Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Characterization
2.2.1. Thermal Annealing
2.2.2. Atomic Force Microscopy
2.2.3. Scanning Electron Microscopy
2.2.4. X-ray Diffraction
2.2.5. Raman Spectroscopy
3. Results and Discussion
3.1. Study of as-Grown TiO2 versus Thickness
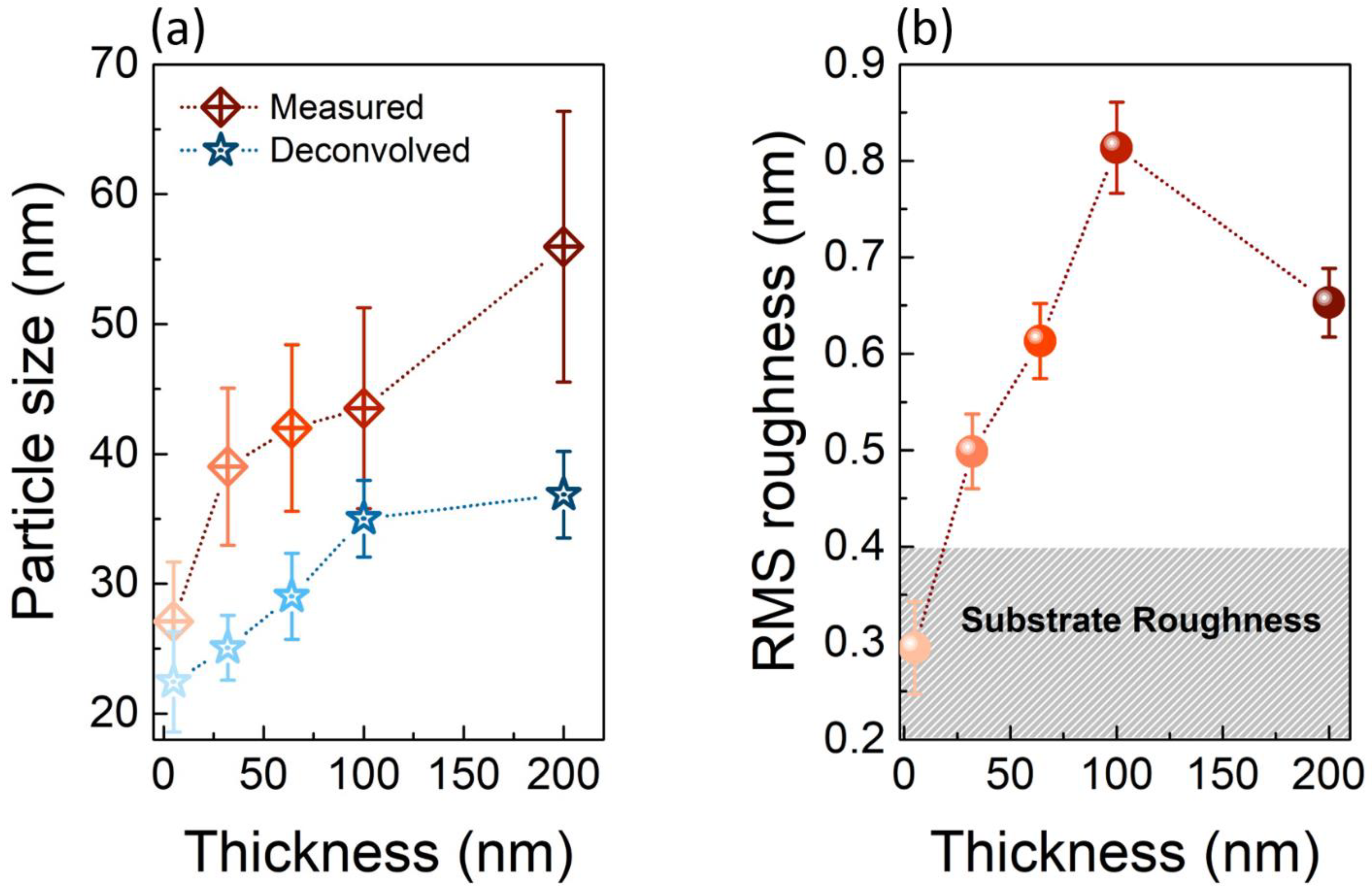
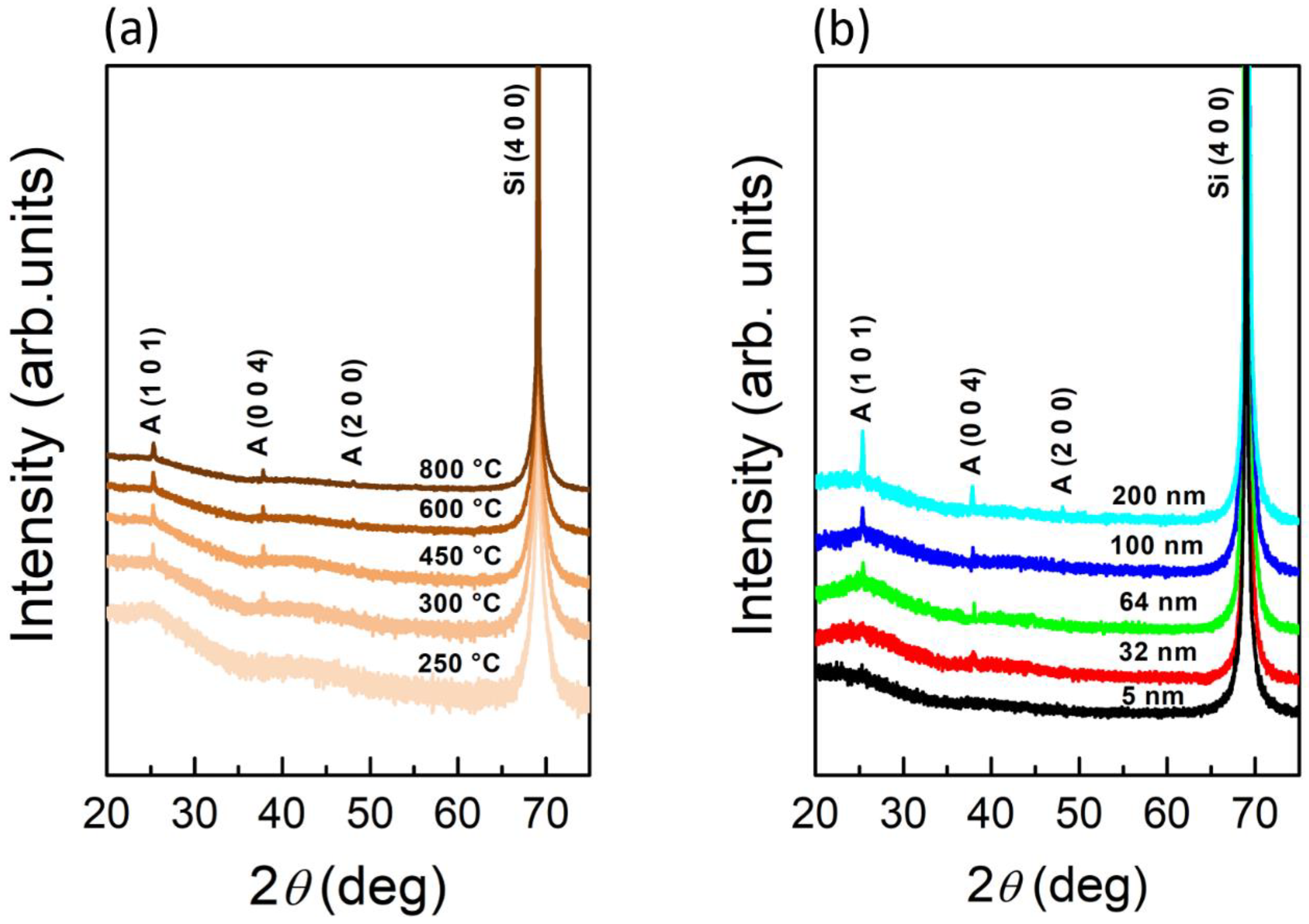
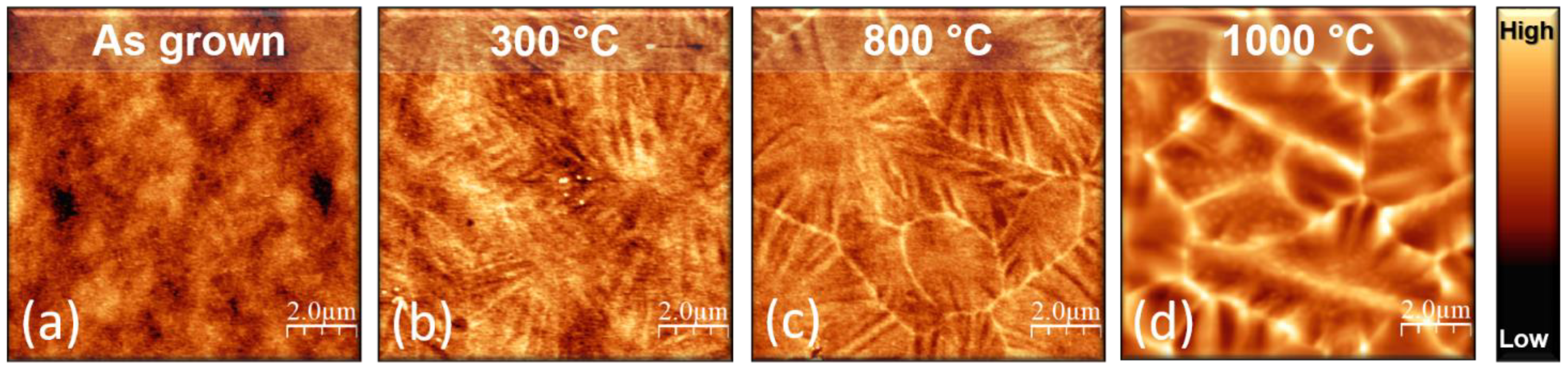
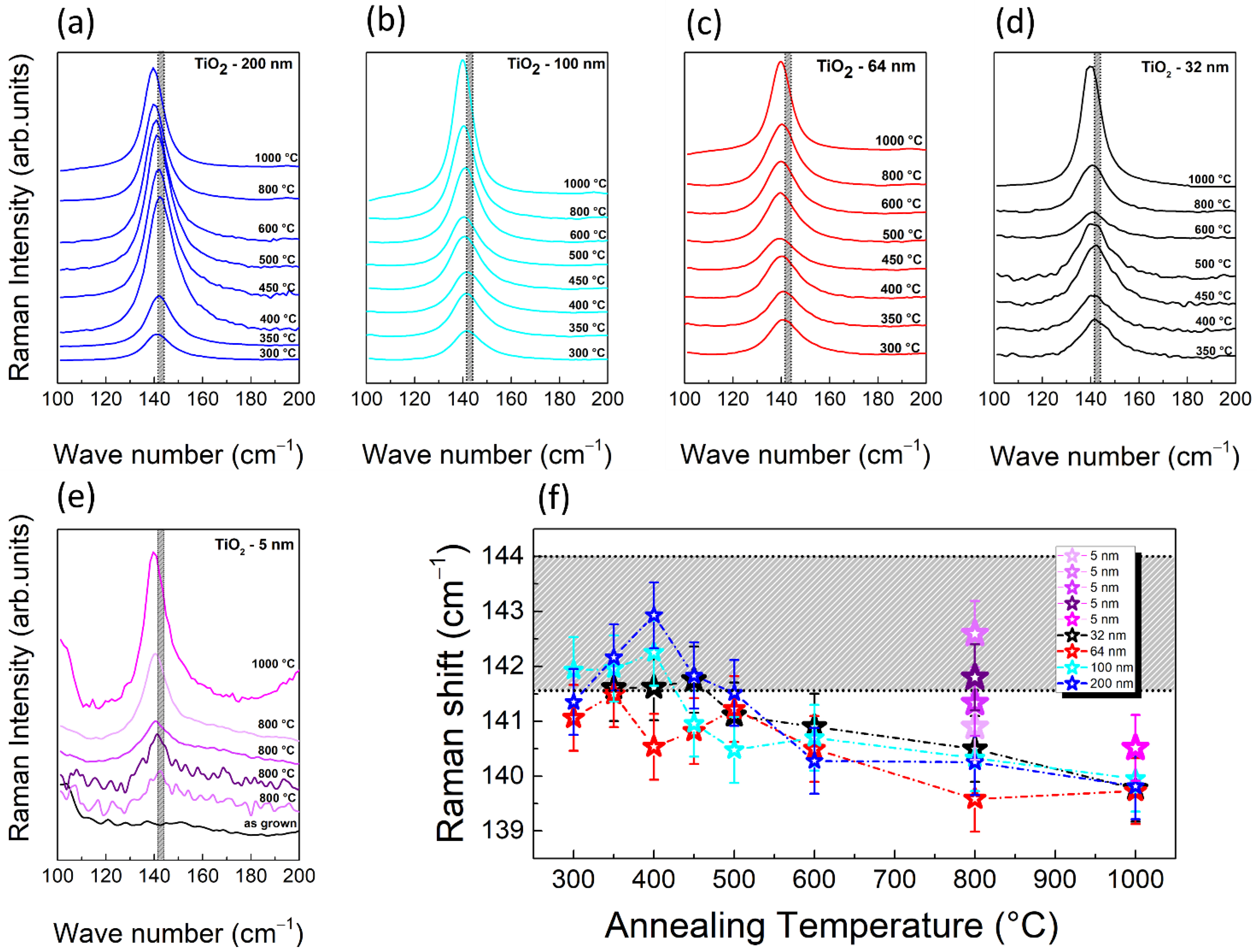
3.2. Study of TiO2 Crystallization Onset versus Thickness
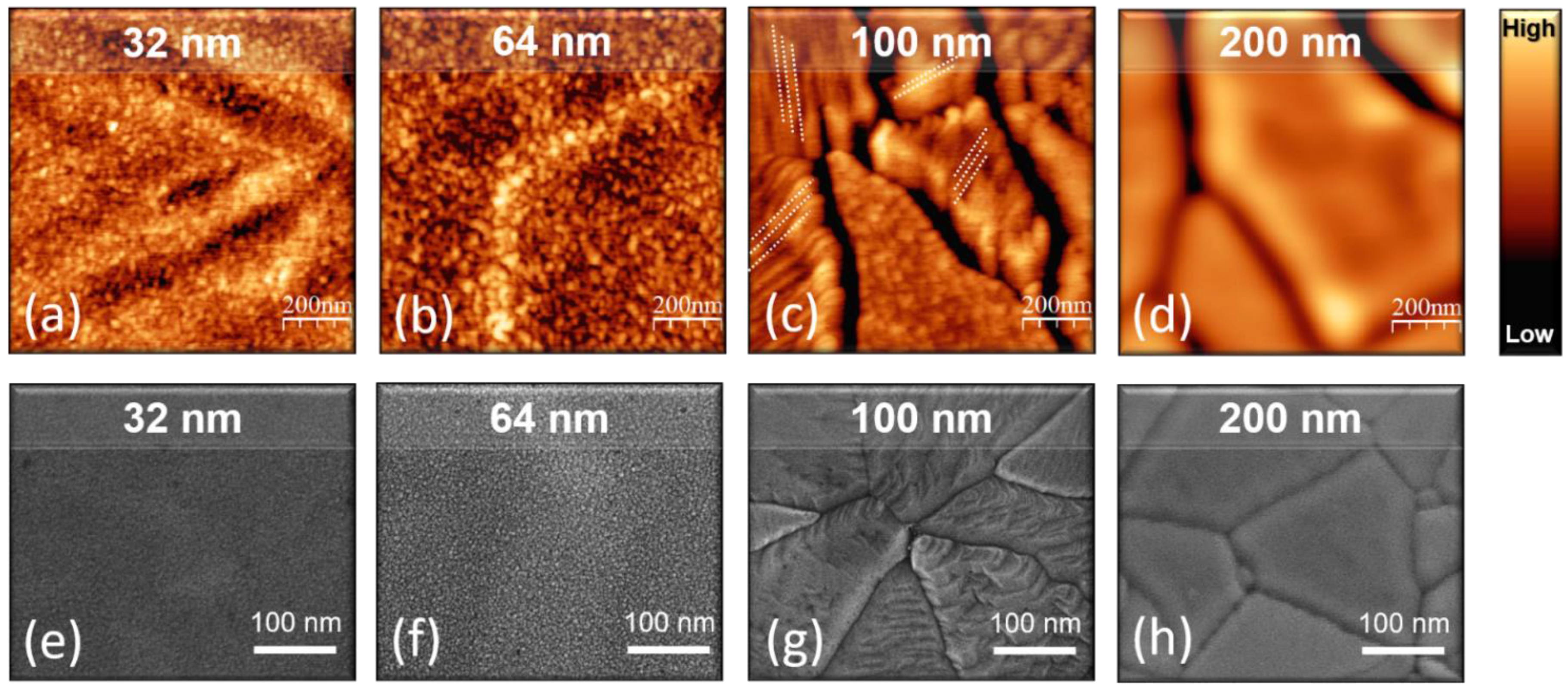
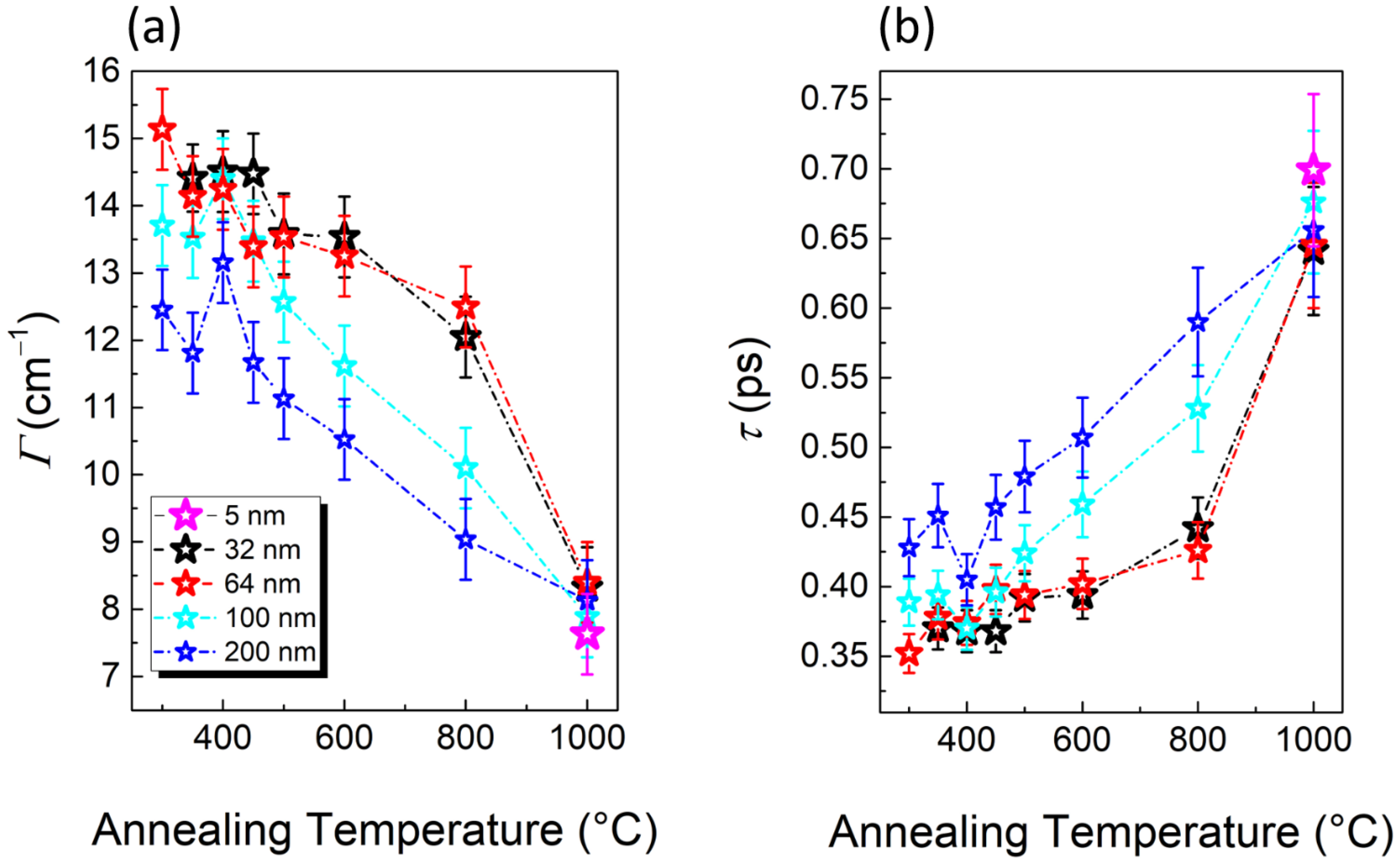
3.3. Study of TiO2 Crystallization Evolution versus Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–345. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Orudzhev, F.; Ramazanov, S.; Sobola, D.; Isaev, A.; Wang, C.; Magomedova, A.; Kadiev, M.; Kaviyarasu, K. Atomic Layer Deposition of Mixed-Layered Aurivillius Phase on TiO2 Nanotubes: Synthesis Characterization and Photoelectrocatalytic Properties. Nanomaterials 2020, 10, 2183. [Google Scholar] [CrossRef]
- Malinowski, S.; Presečki, I.; Jajčinović, I.; Brnardić, I.; Mandić, V.; Grčić, I. Intensification of Dihydroxybenzenes Degradation over Immobilized TiO2 Based Photocatalysts under Simulated Solar Light. Appl. Sci. 2020, 10, 7571. [Google Scholar] [CrossRef]
- Atalay, S.; Izgi, T.; Kolat, V.S.; Erdemoglu, S.; Inan, O.O. Magnetoelastic humidity sensors with TiO2 nanotube sensing layers. Sensors 2020, 20, 425. [Google Scholar] [CrossRef]
