Photodehydrogenation of Ethanol over Cu2O/TiO2 Heterostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Photocatalysts
2.3. Structural and Chemical Characterization
2.4. Photoelectrochemical Measurements
2.5. Photocatalytic Test
2.6. Apparent Quantum Yield (AQY) Calculation
3. Results and Discussion
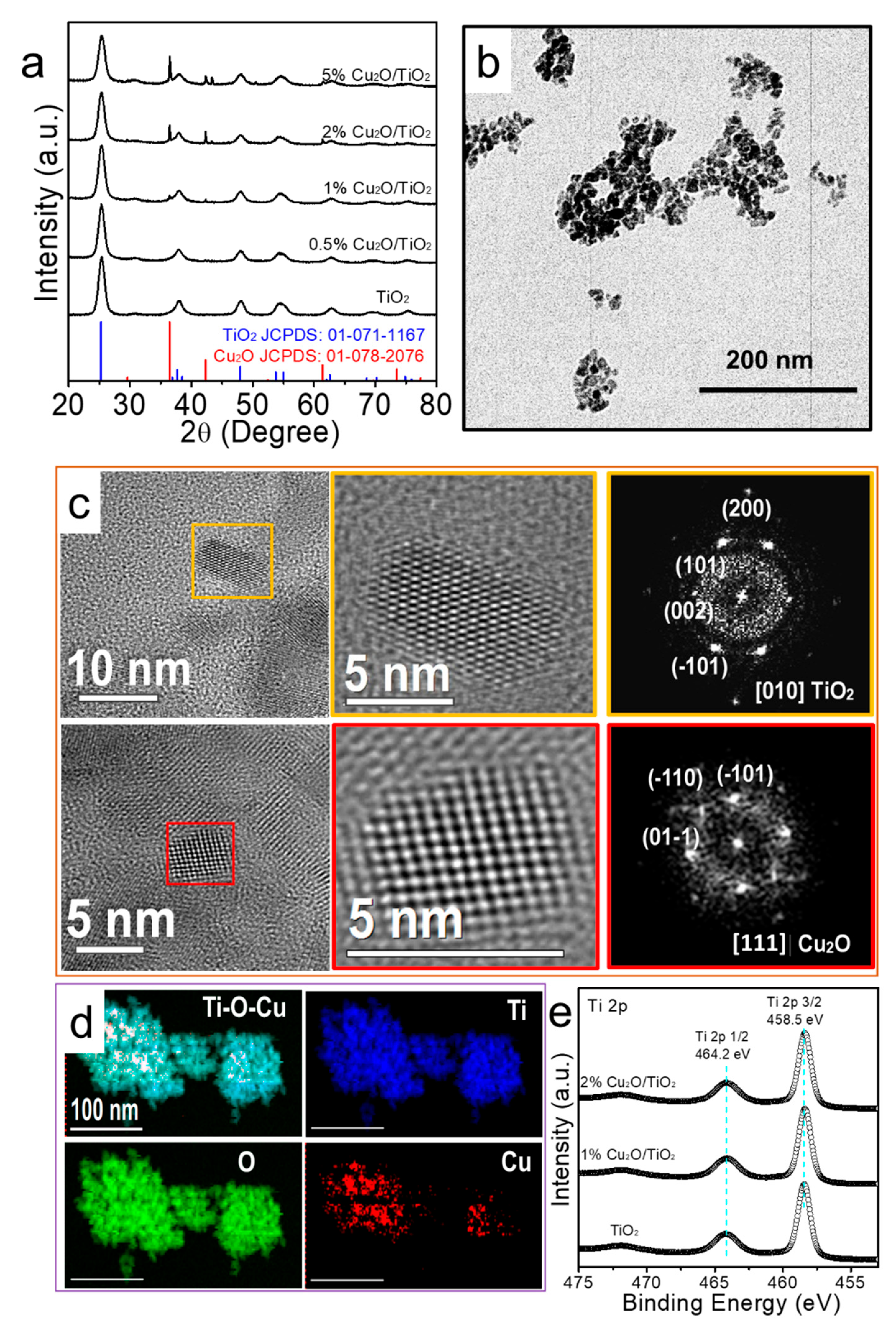
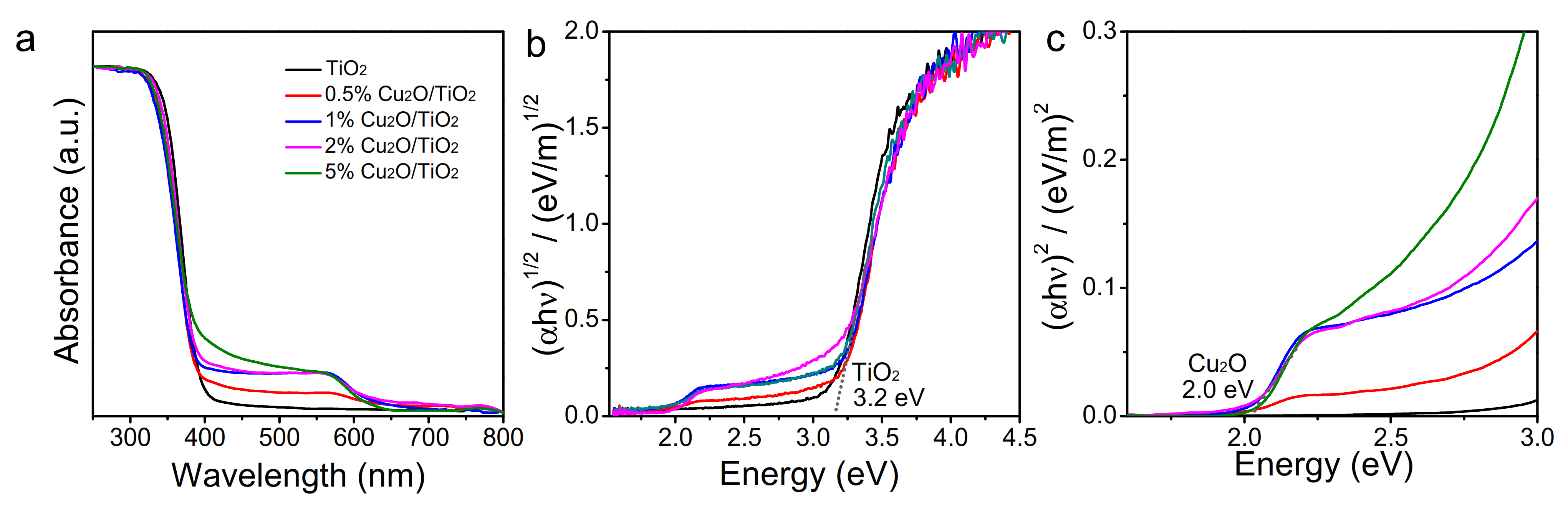
3.1. Structural, Chemical and Optical Properties
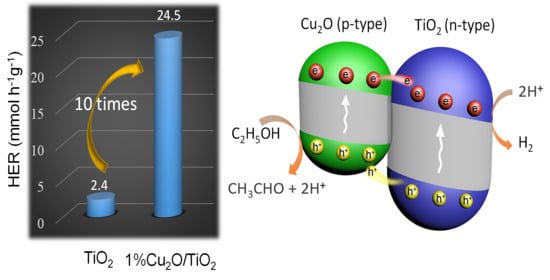
3.2. Photocatalytic Activity
3.3. Photoluminescence and Photoconductivity
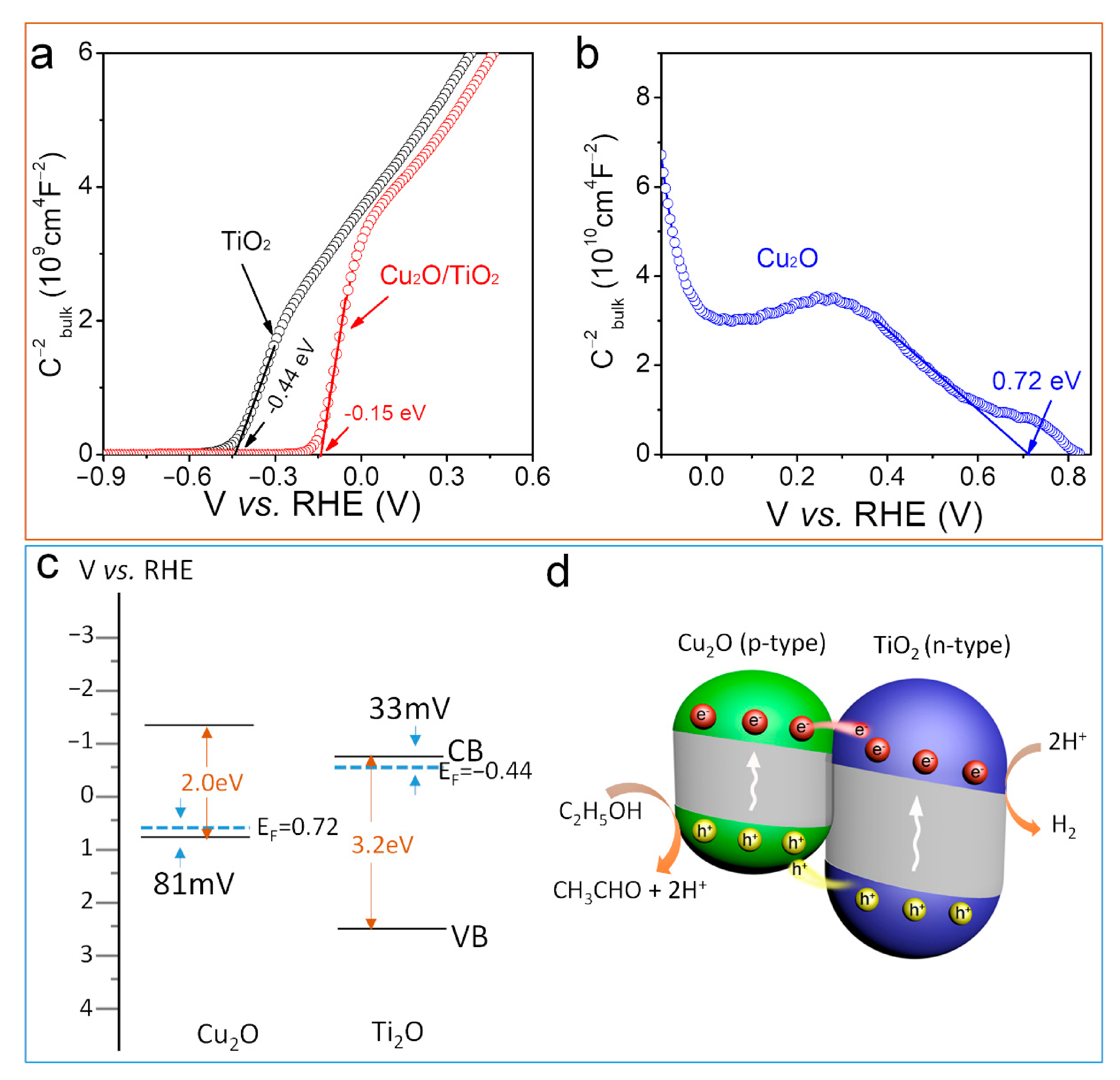
3.4. Determination of Heterojunction Band Position
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Wang, B.; Yuan, H.; Sun, Y.; Yang, D.; Cui, X.; Shi, F. The Catalytic Dehydrogenation of Ethanol by Heterogeneous Catalysts. Catal. Sci. Technol. 2021, 11, 1652–1664. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic Production of Hydrogen from Biomass-Derived Feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt Doped TiO2: A Stable and Efficient Photocatalyst for Continuous Hydrogen Production from Glycerol: Water Mixtures under Solar Light Irradiation. Int. J. Hydrog. Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schaeffer, H.; Hayes, G.; Ismat-Shah, S. Photocatalytic Degradation of 2-Chlorophenol by Co-doped TiO2 Nanoparticles. Appl. Catal. B Environ. 2005, 57, 23–30. [Google Scholar] [CrossRef]
- Nakhate, G.G.; Nikam, V.S.; Kanade, K.G.; Arbuj, S.; Kale, B.B.; Baeg, J.O. Hydrothermally Derived Nanosized Ni-doped TiO2: A Visible Light Driven Photocatalyst for Methylene Blue Degradation. Mater. Chem. Phys. 2010, 124, 976–981. [Google Scholar] [CrossRef]
- Ampelli, C.; Genovese, C.; Passalacqua, R.; Perathoner, S.; Centi, G. A gas-phase reactor powered by solar energy and ethanol for H2 production. Appl. Therm. Eng. 2014, 70, 1270–1275. [Google Scholar] [CrossRef]
