1. Introduction
Aluminum oxide (Al
2O
3) has extensively been used as a heterogeneous catalyst in diverse catalytic reactions of CO oxidation [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19], CO
2 reduction [
20,
21,
22,
23], CO
2 methanation/hydrogenation [
20,
24,
25,
26,
27], and preferential oxidation of CO [
28,
29]. The efforts to increase the catalytic activity of the metal oxide have been devoted to the modification of the metal surface by loading of transition metals in groups of 9 (Co, Rh, and Ir), 10 (Ni, Pd, and Cu), and 11 (Cu, Ag, and Au). The morphology of a metal oxide support has also been a key factor for the enhancement of the catalytic activity [
30]. It was reported that the role of an overlayer metal becomes different when the support metal oxide is different [
2]. The relative catalytic activities of overlayer metals also become different when the catalytic application areas are different for the application to CO oxidation using transition metal-loaded Al
2O
3 catalysts.
Chen et al. prepared Pt nanoparticles (NPs) on Al
2O
3 and observed 100% CO conversion at −20 °C [
1]. For the extremely high activity compared with a commercial Pt/Al
2O
3, based on the experimental and the density functional theory (DFT) calculations, they proposed that CO was initially adsorbed on Pt(OH) kink sites and reacted with OH to release gaseous CO
2. Afterward, OH was regenerated by activation of O
2 on terrace sites. Lou and Liu studied CO oxidation of single Pt atoms dispersed on Fe
2O
3 (highly reducible), ZnO (reducible), and γ-Al
2O
3 (irreducible) supports, and observed that the catalytic activity was in the order of Pt/γ-Al
2O
3 < Pt/ZnO < Pt/Fe
2O
3 [
2], where the highly reducible support showed the highest catalytic activity. Chen et al. tested Pt/Al
2O
3 for preferential oxidation (PROX) of CO in H
2 [
29]. They concluded that CO conversion and CO
2 selectivity reached up to 100% in a wide range of −30 °C to 120 °C. The high performance was attributed to a combination of Pt(OH) and metallic Pt on the Al
2O
3 support. Therefore, the adsorption of CO and the activation of O
2 were optimally tuned to maximize the performance. For monodispersed single Pt atoms on θ-Al
2O
3, Moses-DeBusk et al. found that the CO oxidation did not follow a conventional Langmuir-Hinshelwood mechanism [
11]. The Pt atom was first oxygenated, and then CO was bound to form a carbonate (CO
3), which dissociated to generate gaseous CO
2 [
11]. Yang et al. employed the DFT calculation to investigate the relative CO oxidation for single-atom catalysts of Ni/γ-Al
2O
3 and Pd/γ-Al
2O
3 [
7]. They reported that Ni showed an unexpectedly higher CO oxidation activity than the Pd. Ananth et al. synthesized Ag
2O/γ-Al
2O
3 and (Ag
2O + RuO
2)/γ-Al
2O
3 catalysts and tested the CO oxidation performances to show that the catalytic activity was increased by the addition of RuO
2 [
6]. Han et al. reported a high CO oxidation activity at 30 °C for NiO (≤1 nm) on mesoporous Al
2O
3 prepared using atomic layer deposition [
16]. The deactivation was found to be lowered with increasing the pre-annealing temperature.
For the application of Al
2O
3 to CO
2 reduction, Zhao et al. synthesized Au/Al
2O
3/TiO
2 nanocomposites, where the atomic-layer Al
2O
3 was sandwiched between the two layers [
21]. They tested the photocatalytic CO
2 reduction activity and observed CO (major) and CH
4 (minor) as products. It was concluded that the charge transfer and surface charge recombination were highly influenced by Al
2O
3 interlayer thickness. Therefore, the maximum photocatalytic activity (37 μmol/g of CO and 2 μmol/g of CH
4) was obtained by achieving optimum Al
2O
3 thickness (5 Å). Kwak et al. performed a temperature-programmed CO
2 reduction with H
2 on Ru/Al
2O
3 catalysts and observed CO and CH
4 formation yields with activation energies of 82 kJ/mol and 62 kJ/mol, respectively [
20]. It was found that CO formation selectivity was increased with increasing Ru metal dispersion but decreased with increasing Ru clustering and concluded that CO was not an intermediate species for CH
4 formation. Chein and Wang tested CO
2 methanation activities using Ni/Al
2O
3, Ru/Al
2O
3, and Ru-Ni/Al
2O
3 catalysts [
27] and found that the hybrid bimetallic Ru-Ni showed higher performance than the monometallic catalysts.
