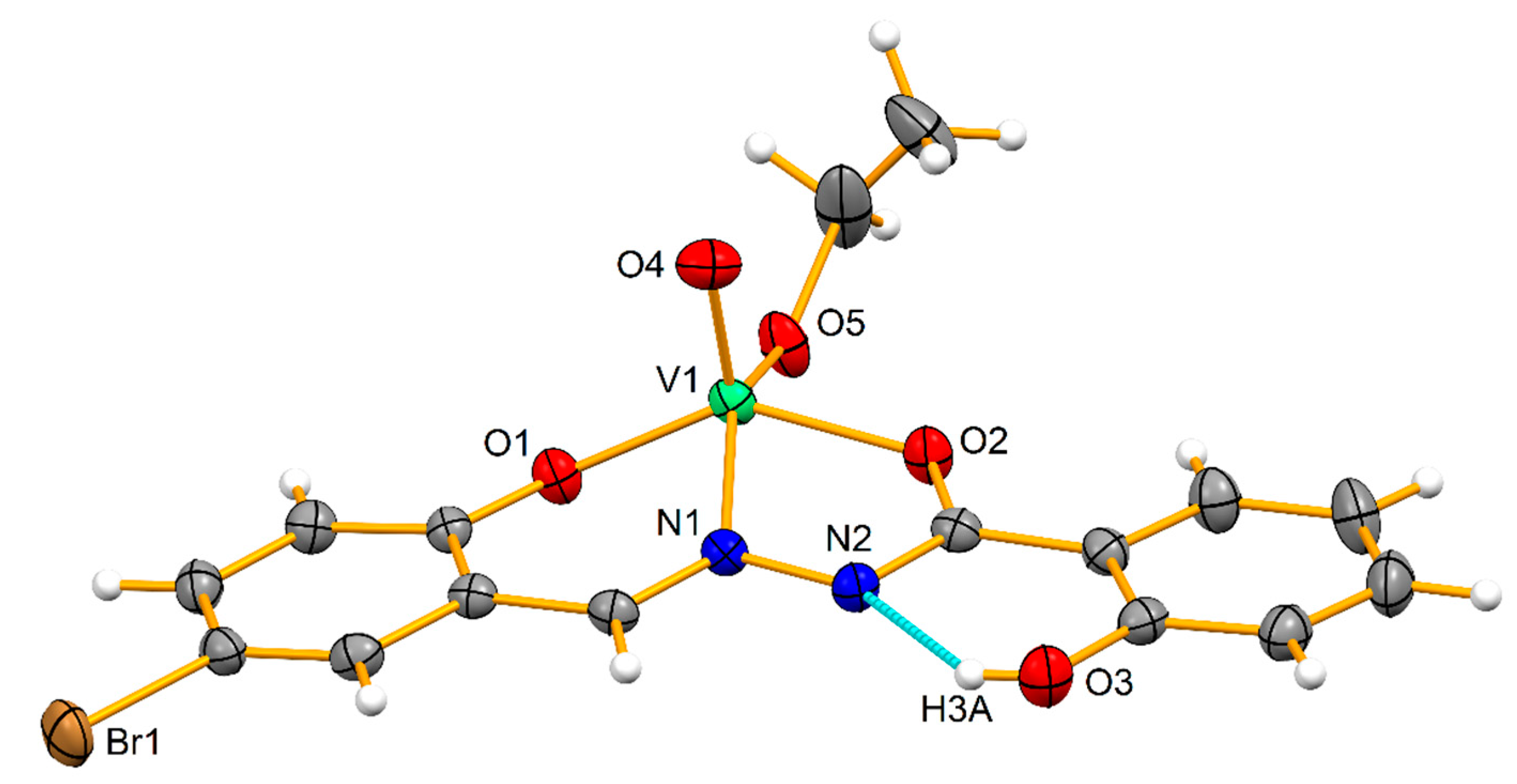
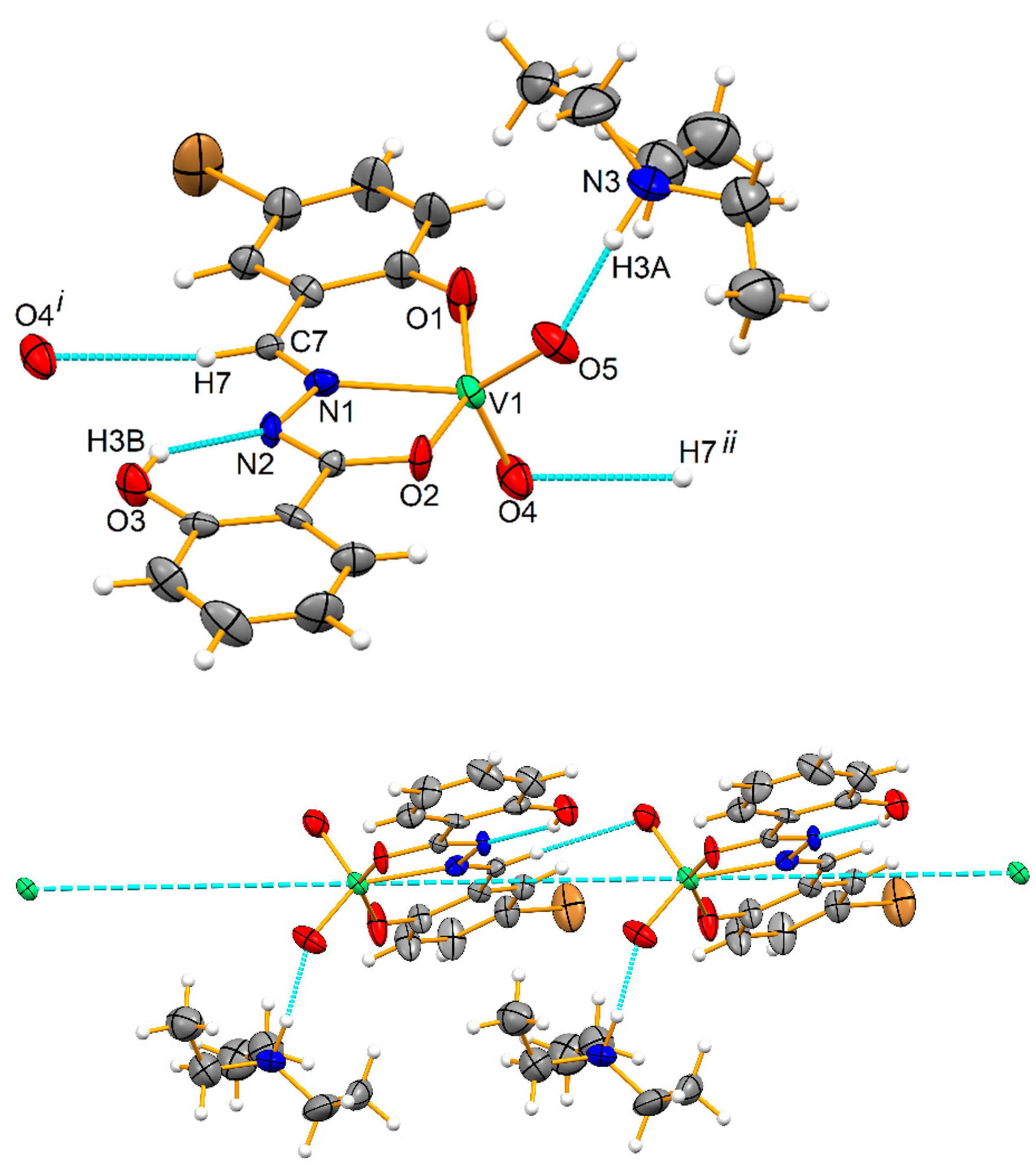
3.1. X-ray Structures
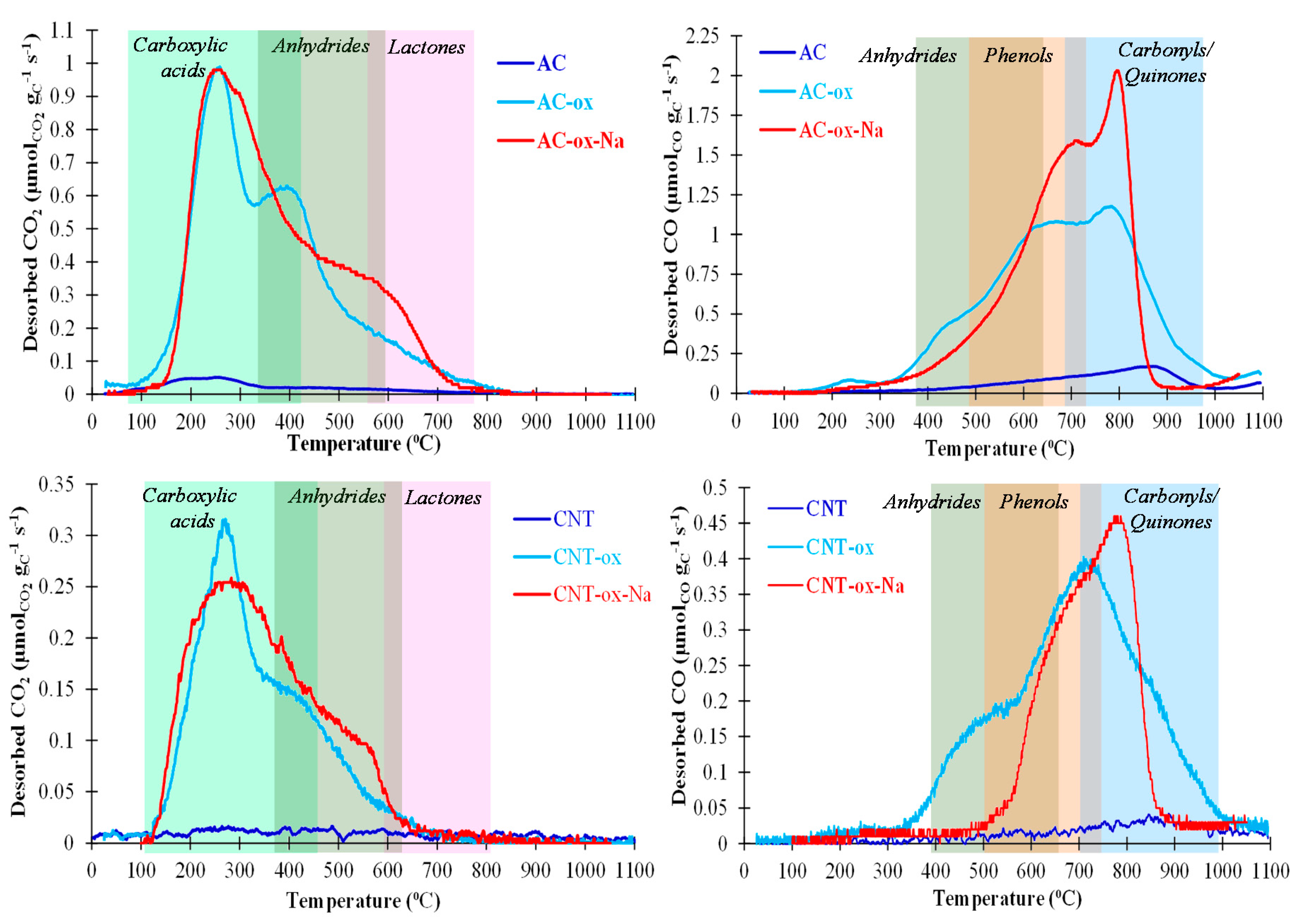
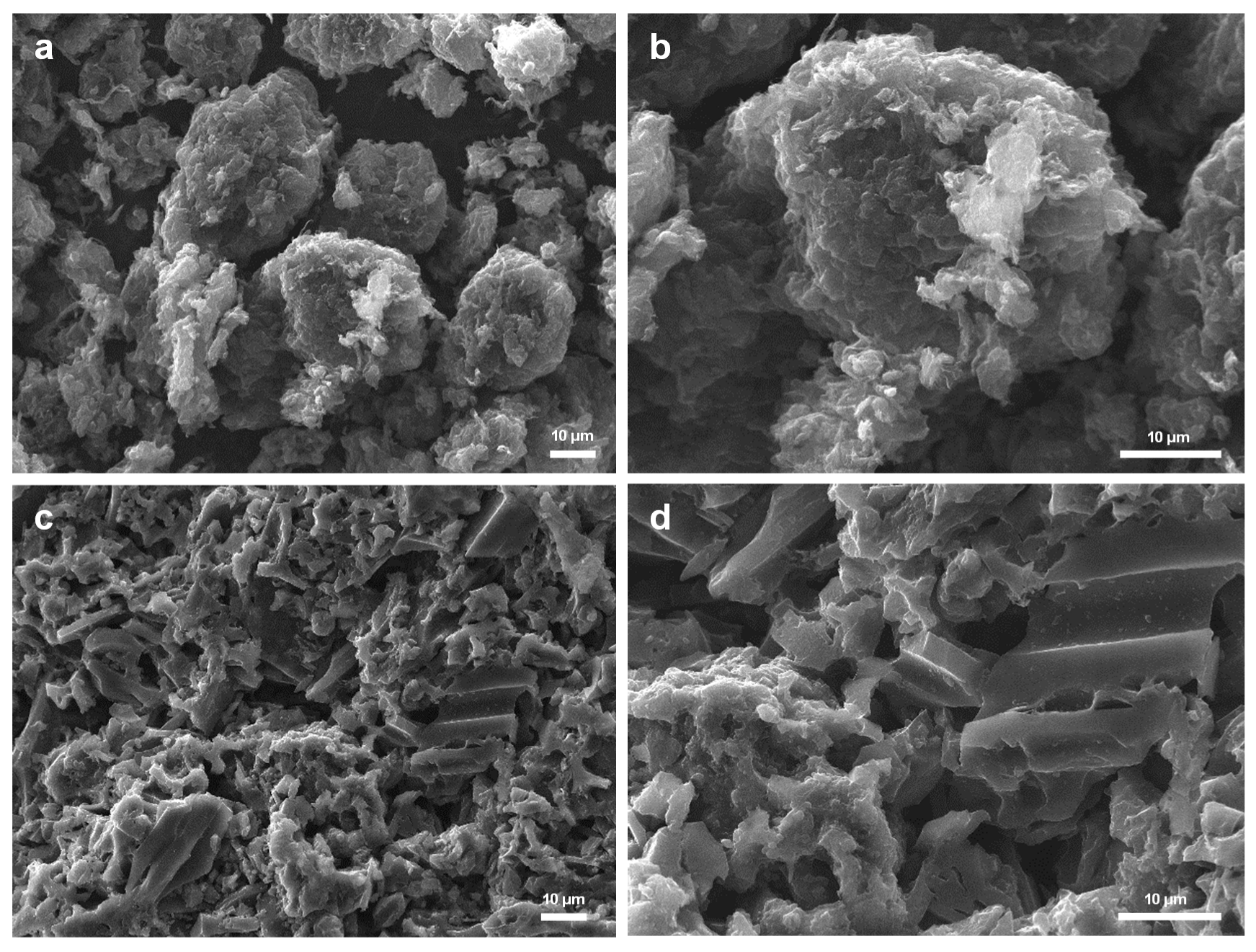
3.2. Characterization of Carbon Supports and Heterogenized Materials
3.3. Heterogenization Efficiency
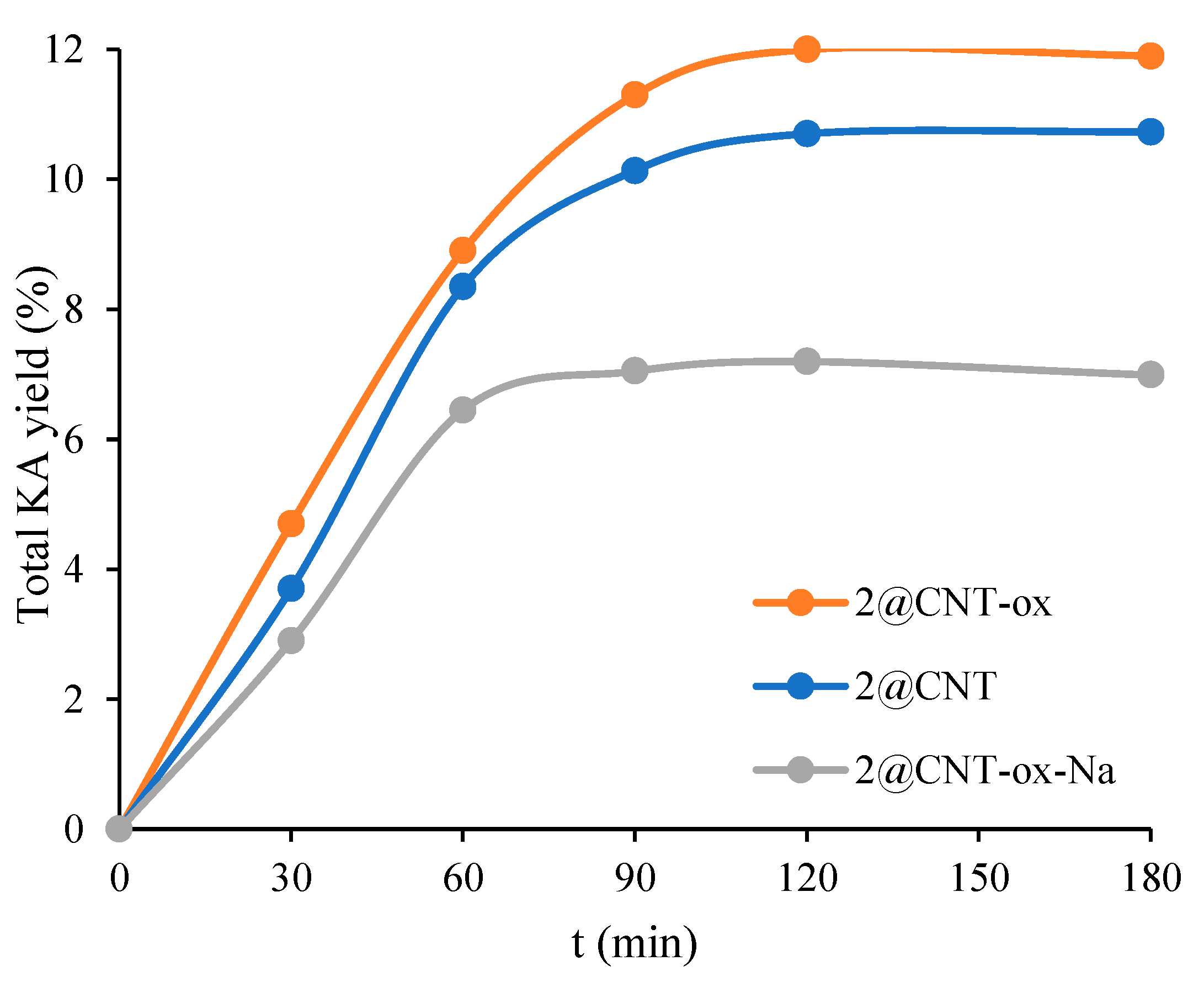
3.4. Microwave-Assisted Oxidation of Cyclohexane
3.5. Catalytic Tests for the Homogeneous Catalysts
3.6. Catalytic Tests for the Heterogenized Catalysts
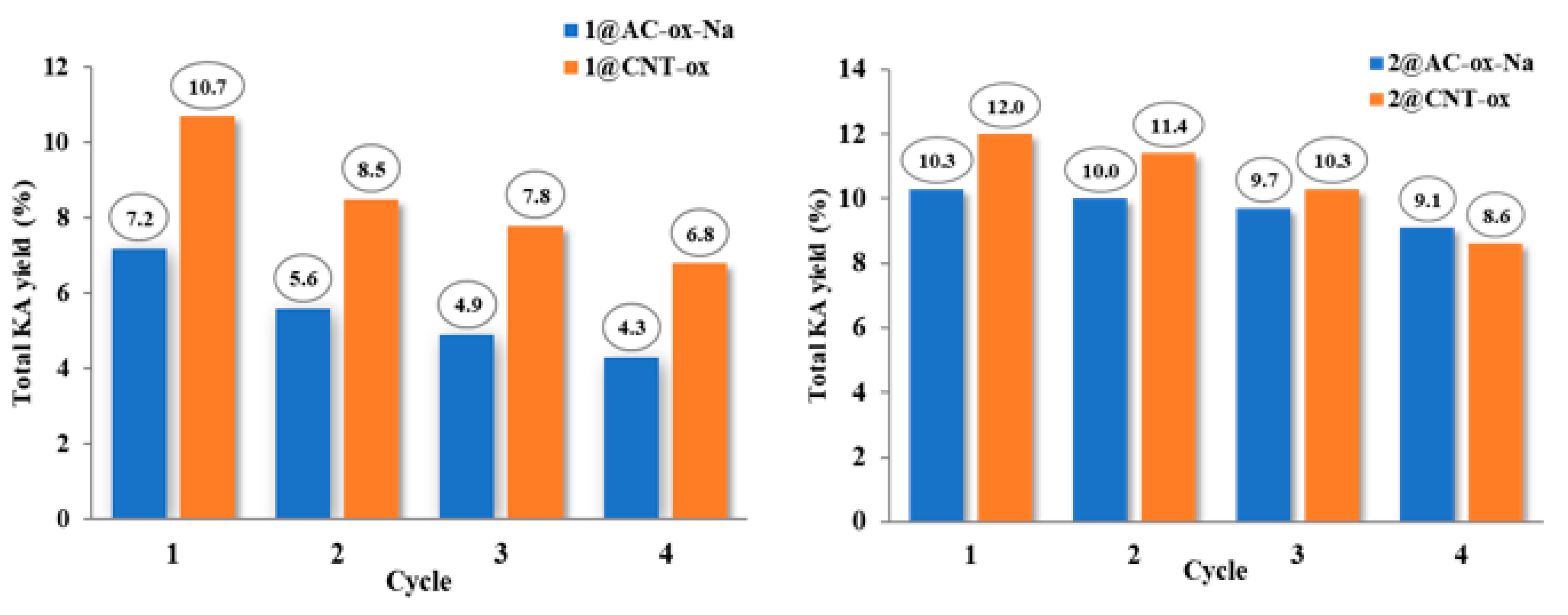
3.7. Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutradhar, M.; da Silva, J.A.L.; Pombeiro, A.J.L. (Eds.) Vanadium Catalysis; Royal Society of Chemistry: London, UK, 2021; ISBN 978-1-78801-857-9. [Google Scholar]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Ligtenbarg, A.G.; Hage, R.; Feringa, B.L. Catalytic oxidations by vanadium complexes. Coord. Chem. Rev. 2003, 237, 89–101. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J. Vanadium Complexes in Catalytic Oxidations. Elsevier Ref. Modul. Chem. Mol. Sci. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective vanadium-catalyzed transformations. An update. Coord. Chem. Rev. 2020, 418, 213395. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301–302, 200–239. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J. Coordination chemistry of non-oxido, oxido and dioxidovanadium(IV/V) complexes with azine fragment ligands. Coord. Chem. Rev. 2014, 265, 89–124. [Google Scholar] [CrossRef]