- Bao, S.J.; Li, C.M.; Zang, J.F.; Cui, X.Q.; Qiao, Y.; Guo, J. New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Guo, C.; Kong, M. Fabrication of Ultralow Stress TiO2/SiO2 Optical Coatings by Plasma Ion-Assisted Deposition. Coatings 2020, 10, 720. [Google Scholar] [CrossRef]
- Kulczyk-Malecka, J.; Kelly, P.J.; West, G.; Clarke, G.C.; Ridealgh, J.A. Characterisation studies of the structure and properties of as-deposited and annealed pulsed magnetron sputtered titania coatings. Coatings 2013, 3, 166–176. [Google Scholar] [CrossRef]
- Pierro, V.; Fiumara, V.; Chiadini, F.; Bobba, F.; Carapella, G.; Di Giorgio, C.; Durante, O.; Fittipaldi, R.; Mejuto Villa, E.; Neilson, J.; et al. On the performance limits of coatings for gravitational wave detectors made of alternating layers of two materials. Opt. Mater. 2019, 96, 109269. [Google Scholar] [CrossRef]
- Pierro, V.; Fiumara, V.; Chiadini, F.; Granata, V.; Di Giorgio, C.; Durante, O.; Neilson, J.; Fittipaldi, R.; Carapella, G.; Bobba, F.; et al. Ternary Quarter Wavelength Coatings for Gravitational Wave Detector Mirrors: Design Optimization via Exhaustive Search. arXiv 2021, arXiv:2012.02146. accepted by Phys. Rev. Res. [Google Scholar]
- Harry, G.; Bodiya, T.P.; DeSalvo, R. Optical Coatings and Thermal Noise in Precision Measurement, 1st ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Tsvetkov, N.; Larina, L.; Ku Kang, J.; Shevaleevskiy, O. Sol-gel processed TiO2 nanotube photoelectrodes for dye-sensitized solar cells with enhanced photovoltaic performance. Nanomaterials 2020, 10, 296. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Xu, C.; Fan, D.; Cao, Y.; Xue, W. Laser sintering of TiO2 films for flexible dye-sensitized solar cells. Appl. Sci. 2019, 9, 823. [Google Scholar] [CrossRef]
- Shin, J.; Kim, I.; Biju, K.P.; Jo, M.; Park, J.; Lee, J.; Hwang, H. TiO2-based metal-insulator-metal selection device for bipolar resistive random access memory cross-point application. J. Appl. Phys. 2011, 109, 033712. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The preparation of TiO2 film by the sol-gel method and evaluation of its self-cleaning property. Materials 2018, 11, 450. [Google Scholar] [CrossRef]
- Liao, T.W.; Verbruggen, S.W.; Claes, N.; Yadav, A.; Grandjean, D.; Bals, S.; Lievens, P. TiO2 films modified with Au nanoclusters as self-cleaning surfaces under visible light. Nanomaterials 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Prasai, B.; Cai, B.; Underwood, M.K.; Lewis, J.P.; Drabold, D.A. Properties of amorphous and crystalline titanium dioxide from first principles. J. Mater. Sci. 2012, 47, 7515–7521. [Google Scholar] [CrossRef]
- Casu, A.; Lamberti, A.; Stassi, S.; Falqui, A. Crystallization of TiO2 nanotubes by in situ heating TEM. Nanomaterials 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Dorow-Gerspach, D.; Mergel, D.; Wuttig, M. Effects of Different Amounts of Nb Doping on Electrical, Optical and Structural Properties in Sputtered TiO2− x Films. Crystals 2021, 11, 301. [Google Scholar] [CrossRef]
