Assessment of the Biocompatibility of Cucurbiturils in Blood Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of PBMC
2.2. PBMC Cytotoxicity Assay
2.3. Apoptosis of the PBMC Assay
2.4. Hemolysis Assay
2.5. Statistical Analysis
3. Results
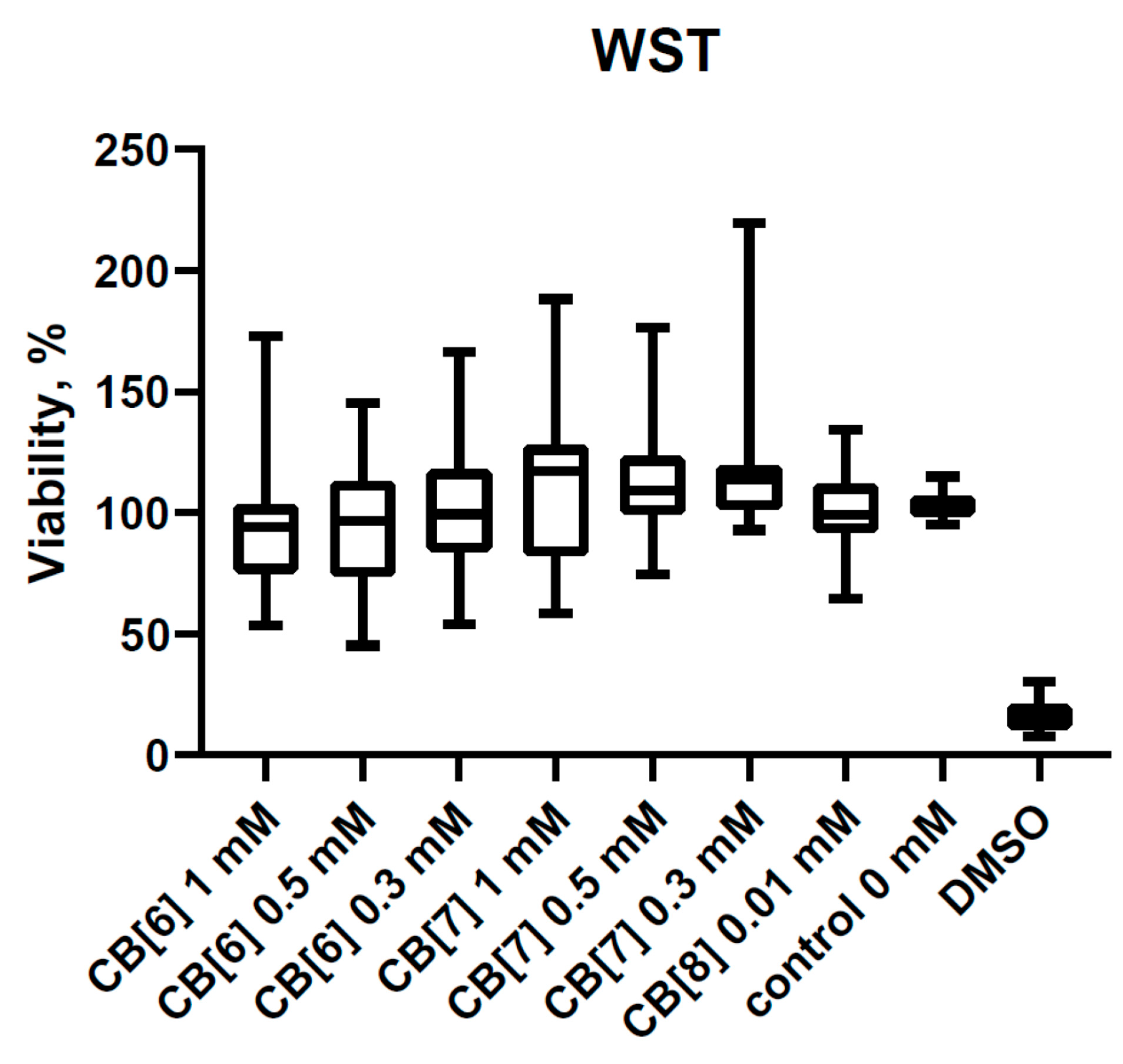
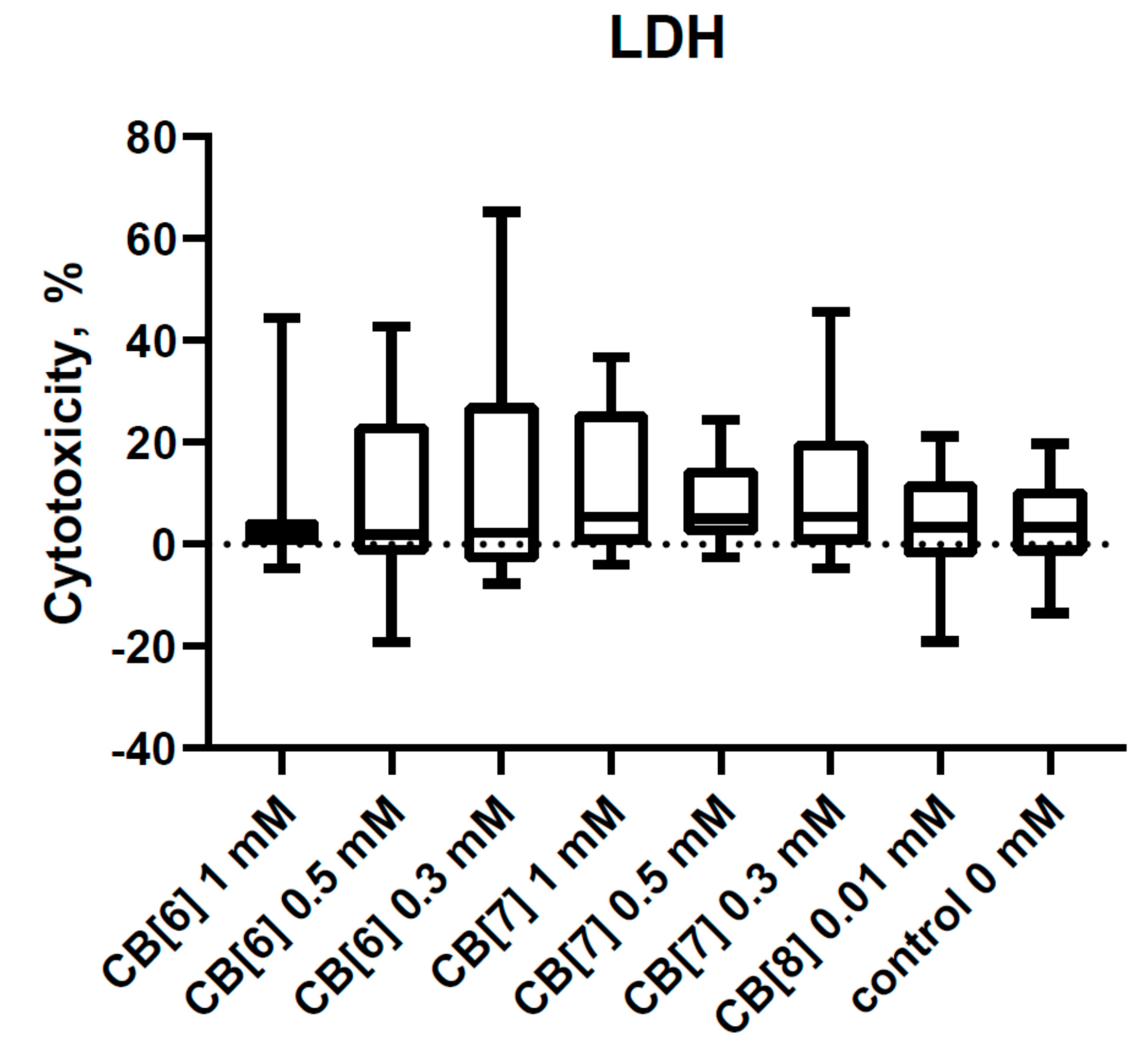
3.1. Cytotoxic Effect of Cucurbit[6,7,8]urils on PBMCs
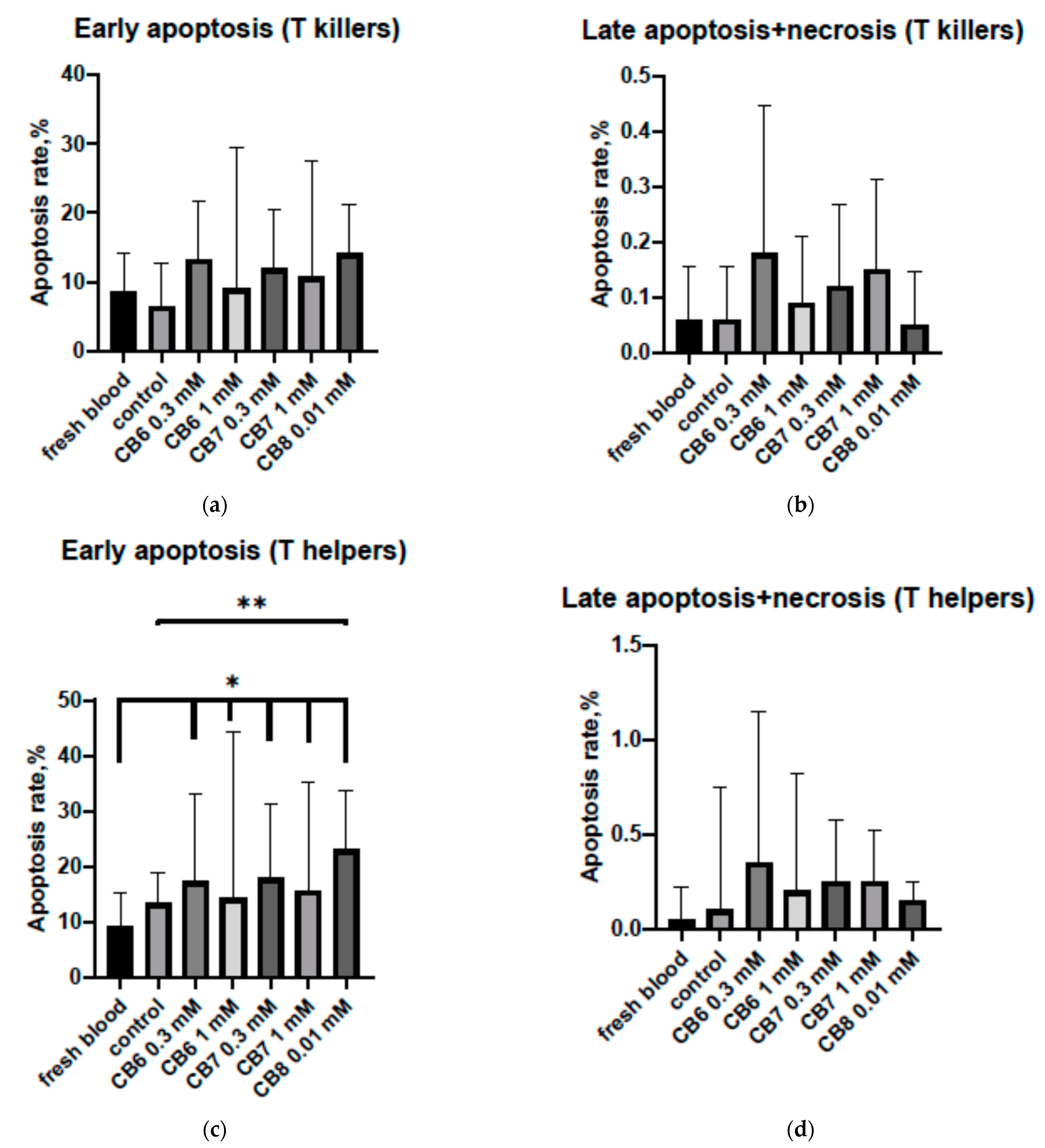
3.2. Effect of Cucurbit[6,7,8]urils on the Apoptosis of PBMCs
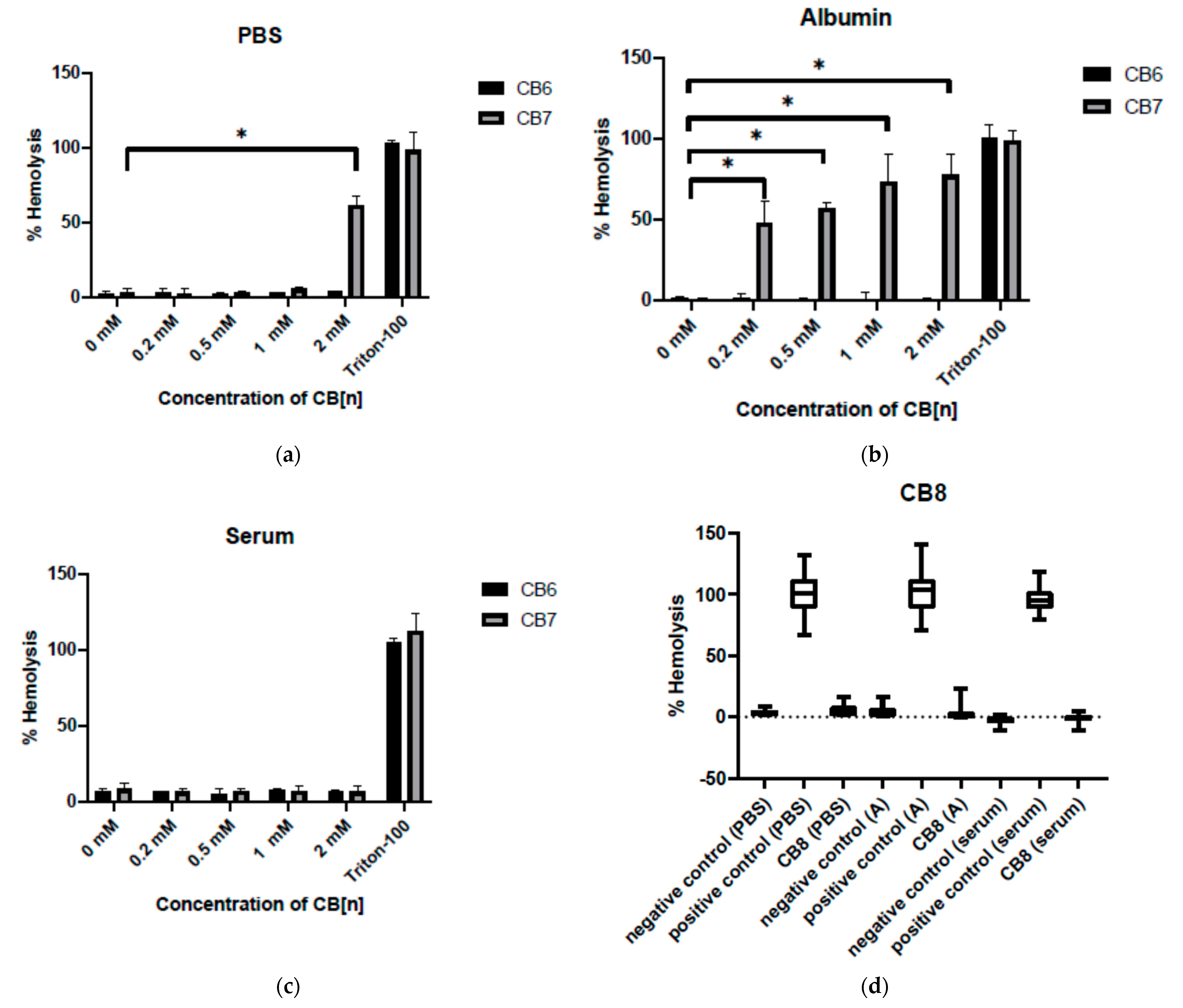
3.3. Effect of Cucurbit[6,7,8]urils on the Hemolysis of RBC
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Behrend, R.; Meyer, E.; Rusche, F. I Ueber Condensations producte aus Glycoluril und Formaldehyd. Justus Liebig’s Ann. Chem. 1905, 339, 1–37. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.; Zhao, H.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]uril. ACS Appl. Mater. Interfaces 2017, 9, 8602–8608. [Google Scholar] [CrossRef]
- Wheate, N.J.; Buck, D.P.; Day, A.I.; Collins, J.G. Cucurbit[n]uril binding of platinum anticancer complexes. Dalton Trans. 2006, 3, 451–458. [Google Scholar] [CrossRef]
- Chang, Y.X.; Zhang, X.M.; Duan, X.C.; Liu, F.; Du, L.M. Supramolecular interaction of methotrexate with cucurbit[7]uril and analytical application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 131–137. [Google Scholar] [CrossRef]
- Ahmadian, N.; Amininasab, M.; Mehrnejad, F. Paclitaxel interaction with cucurbit[7]uril and acyclic Cucurbit[4]urilnanocontainers: A computational approach. Mol. Graph. Model. 2019, 90, 210–218. [Google Scholar] [CrossRef]