- Martins, L.M. C-scorpionate complexes: Ever young catalytic tools. Coord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Schwendt, P.; Tatiersky, J.; Krivosudský, L.; Šimuneková, M. Peroxido complexes of vanadium. Coord. Chem. Rev. 2016, 318, 135–157. [Google Scholar] [CrossRef]
- Grant, J.T.; Venegas, J.M.; McDermott, W.P.; Hermans, I. Aerobic Oxidations of Light Alkanes over Solid Metal Oxide Catalysts. Chem. Rev. 2018, 118, 2769–2815. [Google Scholar] [CrossRef]
- Da Silva, J.A.L.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Oxovanadium complexes in catalytic oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Conte, V.; Coletti, A.; Floris, B.; Licini, G.; Zonta, C. Mechanistic aspects of vanadium catalysed oxidations with peroxides. Coord. Chem. Rev. 2011, 255, 2165–2177. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Guedes Da Silva, M.F.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Oxidovanadium(V) Complexes Anchored on Carbon Materials as Catalysts for the Oxidation of 1-Phenylethanol. ChemCatChem 2016, 8, 2254–2266. [Google Scholar] [CrossRef]
- Wang, J.; Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Supported C-Scorpionate Vanadium(IV) Complexes as Reusable Catalysts for Xylene Oxidation. Chem. Asian J. 2017, 12, 1915–1919. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; De Almeida, M.P.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-Scorpionate FeIIComplex on Carbon Materials for Cyclohexane Oxidation with Hydrogen Peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M. Sustainability in Catalytic Cyclohexane Oxidation: The Contribution of Porous Support Materials. Catalysts 2019, 10, 2. [Google Scholar] [CrossRef]