- Park, J.S.; Maeng, W.J.; Kim, H.S.; Park, J.S. Review of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Films 2012, 520, 1679–1693. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Chu, C.; Majeed, M.K.; Yang, J. Mesoporous Amorphous silicon: A simple synthesis of a high-rate and long-life anode material for lithium-ion batteries. Angew. Chem. Ger. Edit. 2016, 128, 14269–14272. [Google Scholar] [CrossRef]
- Raoux, S. Phase change materials. Ann. Rev. Mater. Res. 2009, 39, 25–48. [Google Scholar] [CrossRef]
- Thielsch, R.; Gatto, A.; Heber, J.; Kaiser, N. A comparative study of the UV optical and structural properties of SiO2, Al2O3, and HfO2 single layers deposited by reactive evaporation, ion-assisted deposition and plasma ion-assisted deposition. Thin Solid Films 2002, 410, 86–93. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, H.C.; Jaing, C.C. Effect of thermal annealing on the optical properties and residual stress of TiO2 films produced by ion-assisted deposition. Appl. Opt. 2005, 44, 2996–3000. [Google Scholar] [CrossRef]
- Martin, P.J.; MacLeod, H.A.; Netterfield, R.P.; Pacey, C.G.; Sainty, W.G. Ion-beam-assisted deposition of thin films. Appl. Opt. 1983, 22, 178–184. [Google Scholar] [CrossRef]
- Wei, C.H.; Chang, C.M. Polycrystalline TiO2 Thin Films with Different Thicknesses Deposited on Unheated Substrates Using RF Magnetron Sputtering. Mater. Trans. 2011, 3, 554–559. [Google Scholar] [CrossRef]