- Li, S.; Kuok, K.I.; Ji, X.; Xu, A.; Yin, H.; Zheng, J.; Tan, H.; Wang, R. Supramolecular Modulation of Antibacterial Activity of Ambroxol by Cucurbit[7]uril. Chempluschem 2020, 85, 679–683. [Google Scholar] [CrossRef]
- Li, S.; Chan, J.Y.; Li, Y.; Bardelang, D.; Zheng, J.; Yew, W.W.; Chan, D.P.; Lee, S.M.; Wang, R. Complexation of clofazimine by macrocyclic cucurbit[7]uril reduced its cardiotoxicity without affecting the antimycobacterial efficacy. Org. Biomol. Chem. 2016, 14, 7563. [Google Scholar] [CrossRef]
- ZdarovaKarasova, J.; Hepnarova, V.; Andrys, R.; Lisa, M.; Jost, P.; Muckova, L.; Pejchal, J.; Herman, D.; Jun, D.; Kassa, J.; et al. Encapsulation of oxime K027 into cucurbit[7]uril: In vivo evaluation of safety, absorption, brain distribution and reactivation effectiveness. Toxicol. Lett. 2020, 320, 64–72. [Google Scholar] [CrossRef]
- Zeng, Z.; Xie, J.; Luo, G.; Tao, Z.; Zhang, Q. Host-guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A. Beilstein. J. Org. Chem. 2020, 16, 2332–2337. [Google Scholar] [CrossRef]
- Konda, S.K.; Maliki, R.; McGrath, S.; Parker, B.S.; Robinson, T.; Spurling, A.; Cheong, A.; Lock, P.; Pigram, P.J.; Phillips, D.R.; et al. Encapsulation of Mitoxantrone within Cucurbit[8]uril Decreases Toxicity and Enhances Survival in a Mouse Model of Cancer. ACS Med. Chem. Lett. 2017, 8, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, N.; Zhao, W.; Ding, Y.F.; Zheng, Y.; Wang, L.H.; Zheng, J.; Wang, R. An eco-friendly in situ activatable antibiotic via cucurbit[8]uril-mediated supramolecular crosslinking of branched polyethylenimine. Chem. Commun. 2017, 53, 5870–5873. [Google Scholar] [CrossRef] [PubMed]