- Ying Toe, C.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing Photoreforming of Organics: Highlights on Photocatalyst and System Designs for Selective Oxidation Reactions. Energy Environ. Sci. 2021, 14, 1140. [Google Scholar] [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Delgado, J.J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Aqueous Solutions. ChemCatChem 2011, 3, 574–577. [Google Scholar] [CrossRef]
- Sangpour, P.; Hashemi, F.; Moshfegh, A.Z. Photoenhanced Degradation of Methylene Blue on Cosputtered M:TiO2 (M = Au, Ag, Cu) Nanocomposite Systems: A Comparative Study. J. Phys. Chem. C 2010, 114, 13955–13961. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 Nanotubes Photoanode for Enhanced Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol Production from Agricultural Wastes: An Overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sust. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Rulkens, W. Sewage Sludge as a Biomass Resource for the Production of Energy: Overview and Assessment of the Various Options. Energy Fuels 2008, 22, 9–15. [Google Scholar] [CrossRef]
- Ampelli, C.; Passalacqua, R.; Genovese, C.; Perathoner, S.; Centi, G.; Montini, T.; Gombac, V.; Delgado Jaen, J.J.; Fornasiero, P. H2 Production by Selective Photo-Dehydrogenation of Ethanol in Gas and Liquid Phase on CuOx/TiO2 Nanocomposites. RSC Adv. 2013, 3, 21776–21788. [Google Scholar] [CrossRef]
- Muscetta, M.; Andreozzi, R.; Clarizia, L.; Di Somma, I.; Marotta, R. Hydrogen Production through Photoreforming Processes over Cu2O/TiO2 Composite Materials: A Mini-Review. Int. J. Hydrog. Energy 2020, 45, 28531–28552. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalysis for Hydrogen Production and CO2 Reduction: The Case of Copper-Catalysts. ChemCatChem 2019, 11, 368–382. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Feng, B. Facile Synthesis of CuxO(x = 1, 2)/TiO2 Nanotube Arrays as an Efficient Visible-Light Driven Photocatalysts. J. Porous Mater. 2017, 24, 97–102. [Google Scholar] [CrossRef]
