Deep-Ultraviolet Transparent Conductive MWCNT/SiO2 Composite Thin Film Fabricated by UV Irradiation at Ambient Temperature onto Spin-Coated Molecular Precursor Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SiO2 Precursor Solution (SSilica)
2.3. Preparation of MWCNT/SiO2 Composite Precursor Solution (SCOMP)
2.4. Fabrication of MWCNT/SiO2 Composite Thin Films (FCOMP) by UV-Light Irradiation and Heat Treatment (F’COMP)
2.5. Optical Characterization of Solutions and Thin Films
2.6. Structural Characterization of Thin Films
2.7. Surface Morphologies, Film Thicknesses, and Pencil Hardnesses of FCOMP and FCNT
2.8. Electrical Resistivities of FCOMP, F’COMP, FCNT, and F’CNT
2.9. Chemical Stabilities of FCOMP and F’COMP against Acids and Bases in Water
3. Results and Discussion
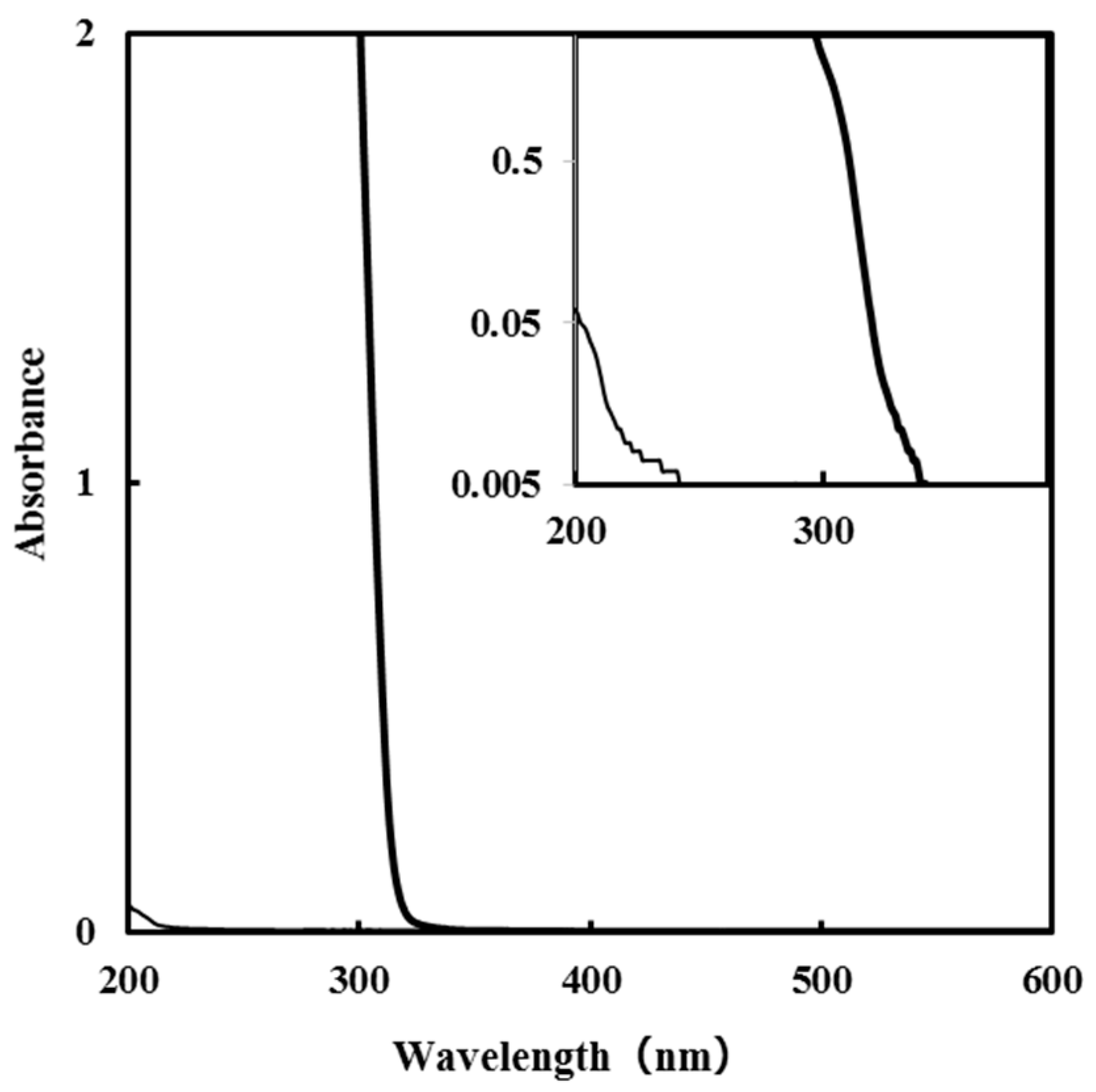
3.1. Absorption Spectra of SSilica
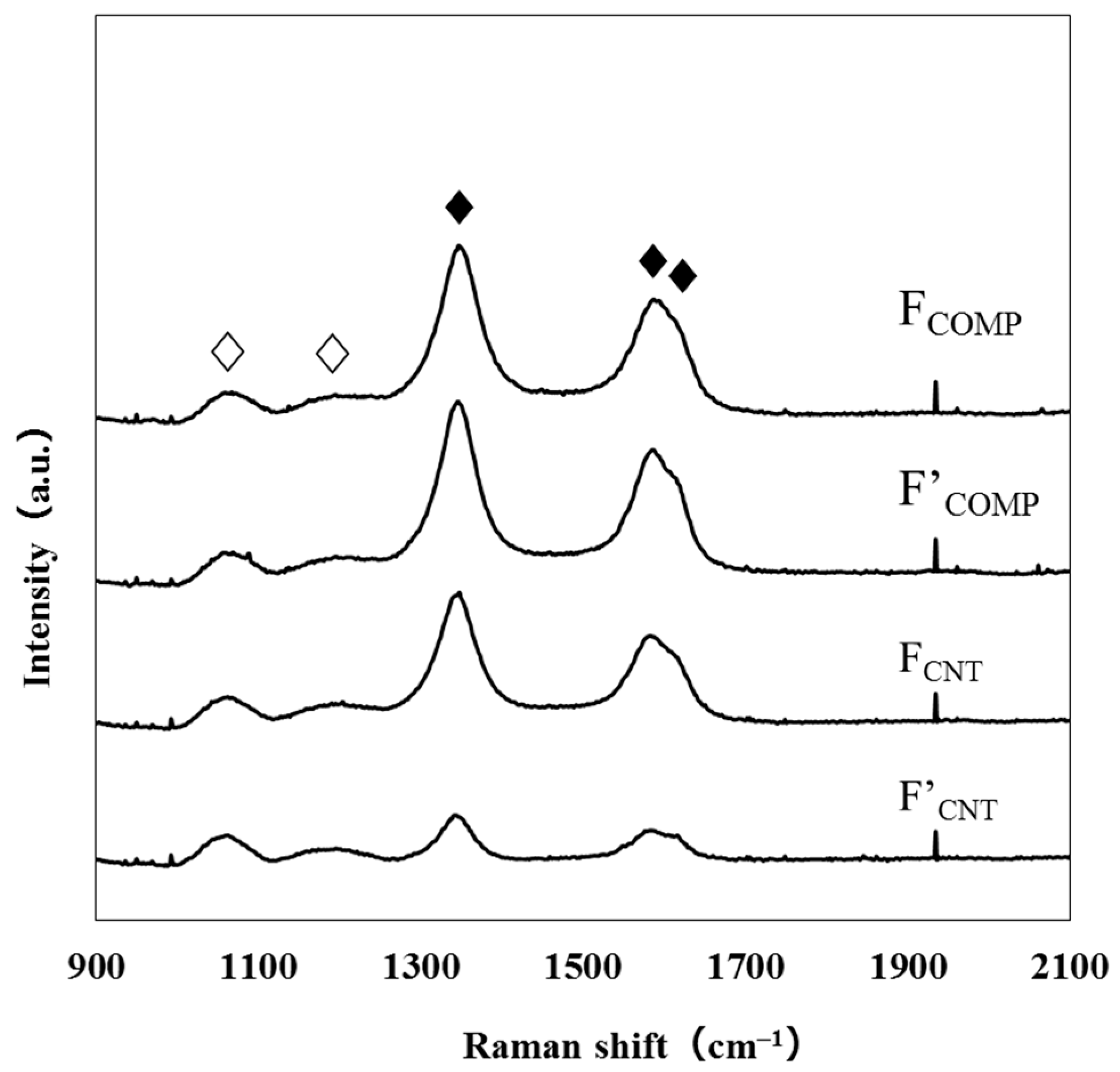
3.2. Structural Characterization of FCOMP and F’COMP
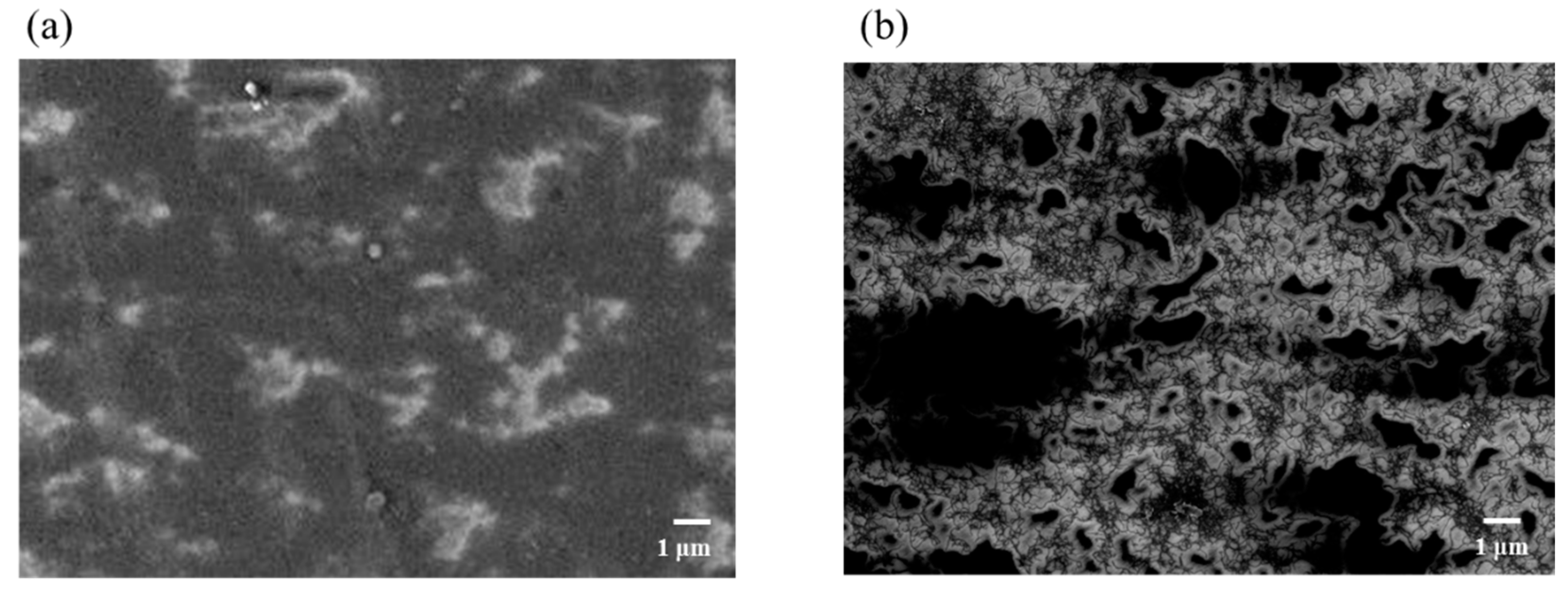
3.3. Surface Morphologies of FCOMP and FCNT
3.4. Film Thicknesses, Pencil Hardnesses, and Electrical Resistivities of FCOMP and F’COMP, and Their Chemical Stabilities
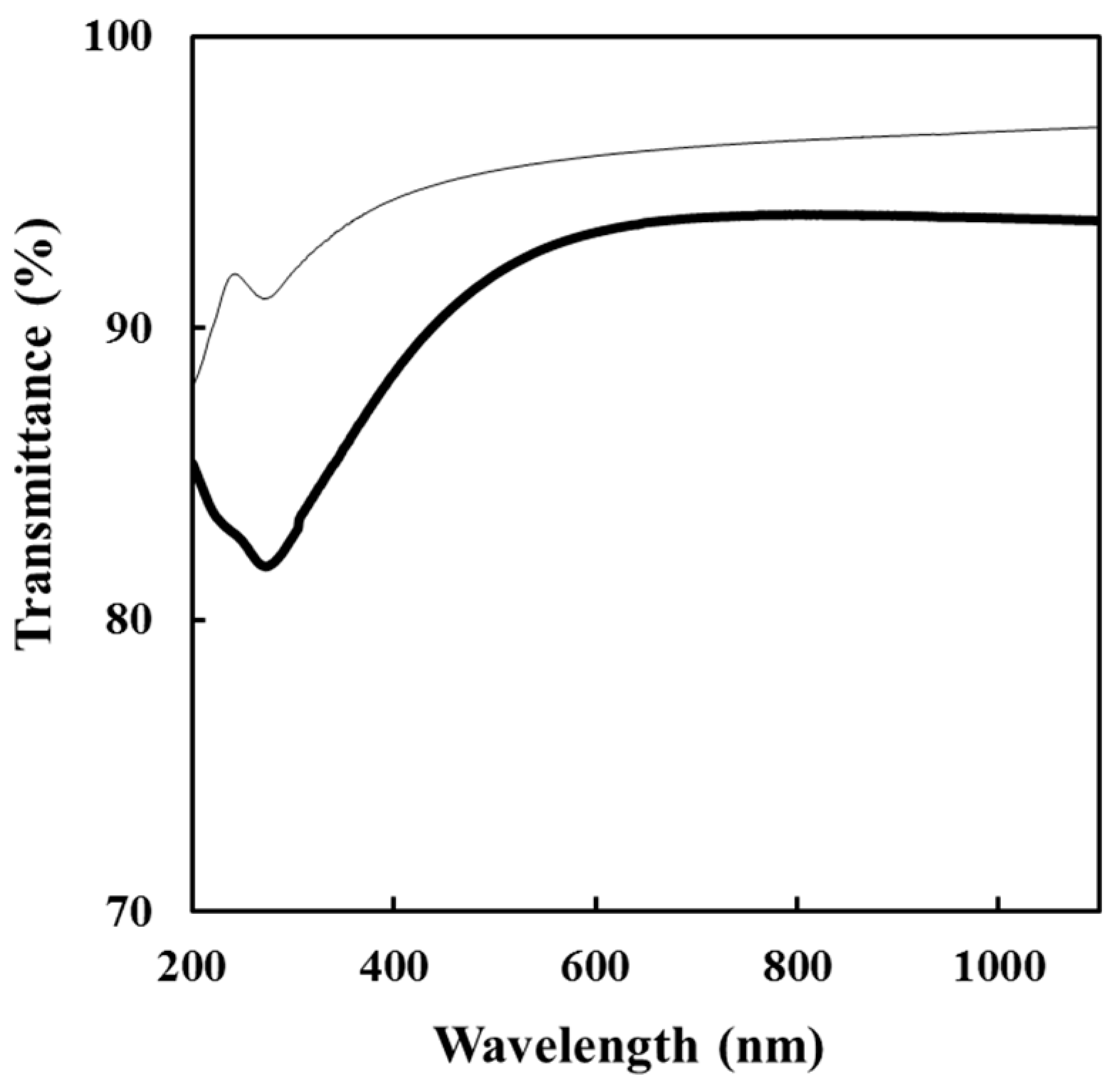