- Mikhelashvili, V.; Eisenstein, G. Effects of annealing conditions on optical and electrical characteristics of titanium dioxide films deposited by electron beam evaporation. J. Appl. Phys. 2001, 89, 3256–3269. [Google Scholar] [CrossRef]
- Mahdjoub, N.; Allen, N.; Kelly, P.; Vishnyakov, V. Thermally induced phase and photocatalytic activity evolution of polymorphous titania. J. Photoch. Photobio. A 2010, 210, 125–129. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Im, H.S. The effect of target density and its morphology on TiO2 thin films grown on Si (100) by PLD. Appl. Surf. Sci. 1999, 151, 6–16. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Sekiya, T.; Ohta, S.; Kamei, S.; Hanakawa, M.; Kurita, S. Raman spectroscopy and phase transition of anatase TiO2 under high pressure. J. Phys. Chem. Solids 2001, 62, 717–721. [Google Scholar] [CrossRef]
- Mechiakh, R.; Meriche, F.; Kremer, R.; Bensaha, R.; Boudine, B.; Boudrioua, A. TiO2 thin films prepared by sol–gel method for waveguiding applications: Correlation between the structural and optical properties. Opt. Mater. 2007, 30, 645–651. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stabil. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Wang, S.F.; Hsu, Y.F.; Lee, Y.S. Microstructural evolution and optical properties of doped TiO2 films prepared by RF magnetron sputtering. Ceram. Int. 2006, 32, 121–125. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, K.W.; Stroscio, M.A.; Dutta, M. Optical-phonon confinement and scattering in wurtzite heterostructures. Phys. Rev. B 1998, 58, 4860. [Google Scholar] [CrossRef]