- Kubacka, A.; Muñoz, M.J.; Muñoz-Batista, M.; Fernández-García, M.; Obregón, S.; Colón, G. Evolution of H2 Photoproduction with Cu Content on CuOx-TiO2 Composite Catalysts Prepared by a Microemulsion Method. Appl. Catal. B Environ. 2015, 163, 214–222. [Google Scholar] [CrossRef]
- Sorcar, S.; Yoriya, S.; Lee, H.; Grimes, C.A.; Feng, S.P. A Review of Recent Progress in Gas Phase CO2 Reduction and Suggestions on Future Advancement. Mater. Today Chem. 2020, 16, 100264. [Google Scholar] [CrossRef]
- Xing, J.; Chen, Z.P.; Xiao, F.Y.; Ma, X.Y.; Wen, C.Z.; Li, Z.; Yang, H.G. Cu-Cu2O-TiO2 Nanojunction Systems with an Unusual Electron-Hole Transportation Pathway and Enhanced Photocatalytic Properties. Chem. Asian J. 2013, 8, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Obregón, S.; Muñoz, M.J.; Muñoz-Batista, M.; Fernández-García, M.; Kubacka, A.; Colón, G. Cu-TiO2 Systems for the Photocatalytic H2 Production: Influence of Structural and Surface Support Features. Appl. Catal. B Environ. 2015, 179, 468–478. [Google Scholar] [CrossRef]
- Bonmatí, E.; Casanovas, A.; Angurell, I.; Llorca, J. Hydrogen Photoproduction from Ethanol-Water Mixtures over Au-Cu Alloy Nanoparticles Supported on TiO2. Top. Catal. 2015, 58, 77–84. [Google Scholar] [CrossRef]
- Sahu, M.; Biswas, P. Single-Step Processing of Copper-Doped Titania Nanomaterials in a Flame Aerosol Reactor. Nanoscale Res. Lett. 2011, 6, 441. [Google Scholar] [CrossRef]
- Brant, A.T.; Yang, S.; Giles, N.C.; Iqbal, M.Z.; Manivannan, A.; Halliburton, L.E. Oxygen Vacancies Adjacent to Cu2+ ions in TiO2 (rutile) Crystals. J. Appl. Phys. 2011, 109, 073711. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Huang, W. Influences of TiO2 Phase Structures on the Structures and Photocatalytic Hydrogen Production of CuOx/TiO2 Photocatalysts. Appl. Surf. Sci. 2016, 389, 760–767. [Google Scholar] [CrossRef]