Although numerous detailed in-depth studies have been performed using transition metal-loaded Al2O3 catalysts, no systematic comparison studies have been reported among diverse (M = Co, Ni, Cu, Rh, Pd, Ag, Ir, Pt, and Au) transition metal-loaded Al2O3 catalysts prepared by the same synthesis method. Motivated by this, we synthesized transition metal-loaded Al2O3 nanosheets and evaluated thermal CO oxidation activity as well as photocatalytic CO2 reduction activity. Consequently, the roles of overlayer transition metals were comparatively investigated in two totally different application reactions. Thereby, the present pre-screening test results provided useful information on the quick-selection of catalysts for thermal CO oxidation and photocatalytic CO2 reduction.
3. Results and Discussion
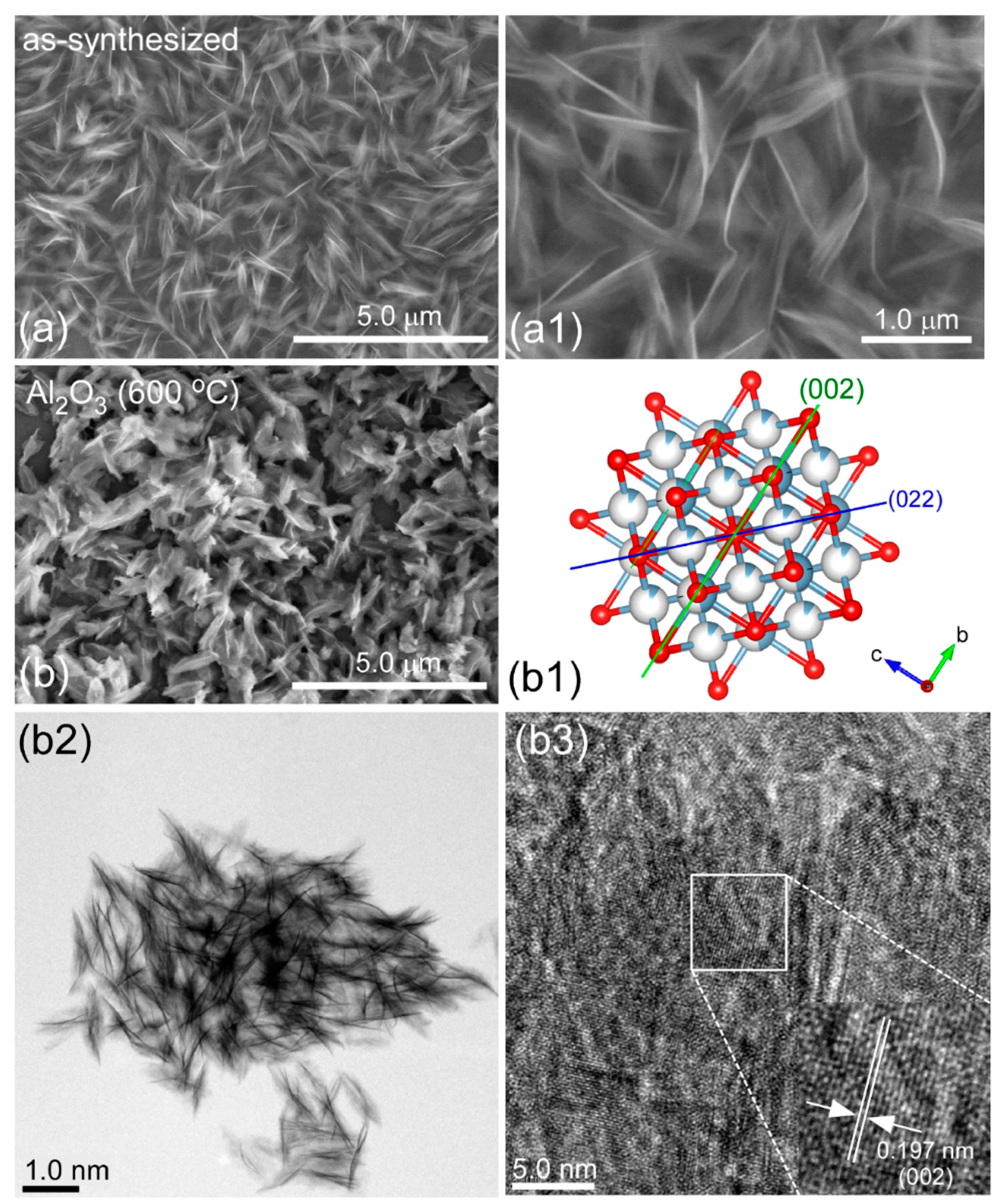
Figure 1(a,a1) show the SEM image of the as-prepared Al-precursor with a morphology of cotton-like nanostructures.