- Tanino, H.; Kuprin, A.; Deai, H.; Koshida, N. Raman study of free-standing porous silicon. Phys. Rev. B 1996, 53, 1937. [Google Scholar] [CrossRef]
- Colombi, P.; Alessandri, I.; Bergese, P.; Federici, S.; Depero, L.E. Self-assembled polystyrene nanospheres for the evaluation of atomic force microscopy tip curvature radius. Meas. Sci. Technol. 2009, 20, 84015. [Google Scholar] [CrossRef]
- Ostwald, W.Z. Blocking of Ostwald ripening allowing long-term stabilization. Phys. Chem. 1901, 37, 385. [Google Scholar]
- Zacharias, M.; Streitenberger, P. Crystallization of amorphous superlattices in the limit of ultrathin films with oxide interfaces. Phys. Rev. B 2000, 62, 8391. [Google Scholar] [CrossRef]
- Nichtová, L.; Kužel, R.; Matěj, Z.; Šícha, J.; Musil, Z. Time and thickness dependence of crystallization of amorphous magnetron deposited TiO2 thin films. Kristallogr. Suppl. 2009, 30, 235–240. [Google Scholar] [CrossRef]
- Reklaitis, I.; Radiunas, E.; Malinauskas, T.; Stanionytė, S.; Juška, G.; Ritasalo, R.; Pilvi, T.; Taeger, S.; Strassburge, M.; Tomašiūnas, R. A comparative study on atomic layer deposited oxide film morphology and their electrical breakdown. Surf. Coat. Tech. 2020, 399, 126123. [Google Scholar] [CrossRef]
- Kim, Y.H.; Osada, M.; Dong, L.; Kim, H.J.; Sasaki, T. High-temperature dielectric responses of molecularly-thin titania nanosheet. J. Ceram. Soc. Jpn. 2015, 123, 335–339. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Zhang, K.; Liu, C.; Yu, A.; Peng, M.; Zhai, J. Raman study of 2D anatase TiO2 nanosheets. Phys. Chem. Chem. Phys. 2016, 18, 32178–32184. [Google Scholar] [CrossRef] [PubMed]