- Losito, I.; Amorisco, A.; Palmisano, F.; Zambonin, P.G. X-ray Photoelectron Spectroscopy Characterization of Composite TiO2-Poly(vinylidenefluoride) Films Synthesised for Applications in Pesticide Photocatalytic Degradation. Appl. Surf. Sci. 2005, 240, 180–188. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Chen, H.-R.; Zeng, Z.-X.; Lei, B. Investigation on Mechanism of Photocatalytic Activity Enhancement of Nanometer Cerium-doped Titania. Appl. Surf. Sci. 2006, 252, 8565–8570. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Liang, D.-H.; Liu, M.-L.; Liu, D.-Z. Preparation and Characterization of Cu2O-TiO2: Efficient Photocatalytic Degradation of Methylene Blue. Mater. Res. Bull. 2008, 43, 3474–3482. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 Heterostructures for CO2 Reduction through a Direct Z-scheme: Protecting Cu2O from Photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface Oxidation and Reduction of CuO and Cu2O Studied Using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Z.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Yang, X.; Ma, J. Synthesis of Cu2O Octadecahedron/TiO2 Quantum Dot Heterojunctions with High Visible Light Photocatalytic Activity and High Stability. ACS Appl. Mater. Interfaces 2016, 8, 91–101. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.N.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The Effect of Gold Loading and Particle Size on Photocatalytic Hydrogen Production from Ethanol over Au/TiO2 Nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, Z.; Wang, C.; Wan, F.; Li, Y.; Wu, L.; Zhi, J.-F.; Zhang, X.; Chen, S.; Liu, Y. Simple Ethanol Impregnation Treatment Can Enhance Photocatalytic Activity of TiO2 Nanoparticles under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 7752–7758. [Google Scholar] [CrossRef]
- Lettieri, S.; Gargiulo, V.; Alfè, M.; Amati, M.; Zeller, P.; Maraloiu, V.-A.; Borbone, F.; Pavone, M.; Muñ Oz-García, A.B.; Maddalena, P. Simple Ethanol Refluxing Method for Production of Blue-Colored Titanium Dioxide with Oxygen Vacancies and Visible Light-Driven Photocatalytic Properties. J. Phys. Chem. C 2020, 124, 3564–3576. [Google Scholar] [CrossRef]