Figure 1b shows the sample after thermal annealing at 600 °C, abbreviated as bare Al
2O
3. It appears that the morphology showed no significant change, but the nanosheets became somewhat compacted. The Brunauer-Emmett-Teller (BET) surface area of bare Al
2O
3 was measured to be 154.4 m
2/g. The corresponding transmission electron microscope (TEM) image clearly showed the morphology of nanosheets. For the high-resolution TEM image of bare Al
2O
3, clear lattice fringes were seen, and the lattice spacing was estimated to be 0.197 nm. This was well-matched to the (002) crystal plane of cubic phase gamma-Al
2O
3. This was further discussed in detail below. The structure projection of the (002) and (022) planes for Al
2O
3 are shown in
Figure 1(b1) for visual understanding.
Figure 2 shows the SEM and TEM images of selected Ni- and Rh-loaded Al
2O
3 nanosheets. The SEM images of other M-loaded Al
2O
3 nanosheets are provided in the Supporting Information,
Figure S1. The SEM image (
Figure 2a) of Rh-Al
2O
3 showed small nanoparticles embedded on the nanosheets. The nanoparticles appeared as a result of Rh particle formation. The color of burlywood was clearly different from the white color for bare Al
2O
3. On the other hand, the SEM image (
Figure 2b) of Ni-Al
2O
3 nanosheets showed only cotton-like nanosheets. It was difficult to discriminate Ni species from the bare Al
2O
3 support. However, the color clearly changed from white to pale blue upon Ni-loading. The photos and optical microscope images of the M-loaded Al
2O
3 nanosheets are provided in the Supporting Information,
Figures S2 and S3, respectively. Although the SEM images showed no clear metal embedment, the color change was a clear indication of metal-loading on the Al
2O
3 support. The metal-loading was also confirmed by the X-ray photoelectron spectroscopy (XPS) data, discussed below.
The TEM images (
Figure 2(a1)) of Rh-Al
2O
3 clearly showed NPs (with a size of ~20 nm) embedded onto the nanosheets. For the HRTEM image (
Figure 2(b2)) of an Rh-NP, clear lattice fringes were observed, and the distances were estimated to be 0.263 nm and 0.254 nm. These distances matched well with the (114) and (200) crystal planes of orthorhombic (Pbca) Rh
2O
3 (ICSD ref. 98-000-9206), respectively. This indicated that Rh was embedded not in the metallic form but rather in the oxide form. This was further confirmed by the XPS data below. The fast Fourier transform (FFT) pattern of the HRTEM image reflected the crystallinity of the Rh oxide. The TEM images (
Figure 2(b1)) of Ni-Al
2O
3 nanosheets showed only nanosheet morphology, consistent with the corresponding SEM image (
Figure 2b).
For the HRTEM image in
Figure 2(b2), the lattice fringes with distances of 0.227 nm and 0.196 nm matched well with the (111) and (002) crystal planes of the cubic phase γ-Al
2O
3. The lattices showed poor crystallinity compared with those of bare Al
2O
3, seen in
Figure 1(b3). Interestingly, some areas (dotted circles) showed very poor crystallinity, and these appeared like amorphous particles. This is likely an indication of Ni embedment on the Al
2O
3 nanosheets. Very similarly for Co-Al
2O
3 nanosheets, although particles were not clearly seen in the TEM image (Supporting Information,
Figure S4), the corresponding HRTEM image showed the areas with very poor crystallinity. The areas appeared like Co-embedment in the Al
2O
3 support.