- Choong, E.S. 5 Immobilisation of chiral catalysts: Easy recycling of catalyst and improvement of catalytic efficiencies. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2005, 101, 143–173. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.; Freitas, M.M.; Órfão, J. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Surface Chemistry of Activated Carbons. In Activated Carbon: Classifications, Properties and Applications; Kwiatkowski, J.F., Ed.; Nova Science Pub Inc.: New York, NY, USA, 2011; pp. 125–168. [Google Scholar]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F. Synthesis and functionalization of carbon xerogels to be used as supports for fuel cell catalysts. J. Energy Chem. 2013, 22, 195–201. [Google Scholar] [CrossRef]
- Silva, T.F.; Mac Leod, T.C.; Martins, L.M.; Guedes Da Silva, M.F.C.; Schiavon, M.A.; Pombeiro, A.J. Pyrazole or tris(pyrazolyl)ethanol oxo-vanadium(IV) complexes as homogeneous or supported catalysts for oxidation of cyclohexane under mild conditions. J. Mol. Catal. A Chem. 2013, 367, 52–60. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zheng, D.; Ding, H.; Wang, X.; Yang, X.; Huo, Q.; Guan, J.; Kan, Q. Enhanced Aerobic Epoxidation of Styrene with Copper(II), Cobalt(II), Iron(III), or Oxovanadium(IV) Salen Complexes Immobilized onto Carbon-Coated Fe3O4 Nanoparticles Hybridized with Graphene Sheets. ChemPlusChem 2014, 79, 716–724. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.; Xiong, L.; He, Z.; Wang, T.; Xu, Y.; Huang, K. Oxo-vanadium (IV) complex supported by microporous organic nanotube frameworks: A high selective heterogeneous catalyst for the oxidation of thiols to disulfides. Microporous Mesoporous Mater. 2018, 255, 103–109. [Google Scholar] [CrossRef]