- Wrana, D.; Rodenbücher, C.; Jany, B.R.; Kryshtal, O.; Cempura, G.; Kruk, A.; Indyka, P.; Szot, K.; Krok, F. A bottom-up process of self-formation of highly conductive titanium oxide (TiO2) nanowires on reduced SrTiO3. Nanoscale 2019, 11, 89–97. [Google Scholar] [CrossRef] [PubMed]
- RRUFF. Available online: https://rruff.info/chem=Ti,O/display=default/R070582 (accessed on 18 April 2021).
- Matěj, Z.; Kužel, R.; Nichtová, L. X-ray diffraction analysis of residual stress in thin polycrystalline anatase films and elastic anisotropy of anatase. Metall. Mater. Trans. A 2011, 42, 3323–3332. [Google Scholar] [CrossRef]
- Kužel, R.; Nichtová, L.; Matěj, Z.; Musil, J. In-situ X-ray diffraction studies of time and thickness dependence of crystallization of amorphous TiO2 thin films and stress evolution. Thin Solid Films 2010, 519, 1649–1654. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Menendez, J.; Cardona, M. Temperature dependence of the first-order Raman scattering by phonons in Si, Ge, and α− S n: Anharmonic effects. Phys. Rev. B 1984, 29, 2051. [Google Scholar] [CrossRef]
- Klemens, P.G. Anharmonic decay of optical phonons. Phys. Rev. 1966, 148, 845. [Google Scholar] [CrossRef]
- Maradudin, A.A. Theoretical and Experimental Aspects of the Effects of Point Defects and Disorder on the Vibrations of Crystals. Solid State Phys. 1966, 18, 273–420. [Google Scholar]