The BET surface areas of Ni-Al2O3 and Rh-Al2O3 nanosheets were measured to be 151.2 m2/g and 153.8 m2/g, respectively. The surface areas were very similar to that of bare Al2O3. This indicated that the surface area was not significantly impacted by the metal-loading.
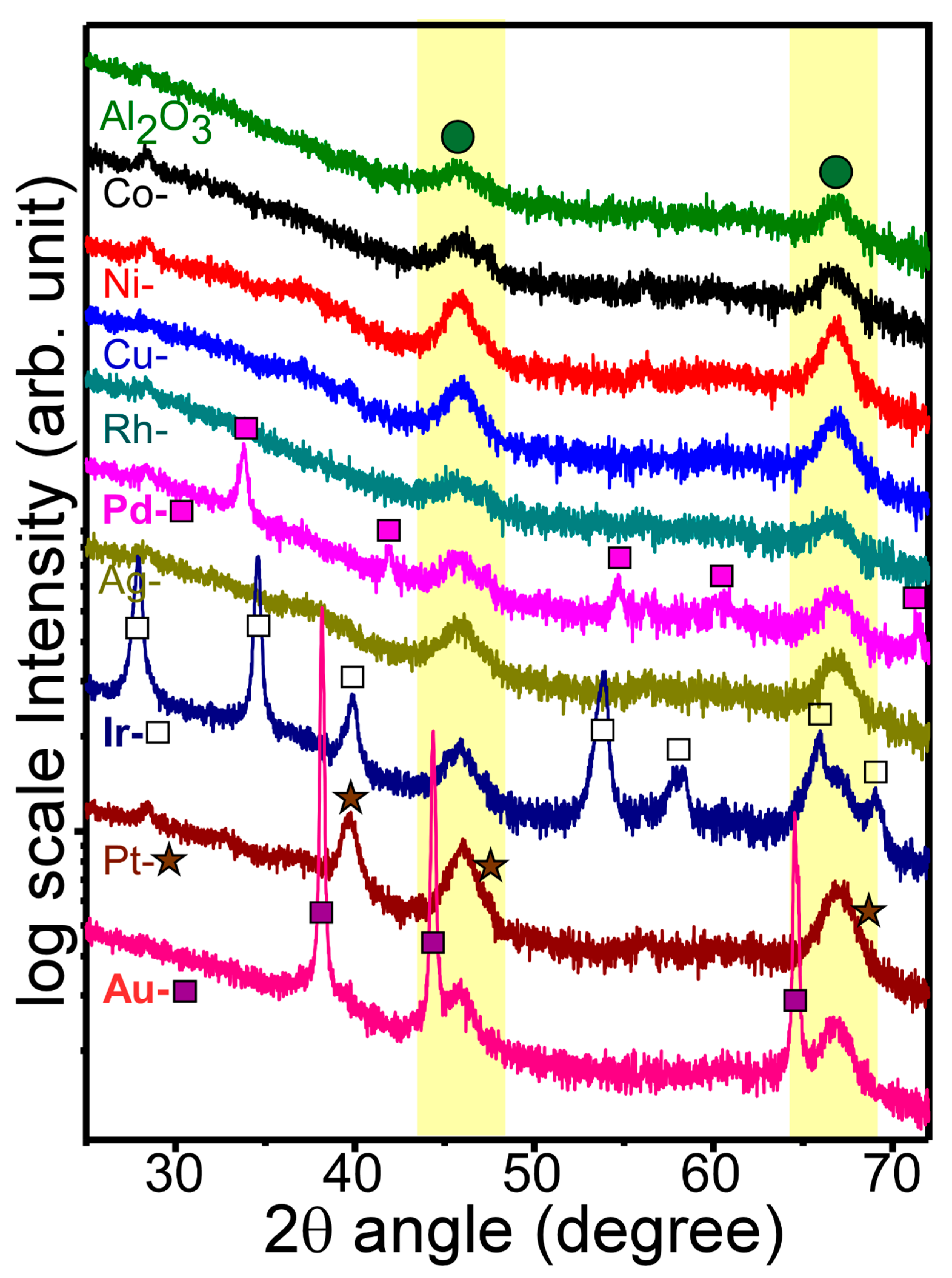
Figure 3 displays the X-ray diffraction patterns of bare Al
2O
3 and M-loaded Al
2O
3 nanosheets. For the XRD patterns of bare Al
2O
3, two distinctive peaks were observed at 2θ = 45.9° and 67.0°. These two peaks could be assigned to the (002) and (022) planes of cubic phase (Fm-3m) γ-Al
2O
3, (ICSD ref. 98-003-0267), respectively. The XRD result was in good consistency with the HRTEM result of the bare Al
2O
3 nanosheet. For the XRD profiles of M-loaded Al
2O
3 nanosheets, two peaks were commonly observed, as expected. Interestingly, Co, Ni, Cu, Rh, and Ag-loaded samples showed no significant extra peaks in the corresponding XRD profiles. These results indicated that the metals were loaded with an amorphous oxide state (discussed below in XPS) or embedded very uniformly without forming good crystal phases. In addition, because the metal amount was only 2 mol%, the XRD patterns could not be clearly observed when the phase was an amorphous oxide form. As seen in the HRTEM images of Ni-Al
2O
3 and Co-Al
2O
3 nanosheets discussed above (
Figure 2(b2) and
Figure S4, respectively), the particle-like areas showed very poor crystallinity. On the other hand, Pd, Ir, Pt, and Au-loaded samples showed new peaks in the corresponding XRD profiles.