- Chandra, F.; Dutta, T.; Koner, A.L. Supramolecular Encapsulation of a Neurotransmitter Serotonin by Cucurbit[7]uril. Front Chem. 2020, 8, 582757. [Google Scholar] [CrossRef]
- Yin, H.; Wang, R.; Wan, J.; Zheng, Y.; Ouyang, D.; Wang, R. Molecular Encapsulation of Histamine H2-Receptor Antagonists by Cucurbit[7]Uril: An Experimental and Computational Study. Molecules 2016, 21, 1178. [Google Scholar] [CrossRef]
- Kwong, C.H.T.; Mu, J.; Li, S.; Fang, Y.; Liu, Q.; Zhang, X.; Kam, H.; Lee, S.M.Y.; Chen, Y.; Deng, F.; et al. Reviving chloroquine for anti-SARS-CoV-2 treatment with cucurbit[7]uril-based supramolecular formulation. Chin. Chem. Lett. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Ma, D.D.; Yang, W.X. Engineered nanoparticles induce cell apoptosis: Potential for cancer therapy. Oncotarget. 2016, 7, 40882–40903. [Google Scholar] [CrossRef]
- Schönfelder, U.; Radestock, A.; Elsner, P.; Hipler, U.C. Cyclodextrin-induced apoptosis in human keratinocytes is caspase-8 dependent and accompanied by mitochondrial cytochrome c release. Exp. Dermatol. 2006, 15, 883–890. [Google Scholar] [CrossRef]
- Ercan, A.; Çelebier, M.; Oncul, S.; Varan, G.; Kocak, E.; Benito, J.M.; Bilensoy, E. Polycationiccyclodextrin nanoparticles induce apoptosis and affect antitumoral activity in HepG2 cell line: An evaluation at the molecular level. Int. J. Pharm. 2021, 598, 120379. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, J.H.; Yu, Q.; Wen, X.; Liu, Y. Targeted Polypeptide-Microtubule Aggregation with Cucurbit[8]uril for Enhanced Cell Apoptosis. Angew. Chem. Int. Ed. Engl. 2019, 58, 10553–10557. [Google Scholar] [CrossRef]
- Fink, S.; Reddersen, K.; Wiegand, C.; Elsner, P.; Hipler, U.-C. Evaluation of cell and hemocompatibility of Cucurbiturils. Eur. J. Pharm. Sci. 2020, 146, 105271. [Google Scholar] [CrossRef]
- Gamal-Eldin, M.A.; MacArtney, D.H. Selective molecular recognition of methylated lysines and arginines by cucurbit[6]uril and cucurbit[7]uril in aqueous solution. Org. Biomol. Chem. 2013, 11, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Hennig, A.; Uzunova, V.D.; Nau, W.M. Supramolecular tandem enzyme assays for multiparameter sensor arrays and enantiomeric excess determination of amino acids. Chem. A Eur. J. 2008, 14, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Chinai, J.M.; Taylor, A.B.; Ryno, L.M.; Hargreaves, N.D.; Morris, C.A.; Hart, P.J.; Urbach, A.R. Molecular recognition of insulin by a synthetic receptor. J. Am. Chem. Soc. 2011, 133, 8810–8813. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, E.A.; Pashkina, E.A.; Kanazhevskaya, L.Y.; Masliy, A.N.; Kozlov, V.A. Chemical and biological properties of a supramolecular complex of tuftsin and cucurbit[7]uril. Int. Immunopharmacol. 2017, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Urbach, A.R.; Ramalingam, V. Molecular recognition of amino acids, peptides, and proteins by cucurbit[n]uril receptors. Isr. J. Chem. 2011, 51, 664–678. [Google Scholar] [CrossRef]