3.5. Optical Properties of the FCOMP and FCNT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Guo, S.; Sivakumar, R.; Kitazawa, H.; Kagawa, Y. Electrical properties of silica-based nanocomposites with multiwall carbon nanotubes. J. Am. Ceram. Soc. 2007, 90, 1667–1670. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Shi, L.; Li, Q.; Lu, W.; Qu, S.; Qiu, W.; Zhou, G. Fabrication of thermally robust carbon nanotube (CNT)/SiO2 composite films and their high-temperature mechanical properties. Carbon N. Y. 2019, 147, 236–241. [Google Scholar] [CrossRef]
- Seeger, T.; Köhler, T.; Frauenheim, T.; Grobert, N.; Terrones, M.; Seifert, G. Nanotube composites: Novel SiO2 coated carbon nanotubes. Chem. Commun. 2002, 2, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhang, J.; Pan, Y.; Guo, J. Fabrication and mechanical properties of SiO2 matrix composites reinforced by carbon nanotube. Mater. Sci. Eng. A 2003, 357, 392–396. [Google Scholar] [CrossRef]
- Nagai, H.; Sato, M. The Science of Molecular Precursor Method. Adv. Coat. Mater. 2018, 1–27. [Google Scholar] [CrossRef]
- Wu, H.-J.; Tomiyama, N.; Nagai, H.; Sato, M. Fabrication of a p-type Cu2O thin-film via UV-irradiation of a patternable molecular-precursor film containing Cu(II) complexes. J. Cryst. Growth 2019, 509, 112–117. [Google Scholar] [CrossRef]