- Salavatiniasari, M.; Bazarganipour, M. Synthesis, Characterization and Liquid Phase Oxidation of Cyclohexane with Hydrogen Peroxide over Oxovanadium(IV) Schiff-base Tetradendate Complex Covalently Anchored to Multi-Wall Carbon Nanotubes (MWNTs). Bull. Korean Chem. Soc. 2009, 30, 355–362. [Google Scholar] [CrossRef][Green Version]
- Mungse, H.P.; Verma, S.; Kumar, N.; Sain, B.; Khatri, O.P. Grafting of oxo-vanadium Schiff base on graphene nanosheets and its catalytic activity for the oxidation of alcohols. J. Mater. Chem. 2012, 22, 5427–5433. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Badiei, A.; Saberyan, K. Oxovanadium(IV) salophen complex covalently anchored to multi-wall carbon nanotubes (MWNTs) as heterogeneous catalyst for oxidation of cyclooctene. Chem. Eng. J. 2011, 173, 651–658. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Li, Z.; Yang, X.; Hu, J.; Huo, Q.; Guan, J.; Kan, Q. Oxovanadium(IV), copper(II) or cobalt(II) acetylacetone complexes immobilized on amino-functionalized CMK-3 for the aerobic epoxidation of styrene. Appl. Organomet. Chem. 2015, 29, 698–706. [Google Scholar] [CrossRef]
- Su, H.; Wu, S.; Li, Z.; Huo, Q.; Guan, J.; Kan, Q. Co(II), Fe(III) or VO(II) Schiff base metal complexes immobilized on graphene oxide for styrene epoxidation. Appl. Organomet. Chem. 2015, 29, 462–467. [Google Scholar] [CrossRef]
- Verma, S.; Aila, M.; Kaul, S.; Jain, S.L. Immobilized oxo-vanadium Schiff base on graphene oxide as an efficient and recyclable catalyst for the epoxidation of fatty acids and esters. RSC Adv. 2014, 4, 30598–30604. [Google Scholar] [CrossRef]