- Guil, J.M.; Homs, N.; Llorca, J.; De La Piscina, P.R. Microcalorimetric and Infrared Studies of Ethanol and Acetaldehyde Adsorption to Investigate the Ethanol Steam Reforming on Supported Cobalt Catalysts. J. Phys. Chem. B 2005, 109, 10813–10819. [Google Scholar] [CrossRef]
- Castedo, A.; Casanovas, A.; Angurell, I.; Soler, L.; Llorca, J. Effect of Temperature on the Gas-Phase Photocatalytic H2 Generation using Microreactors under UVA and Sunlight Irradiation. Fuel 2018, 222, 327–333. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef]
- Xu, J.; Li, L.; Yan, Y.; Wang, H.; Wang, X.; Fu, X.; Li, G. Synthesis and Photoluminescence of Well-Dispersible Anatase TiO2 Nanoparticles. J. Colloid Interface Sci. 2008, 318, 29–34. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S. Solvothermal Synthesis of Anatase TiO2-Graphene Oxide Nanocomposites and Their Photocatalytic Performance. J. Alloy. Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Abazović, N.D.; Čomor, M.I.; Dramićanin, M.D.; Jovanović, D.J.; Ahrenkiel, S.P.; Nedeljković, J.M. Photoluminescence of Anatase and Rutile TiO2 Particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of Photocatalytic Performance with the use of Noble-Metal-Decorated TiO2 Nanocrystals as Highly Active Catalysts for Aerobic Oxidation under Visible-Light Irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Xing, C.; Liu, Y.; Zhang, Y.; Wang, X.; Guardia, P.; Yao, L.; Han, X.; Zhang, T.; Arbiol, J.; Soler, L.; et al. A Direct Z-Scheme for the Photocatalytic Hydrogen Production from a Water Ethanol Mixture on CoTiO3/TiO2 Heterostructures. ACS Appl. Mater. Interfaces 2021, 13, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Le Formal, F.; Boudoire, F.; Guijarro, N. Hematite Photoanodes for Solar Water Splitting: A Detailed Spectroelectrochemical Analysis on the pH-Dependent Performance. ACS Appl. Energy Mater. 2019, 2, 6825–6833. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, Y.; Li, J.; Du, R.; Han, X.; Zhang, T.; Arbiol, J.; Divins, N.J.; Llorca, J.; Guijarro, N.; et al. In Situ Electrochemical Oxidation of Cu2S into CuO Nanowires as a Durable and Efficient Electrocatalyst for Oxygen Evolution Reaction. Chem. Mater. 2019, 31, 7732–7743. [Google Scholar] [CrossRef]