- Wu, H.-J.; Tanabe, K.; Nagai, H.; Sato, M. Photo-Induced Super-hydrophilic Thin Films on Quartz Glass by UV Irradiation of Precursor Films Involving a Ti(IV) Complex at Room Temperature. Materials 2019, 12, 348. [Google Scholar] [CrossRef]
- Balkus, K.J.; Gabrielova, I.S.; Bott, S.G. Tris(oxalato) Complexes of Silicon as Precursors to Porous Silicate Materials: Synthesis and Structure. Inorg. Chem. 1995, 34, 5776–5780. [Google Scholar] [CrossRef]
- Seiler, O.; Burschka, C.; Penka, M.; Tacke, R. Dianionic tris[oxalato(2-)]silicate and tris[oxalato(2-)]germanate complexes: Synthesis, properties, and structural characterization in the solid state and in solution. Z. Anorg. Allg. Chem. 2002, 628, 2427–2434. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Wang, L.; Lyu, S.; Ye, W.; Xu, B.B.; Qiu, H.; Chen, L.; Gu, J. Fabrication and investigation on ternary heterogeneous MWCNT@TiO2-C fillers and their silicone rubber wave-absorbing composites. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105714. [Google Scholar] [CrossRef]
- Kocyigit, A.; Orak, I.; Karteri, İ.; Uruş, S. The structural analysis of MWCNT-SiO2 and electrical properties on device application. Curr. Appl. Phys. 2017, 17, 1215–1222. [Google Scholar] [CrossRef]
- Díaz-Corona, N.; Martínez-Juárez, J.; Pérez-Luna, J.G.; Hernández-de la Luz, A.D.; Rabanal, M.E.; Robles-Águila, M.J. Structural, optical and electrical behavior of zinc oxide/MWCNT composite thin films. Opt. Quantum Electron. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Galeener, F.L. Band limits and the vibrational spectra of tetrahedral glasses. Phys. Rev. B 1979, 19, 4292–4297. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Murakami, Y.; Einarsson, E.; Edamura, T.; Maruyama, S. Polarization dependent optical absorption properties of single-walled carbon nanotubes and methodology for the evaluation of their morphology. Carbon N. Y. 2005, 43, 2664–2676. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon N. Y. 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
| Film | Film Thickness | Electrical Resistivity | Pencil Hardness |
|---|---|---|---|
| nm | Ω·cm | ||
| FCOMP | 220 | 0.7 ± 0.1 | 8H |
| F’COMP | 210 | 0.9 ± 0.1 | 8H |
| FCOMP-sa | 180 | 0.7 ± 0.1 | 8H |
| F’COMP-sa | 180 | 0.9 ± 0.1 | 8H |
| FCOMP-wb | 180 | 0.8 ± 0.1 | 4H |
| F’COMP-wb | 180 | 0.9 ± 0.1 | 8H |