For the XRD patterns of Pd-Al
2O
3 nanosheets, several peaks at 2θ = 33.8°, 42.0°, 54.7°, 60.1°, and 71.5° showed good matches with the (011), (110), (112), (013), and (121) crystal planes of tetragonal (p 42/mmc) PdO (ICSD ref. 98-002-9281), respectively [
13]. For Ir-Al
2O
3 nanosheets, several strong XRD peaks were observed at 2θ = 27.9°, 34.6°, 39.9°, 53.9°, 57.9°, 58.3°, 66.0°, 69.0°, and 73.0°, with good matches with the (110), (011), (020), (121), (220), (002), (130), (112), and (031) crystal planes of tetragonal (p 42/mnm) IrO
2 (ICSD ref. 98-008-4577), respectively. For Pt-Al
2O
3 nanosheets, three major peaks were observed at 2θ = 39.8°, 46.2°, and 67.5°, assigned to the (111), (002,) and (022) crystal planes of the cubic (Fm-3m) crystal phase of metallic Pt (ICSD ref. 98-007-6153), respectively. For Au-Al
2O
3 nanosheets, three strong peaks were observed at 2θ = 38.1°, 44.3°, and 64.5°, assigned to the (111), (002), and (022) crystal planes of the cubic (Fm-3m) crystal phase of Au (ICSD ref. 98-061-1624), respectively.
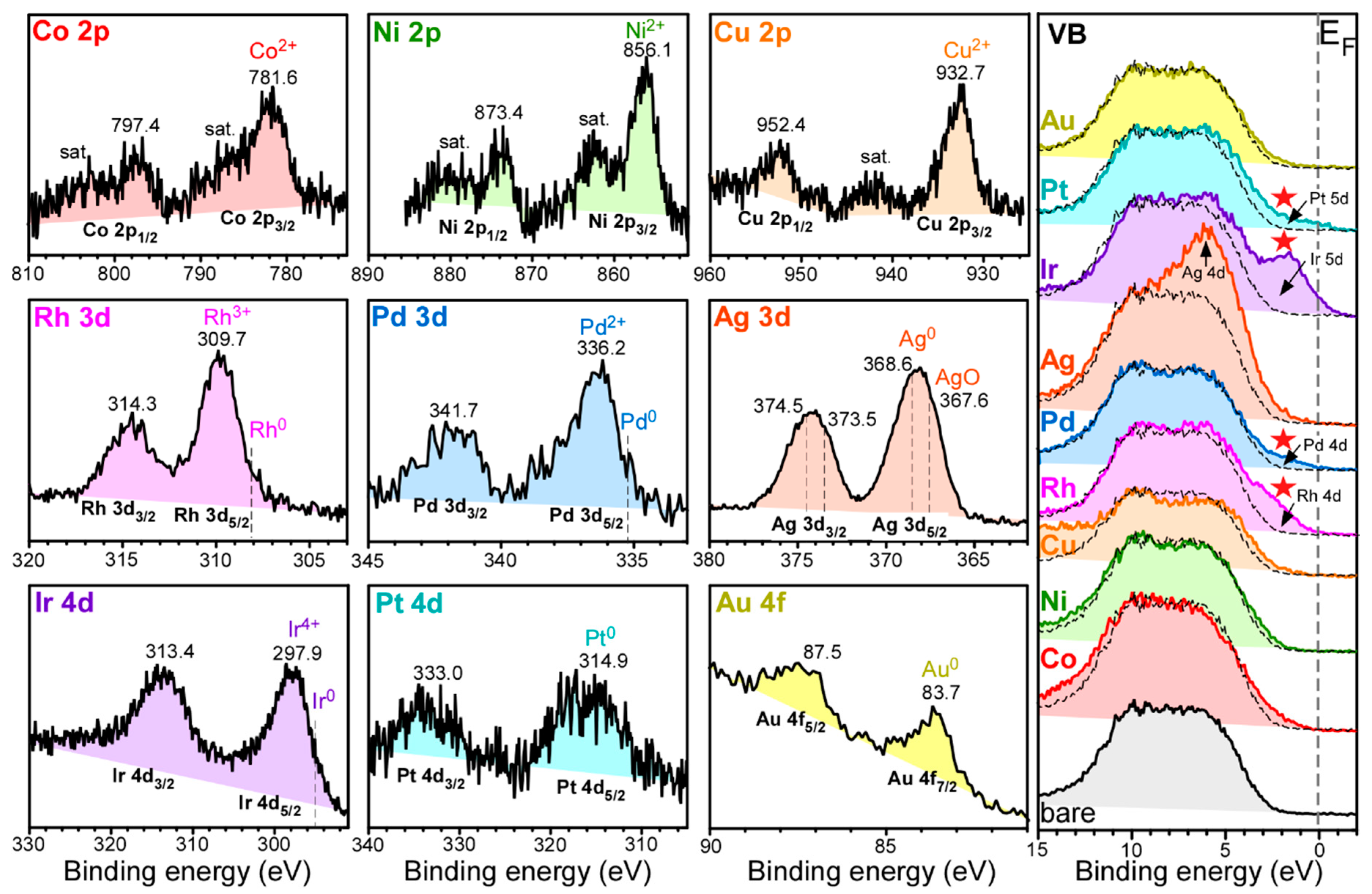
XPS was employed to confirm the loading of the transition metals and examine the oxidation states.
Figure 4 shows Co 2p, Ni 2p, Cu 2p, Rh 3d, Pd 3d, Ag 3d, Ir 4d, Pt 4d, and Au 4d of Co-Al
2O
3, Ni-Al
2O
3, Cu-Al
2O
3, Rh-Al
2O
3, Pd-Al
2O
3, Ag-Al
2O
3, Ir-Al
2O
3, Pt-Al
2O
3, and Au-Al
2O
3 nanosheets, respectively. XPS valence band spectra (