- Pashkina, E.; Aktanova, A.; Blinova, E.; Mirzaeva, I.; Kovalenko, E.; Knauer, N.; Ermakov, A.; Kozlov, V. Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro. Molecules 2020, 25, 3388. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, V.D.; Cullinane, C.; Brix, K.; Nau, W.M.; Day, A.I. Toxicity of cucurbit[7]uril and cucurbit[8]uril: An exploratory in vitro and in vivo study. Org. Biomol. Chem. 2010, 8, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Floriano, R.S.; Isaacs, L.; Rowana, E.G.; Wheate, N.J. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbituril-based macrocyclic drug delivery vehicles. Toxicol. Res. 2014, 3, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, J.Y.; Yang, X.; Ian, W.; Bardelang, D.; Macartney, H.; Lee Simon, M.Y.; Wang, R. Developmental and organ-specific toxicity of cucurbit[7]uril in vivo study on zebrafish models. RSC Adv. 2015, 5, 30067–30074. [Google Scholar] [CrossRef]
- Irie, T.; Otagiri, M.; Sunada, M.; Uekama, K.; Ohtani, Y.; Yamada, Y.; Sugiyama, Y. Cyclodextrin-induced hemolysis and shape changes of human erythrocytes in vitro. Pharmacobiodyn 1982, 5, 741–744. [Google Scholar] [CrossRef]
- Tovani, C.B.; de Souza, J.F.; Cavallini Tde, S.; Demets, G.J.; Ito, A.; Barioni, M.B.; Pazin, W.M.; Zaniquelli, M.E. Comparison between cucurbiturils and β-cyclodextrin interactions with cholesterol molecules present in Langmuir monolayers used as a biomembrane model. Colloids Surf. B Biointerfaces 2013, 111, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, J.; Robinson-Duggon, J.; Tapia, A.; Paiva, C.; Gómez, M.; Bohne, C.; Fuentealba, D. Photochemical behavior of biosupramolecular assemblies of photosensitizers, cucurbit[n]urils and albumins. Phys. Chem. Chem. Phys. 2017, 19, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Jiang, G.; Zhou, Q.; Zhang, B.; Wang, X. Greatly enhanced binding of a cationic porphyrin towards bovine serum albumin by cucurbit[8]uril. Phys. Chem. Chem. Phys. 2010, 12, 13255–13260. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktanova, A.; Abramova, T.; Pashkina, E.; Boeva, O.; Grishina, L.; Kovalenko, E.; Kozlov, V. Assessment of the Biocompatibility of Cucurbiturils in Blood Cells. Nanomaterials 2021, 11, 1356. https://doi.org/10.3390/nano11061356
Aktanova A, Abramova T, Pashkina E, Boeva O, Grishina L, Kovalenko E, Kozlov V. Assessment of the Biocompatibility of Cucurbiturils in Blood Cells. Nanomaterials. 2021; 11(6):1356. https://doi.org/10.3390/nano11061356
Chicago/Turabian StyleAktanova, Alina, Tatjana Abramova, Ekaterina Pashkina, Olga Boeva, Lyubov Grishina, Ekaterina Kovalenko, and Vladimir Kozlov. 2021. "Assessment of the Biocompatibility of Cucurbiturils in Blood Cells" Nanomaterials 11, no. 6: 1356. https://doi.org/10.3390/nano11061356
APA StyleAktanova, A., Abramova, T., Pashkina, E., Boeva, O., Grishina, L., Kovalenko, E., & Kozlov, V. (2021). Assessment of the Biocompatibility of Cucurbiturils in Blood Cells. Nanomaterials, 11(6), 1356. https://doi.org/10.3390/nano11061356