- Dorbes, S.; Pereira, C.; Andrade, M.; Barros, D.C.B.; Pereira, A.; Rebelo, S.; Araujo, J.; Pires, J.; Carvalho, A.; Freire, C. Oxidovanadium(IV) acetylacetonate immobilized onto CMK-3 for heterogeneous epoxidation of geraniol. Microporous Mesoporous Mater. 2012, 160, 67–74. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Ding, H.; Lu, H.; Liu, J.; Huo, Q.; Guan, J.; Kan, Q. Oxovanadium(iv) and iron(iii) salen complexes immobilized on amino-functionalized graphene oxide for the aerobic epoxidation of styrene. New J. Chem. 2013, 37, 4220. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Alegria, E.C.B.A.; Lapa, H.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Cd(ii) coordination compounds as heterogeneous catalysts for microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol. New J. Chem. 2020, 44, 9163–9171. [Google Scholar] [CrossRef]
- Sutradhar, M.; Rajeshwari; Barman, T.R.; Fernandes, A.R.; Paradinha, F.; Roma-Rodrigues, C.; Guedes da Silva, M.F.C.; Pombeiro, A.J. Mixed ligand aroylhydrazone and N-donor heterocyclic Lewis base Cu(II) complexes as potential antiproliferative agents. J. Inorg. Biochem. 2017, 175, 267–275. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Barman, T.R.; Kuznetsov, M.L.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes of different nuclearities in the catalytic oxidation of cyclohexane and cyclohexanol—An experimental and theoretical investigation. New J. Chem. 2019, 43, 17557–17570. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J. Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols. Int. J. Mol. Sci. 2020, 21, 2832. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Iron(iii) and cobalt(iii) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: Catalysts for the microwave assisted oxidation of alcohols. RSC Adv. 2016, 6, 8079–8088. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.; Barman, T.R.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. 1D Copper(II)-Aroylhydrazone Coordination Polymers: Magnetic Properties and Microwave Assisted Oxidation of a Secondary Alcohol. Front. Chem. 2020, 8, 157. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Liu, C.-M.; Kou, H.-Z.; Pombeiro, A.J.L. Cu(ii) complexes of N-rich aroylhydrazone: Magnetism and catalytic activity towards microwave-assisted oxidation of xylenes. Dalton Trans. 2019, 48, 12839–12849. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Alegria, E.C.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Peroxidative Oxidation of Alkanes and Alcohols under Mild Conditions by Di- and Tetranuclear Copper (II) Complexes of Bis (2-Hydroxybenzylidene) Isophthalohydrazide. Molecules 2018, 23, 2699. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.; Barman, T.R.; Scorcelletti, F.; Guedes da Silva, M.F.C.; Pombeiro, A.J. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enol aroylhydrazone Cu(II) complexes. Mol. Catal. 2017, 439, 224–232. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S.; Warren, P. Practical Microwave Synthesis for Organic Chemists; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527314522. [Google Scholar]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis—Catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016.