- Lahmar, H.; Setifi, F.; Azizi, A.; Schmerber, G.; Dinia, A. On the Electrochemical Synthesis and Characterization of p-Cu2O/n-ZnO heterojunction. J. Alloy. Compd. 2017, 718, 36–45. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Yu, C.-H.; Chen, Y.-C.; Lin, Y.-G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible Light Driven Type II Heterostructures and Their Enhanced Photocatalysis Properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Arya, A.; Ningthoujam, R.S.; Tewari, R.; Rao, R.; Chalke, B.; Ayyub, P.; Tripathi, A.K.; et al. pn Heterojunctions in NiO:TiO2 composites with type-II band alignment assisting sunlight driven photocatalytic H2 generation. Appl. Catal. B Environ. 2018, 221, 443–458. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, C.; Zhang, Y.; Liu, Y.; Wang, X.; Li, J.; Martínez-Alanis, P.R.; Spadaro, M.C.; Guardia, P.; Arbiol, J.; Llorca, J.; et al. Photodehydrogenation of Ethanol over Cu2O/TiO2 Heterostructures. Nanomaterials 2021, 11, 1399. https://doi.org/10.3390/nano11061399
Xing C, Zhang Y, Liu Y, Wang X, Li J, Martínez-Alanis PR, Spadaro MC, Guardia P, Arbiol J, Llorca J, et al. Photodehydrogenation of Ethanol over Cu2O/TiO2 Heterostructures. Nanomaterials. 2021; 11(6):1399. https://doi.org/10.3390/nano11061399
Chicago/Turabian StyleXing, Congcong, Yu Zhang, Yongpeng Liu, Xiang Wang, Junshan Li, Paulina R. Martínez-Alanis, Maria Chiara Spadaro, Pablo Guardia, Jordi Arbiol, Jordi Llorca, and et al. 2021. "Photodehydrogenation of Ethanol over Cu2O/TiO2 Heterostructures" Nanomaterials 11, no. 6: 1399. https://doi.org/10.3390/nano11061399
APA StyleXing, C., Zhang, Y., Liu, Y., Wang, X., Li, J., Martínez-Alanis, P. R., Spadaro, M. C., Guardia, P., Arbiol, J., Llorca, J., & Cabot, A. (2021). Photodehydrogenation of Ethanol over Cu2O/TiO2 Heterostructures. Nanomaterials, 11(6), 1399. https://doi.org/10.3390/nano11061399