Figure 4, right panel) are also displayed for the corresponding samples. The survey, Al 2p, O 1s, and C 1s profiles are provided in the Supporting Information,
Figure S5. All the binding energies (BEs) were referenced to the C 1s XPS peak at 284.8 eV. The survey spectra commonly showed the elements of Al, O, and C (surface impurities), as expected. The XPS peaks of the loaded transition metals were very weakly observed.
For Co-Al
2O
3 nanosheets, Co 2p
1/2 and Co 2p
3/2 XPS peaks were observed at binding energies (BEs) of 797.4 eV and 781.6 eV, respectively, with a spin-orbit splitting of 15.8 eV. This could be attributed to Co
2+ of CoO and Co(OH)
2 [
31,
32]. The corresponding satellite peaks for Co
2+ were clearly observed around 803 eV and 786 eV. For Ni-Al
2O
3 nanosheets, Ni 2p
1/2 and Ni 2p
3/2 XPS peaks were observed at binding energies (BEs) of 873.4 eV and 856.1 eV, respectively, with a spin-orbit splitting of 17.3 eV. This could be attributed to Ni
2+ of NiO and Ni(OH)
2 [
15,
31,
32]. The corresponding satellite peaks for Ni
2+ were clearly observed around 880 eV and 862 eV. For Cu-Al
2O
3 nanosheets, Cu 2p
1/2 and Cu 2p
3/2 XPS peaks were observed at binding energies (BEs) of 952.4 eV and 932.7 eV, respectively, with a spin-orbit splitting of 19.7 eV. This could be attributed to Cu
2+ of CuO and Cu(OH)
2 [
32,
33]. The corresponding satellite peak for Cu
2+ was clearly observed around 942 eV. For Co, Ni, and Cu, no metallic XPS peaks were observed. On the basis of XRD, HRTEM, and XPS data, Co, Ni, and Cu appeared to be embedded as an amorphous oxide form.