- Pombeiro, A.J.L.; Guedes da Silva, M.F. (Eds.) Alkane Functionalization; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 9781119378808. [Google Scholar]
- Hermans, I.; Jacobs, P.A.; Peeters, J. To the Core of Autocatalysis in Cyclohexane Autoxidation. Chem. Eur. J. 2006, 12, 4229–4240. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Bruker, APEX2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction; ScienceOpen: Burlington, MA, USA, 2000. [Google Scholar]
- Sheldrick, G.M. SHELX97. Programs for Crystal Structure Analysis (Release 97-2); Royal Society of Chemistry: London, UK, 1997. [Google Scholar]
- Sheldrick, G. A short history ofSHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGXandORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Shul’Pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Shul’Pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’Pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’Pin, G.B.; Kozlov, Y.N.; Shul’Pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef]
- Sutradhar, M.; Shvydkiy, N.V.; Guedes da Silva, M.F.C.; Kirillova, M.V.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’Pin, G.B. A new binuclear oxovanadium(v) complex as a catalyst in combination with pyrazinecarboxylic acid (PCA) for efficient alkane oxygenation by H2O2. Dalton Trans. 2013, 42, 11791. [Google Scholar] [CrossRef]
- Sutradhar, M.; Mukherjee, G.; Drew, M.G.B.; Ghosh, S. Synthesis, Reactivity, and X-ray Crystal Structure of Some Mixed-Ligand Oxovanadium(V) Complexes: First Report of Binuclear Oxovanadium(V) Complexes Containing 4,4′-Bipyridine Type Bridge. Inorg. Chem. 2006, 45, 5150–5161. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Barman, T.R.; Mukherjee, G.; Drew, M.G.; Ghosh, S. Oxidoalkoxidovanadium(V) complexes: Synthesis, characterization and comparison of X-ray crystal structures. Polyhedron 2012, 34, 92–101. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Ghosh, S.; Drew, M.G. Synthesis of a mononuclear oxidovanadium(V) complex by bridge-splitting of the corresponding binuclear precursor. J. Mol. Struct. 2012, 1020, 148–152. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. Available online: https://pubs.rsc.org/en/content/articlelanding/1984/dt/dt9840001349#!divAbstract (accessed on 28 May 2021). [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area and Porosity. J. Electrochem. Soc. 1967, 114, 279C. [Google Scholar] [CrossRef]
- Freire, C.; Silva, A.R. Carbon-Anchored Metal Complex Catalysts. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 267–307. [Google Scholar]
- Dragancea, D.; Talmaci, N.; Shova, S.; Novitchi, G.; Darvasiová, D.; Rapta, P.; Breza, M.; Galanski, M.; Kožíšek, J.; Martins, N.; et al. Vanadium(V) Complexes with Substituted 1,5-bis(2-hydroxybenzaldehyde)carbohydrazones and Their Use As Catalyst Precursors in Oxidation of Cyclohexane. Inorg. Chem. 2016, 55, 9187–9203. [Google Scholar] [CrossRef] [PubMed]
- Rubčić, M.; Milić, D.; Horvat, G.; Đilović, I.; Galic, N.; Tomisic, V.; Cindric, M. Vanadium-induced formation of thiadiazole and thiazoline compounds. Mononuclear and dinuclear oxovanadium(v) complexes with open-chain and cyclized thiosemicarbazone ligands. Dalton Trans. 2009, 44, 9914–9923. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Martins, L.; Carabineiro, S.; Figueiredo, J.; Pombeiro, A. Gold nanoparticles deposited on surface modified carbon materials as reusable catalysts for hydrocarboxylation of cyclohexane. Appl. Catal. A Gen. 2017, 547, 124–131. [Google Scholar] [CrossRef]
- Andrade, M.A.; Mestre, A.S.; Carvalho, A.P.; Pombeiro, A.J.; Martins, L.M. The role of nanoporous carbon materials in catalytic cyclohexane oxidation. Catal. Today 2020, 357, 46–55. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous Catalysts for Liquid-Phase Oxidations: Philosophers’ Stones or Trojan Horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Rezaei, M.; Chermahini, A.N.; Dabbagh, H.A. Green and selective oxidation of cyclohexane over vanadium pyrophosphate supported on mesoporous KIT-6. Chem. Eng. J. 2017, 314, 515–525. [Google Scholar] [CrossRef]