- Cuscó, R.; Alarcón-Lladó, E.; Ibanez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Serrano, J.; Manjón, F.J.; Romero, A.H.; Widulle, F.; Lauck, R.; Cardona, M. Dispersive phonon linewidths: The E2 phonons of ZnO. Phys. Rev. Lett. 2003, 90, 55510. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durante, O.; Di Giorgio, C.; Granata, V.; Neilson, J.; Fittipaldi, R.; Vecchione, A.; Carapella, G.; Chiadini, F.; DeSalvo, R.; Dinelli, F.; et al. Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study. Nanomaterials 2021, 11, 1409. https://doi.org/10.3390/nano11061409
Durante O, Di Giorgio C, Granata V, Neilson J, Fittipaldi R, Vecchione A, Carapella G, Chiadini F, DeSalvo R, Dinelli F, et al. Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study. Nanomaterials. 2021; 11(6):1409. https://doi.org/10.3390/nano11061409
Chicago/Turabian StyleDurante, Ofelia, Cinzia Di Giorgio, Veronica Granata, Joshua Neilson, Rosalba Fittipaldi, Antonio Vecchione, Giovanni Carapella, Francesco Chiadini, Riccardo DeSalvo, Franco Dinelli, and et al. 2021. "Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study" Nanomaterials 11, no. 6: 1409. https://doi.org/10.3390/nano11061409
APA StyleDurante, O., Di Giorgio, C., Granata, V., Neilson, J., Fittipaldi, R., Vecchione, A., Carapella, G., Chiadini, F., DeSalvo, R., Dinelli, F., Fiumara, V., Pierro, V., Pinto, I. M., Principe, M., & Bobba, F. (2021). Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study. Nanomaterials, 11(6), 1409. https://doi.org/10.3390/nano11061409