| F’COMP-sb | 170 | 0.8 ± 0.1 | 8H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, H.; Ogawa, N.; Sato, M. Deep-Ultraviolet Transparent Conductive MWCNT/SiO2 Composite Thin Film Fabricated by UV Irradiation at Ambient Temperature onto Spin-Coated Molecular Precursor Film. Nanomaterials 2021, 11, 1348. https://doi.org/10.3390/nano11051348
Nagai H, Ogawa N, Sato M. Deep-Ultraviolet Transparent Conductive MWCNT/SiO2 Composite Thin Film Fabricated by UV Irradiation at Ambient Temperature onto Spin-Coated Molecular Precursor Film. Nanomaterials. 2021; 11(5):1348. https://doi.org/10.3390/nano11051348
Chicago/Turabian StyleNagai, Hiroki, Naoki Ogawa, and Mitsunobu Sato. 2021. "Deep-Ultraviolet Transparent Conductive MWCNT/SiO2 Composite Thin Film Fabricated by UV Irradiation at Ambient Temperature onto Spin-Coated Molecular Precursor Film" Nanomaterials 11, no. 5: 1348. https://doi.org/10.3390/nano11051348
APA StyleNagai, H., Ogawa, N., & Sato, M. (2021). Deep-Ultraviolet Transparent Conductive MWCNT/SiO2 Composite Thin Film Fabricated by UV Irradiation at Ambient Temperature onto Spin-Coated Molecular Precursor Film. Nanomaterials, 11(5), 1348. https://doi.org/10.3390/nano11051348