- Zhong, W.; Qiao, T.; Dai, J.; Mao, L.; Xu, Q.; Zou, G.; Liu, X.; Yin, D.; Zhao, F. Visible-light-responsive sulfated vanadium-doped TS-1 with hollow structure: Enhanced photocatalytic activity in selective oxidation of cyclohexane. J. Catal. 2015, 330, 208–221. [Google Scholar] [CrossRef]
- Santra, C.; Shah, S.; Mondal, A.; Pandey, J.K.; Panda, A.B.; Maity, S.; Chowdhury, B. Synthesis, characterization of VPO catalyst dispersed on mesoporous silica surface and catalytic activity for cyclohexane oxidation reaction. Microporous Mesoporous Mater. 2016, 223, 121–128. [Google Scholar] [CrossRef]
- Ottaviani, D.; Van-Dúnem, V.; Carvalho, A.P.; Martins, A.; Martins, L.M. Eco-friendly cyclohexane oxidation by a V-scorpionate complex immobilized at hierarchical MOR zeolite. Catal. Today 2020, 348, 37–44. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
| 1 | 2 | |
|---|---|---|
| Empirical formula | C16H14BrN2O5V | C20H25BrN3O5V |
| Formula weight | 445.14 | 518.28 |
| Crystal system | Triclinic | Orthorhombic |
| Space group | P-1 | Pna21 |
| a/Å | 7.5794(15) | 35.988(3) |
| b/Å | 8.6682(17) | 7.1406(6) |
| c/Å | 13.170(3) | 8.9154(8) |
| α/° | 89.896(7) | 90 |
| β/° | 80.633(8) | 90 |
| γ/° | 80.921(7) | 90 |
| V (Å3) | 842.8(3) | 2291.0(3) |
| Z | 2 | 4 |
| Dcalc (g cm−3) | 1.754 | 1.503 |
| F000 | 444 | 1056 |
| μ(Mo Kα) (mm−1) | 2.989 | 2.212 |
| Rfls. collected/unique/observed | 14,774/3453/3025 | 26,703/4074/3415 |
| Rint | 0.0727 | 0.0969 |
| Nº parameters | 250 | 310 |
| Final R1 a, wR2 b (I ≥ 2σ) | 0.0657, 0.1779 | 0.0969, 0.2199 |
| Goodness-of-fit on F2 | 1.029 | 1.157 |
| Sample | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | CO2 Desorbed (µmol/g) | CO Desorbed (µmol/g) |
|---|---|---|---|---|---|
| AC | 974 | 0.67 | 0.348 | 179 | 643 |
| AC-ox | 914 | 0.62 | 0.324 | 2596 | 4930 |
| AC-ox-Na | 610 | 0.35 | 0.251 | 2883 | 5012 |
| CNT | 257 | 2.89 | ~0 | 89 | 142 |
| CNT-ox | 400 | 1.89 | ~0 | 729 | 1475 |
| CNT-ox-Na | 350 | 1.45 | ~0 | 838 | 1079 |
| Samples | Metal (%, p/p) | |
|---|---|---|
| 1 | 2 | |
| AC | 0.25 | 0.87 |
| AC-ox | 0.19 | 0.85 |
| AC-ox-Na | 1.12 | 0.65 |
| CNT | 1.51 | 0.53 |
| CNT-ox | 1.14 | 0.76 |
| CNT-ox-Na | 0.83 | 0.36 |
| Entry. | Catalyst | TON b | TOF (h−1) | Yield (%) c | ||
|---|---|---|---|---|---|---|
| A | K | Total | ||||
| 1 | 1 | 61 | 31 | 3.0 | 3.1 | 6.1 |
| 2 | 1@AC | 50 | 25 | 1.8 | 2.2 | 4.0 |
| 3 | 1@AC-ox | 72 | 36 | 4.0 | 3.2 | 7.2 |
| 4 | 1@AC-ox-Na | 68 | 34 | 3.2 | 3.6 | 6.8 |
| 5 | 1@CNT | 77 | 39 | 4.0 | 3.7 | 7.7 |
| 6 | 1@CNT-ox | 107 | 54 | 5.4 | 5.3 | 10.7 |
| 7 | 1@CNT-ox-Na | 72 | 36 | 3.3 | 3.9 | 7.2 |
| 8 | 2 | 74 | 37 | 2.7 | 4.7 | 7.4 |
| 9 | 2@AC | 40 | 20 | 2.4 | 2.6 | 5.0 |
| 10 | 2@AC-ox | 83 | 42 | 5.0 | 3.3 | 8.3 |
| 11 | 2@AC-ox-Na | 103 | 52 | 4.6 | 5.7 | 10.3 |
| 12 | 2@CNT | 107 | 54 | 5.2 | 5.5 | 10.7 |
| 13 | 2@CNT-ox | 120 | 60 | 7.0 | 5.0 | 12.0 |
| 14 | 2@CNT-ox-Na | 72 | 36 | 3.7 | 3.1 | 7.2 |
| 15 | - | 6 | 3 | 0.4 | 0.2 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, M.; Andrade, M.A.; Carabineiro, S.A.C.; Martins, L.M.D.R.S.; Guedes da Silva, M.d.F.C.; Pombeiro, A.J.L. Oxido- and Dioxido-Vanadium(V) Complexes Supported on Carbon Materials: Reusable Catalysts for the Oxidation of Cyclohexane. Nanomaterials 2021, 11, 1456. https://doi.org/10.3390/nano11061456
Sutradhar M, Andrade MA, Carabineiro SAC, Martins LMDRS, Guedes da Silva MdFC, Pombeiro AJL. Oxido- and Dioxido-Vanadium(V) Complexes Supported on Carbon Materials: Reusable Catalysts for the Oxidation of Cyclohexane. Nanomaterials. 2021; 11(6):1456. https://doi.org/10.3390/nano11061456
Chicago/Turabian StyleSutradhar, Manas, Marta A. Andrade, Sónia A. C. Carabineiro, Luísa M. D. R. S. Martins, Maria de Fátima C. Guedes da Silva, and Armando J. L. Pombeiro. 2021. "Oxido- and Dioxido-Vanadium(V) Complexes Supported on Carbon Materials: Reusable Catalysts for the Oxidation of Cyclohexane" Nanomaterials 11, no. 6: 1456. https://doi.org/10.3390/nano11061456
APA StyleSutradhar, M., Andrade, M. A., Carabineiro, S. A. C., Martins, L. M. D. R. S., Guedes da Silva, M. d. F. C., & Pombeiro, A. J. L. (2021). Oxido- and Dioxido-Vanadium(V) Complexes Supported on Carbon Materials: Reusable Catalysts for the Oxidation of Cyclohexane. Nanomaterials, 11(6), 1456. https://doi.org/10.3390/nano11061456