For Rh-Al
2O
3 nanosheets, Rh 3d
3/2 and Rh 3d
5/2 XPS peaks were observed at BEs of 314.3 eV and 309.7 eV, respectively, with a spin-orbit splitting of 4.6 eV. The XPS BEs were attributed to an oxidation state of Rh
3+ [
32,
34]. As discussed above, the lattice distances in the HRTEM image confirmed orthorhombic Rh
2O
3. An additional weak shoulder peak was seen around 308 eV for Rh 3d
5/2 peak. This could be due to metallic Rh [
32,
34]. On the basis of the XPS and HRTEM data, Rh-species appeared to be consistent with Rh@Rh
2O
3 core-shell type structure.
For Pd-Al
2O
3 nanosheets, Pd 3d
3/2 and Pd 3d
5/2 XPS peaks were observed at BEs of 341.7 eV and 336.2 eV, respectively, with a spin-orbit splitting of 5.5 eV. The XPS peaks were attributed to an oxidation state of Pd
2+ [
32,
34,
35]. There was a good coincidence between the oxidation state of the XPS and the XRD profiles of tetragonal PdO. A weak shoulder of the Pd 3d
5/2 peak was seen around 335.5 eV, plausibly due to metallic Pd [
32]. For Ag-Al
2O
3 nanosheets, Ag 3d
3/2 and Ag 3d
5/2 XPS peaks were observed at BEs of 374.5 eV and 368.6 eV, respectively. This was attributed to metallic Ag [
6,
32,
36]. The shoulder XPS peak at 367.6 eV for Ag 3d
5/2 was plausibly due to AgO [
36]. On the basis of the XPS profile for each M-Al
2O
3 sample, it could be concluded that the transition metal was loaded on the Al
2O
3 support.
For Ir-Al
2O
3 nanosheets, Ir 4d
3/2 and Ir 4d
5/2 XPS peaks were observed at BEs of 313.6 eV and 297.7 eV, respectively. These peaks were assigned to the Ir
4+ oxidation state [
37], in good coincidence with the XRD profiles of tetragonal IrO
2, shown above. A weak shoulder of Ir 4d
5/2 peak was seen around 295 eV, plausibly due to metallic Ir [
37]. For Pt-Al
2O
3 nanosheets, Pt 4d
3/2 and Pt 4d
5/2 XPS peaks were observed at BEs of 332.9 eV and 314.9 eV, respectively, with a spin-orbit splitting of 18.0 eV. The XPS peaks were attributed to metallic Pt [
34,
35], which was well-fitting with the XRD result of metallic Pt. For Au-Al
2O
3 nanosheets, the Au 4f
7/2 and Au 4f
5/2 XPS peaks were observed at BEs of 87.3 eV and 83.6 eV, respectively, with a spin-orbit splitting of 3.7 eV. The XPS peaks were attributed metallic Au [
32]. This result was in good agreement with the XRD profiles of metallic Au, shown above.
For the Al 2p XPS profiles (Supporting Information,
Figure S5), a broad peak was commonly observed around 74.1 eV, attributed to Al of the Al
2O
3 support [
32,
38]. An additional peak at 75.0 eV was observed and attributed to the Al of surface Al-OH species [
32,
38]. For the O 1s XPS profiles (Supporting Information,
Figure S5), a broad peak was commonly observed around 530.9 eV due to lattice O of Al
2O
3 support. A broad shoulder at 532.5 eV was attributed to oxygen defects and surface OH/H
2O species [
39].
The valence band (VB) spectra are shown in
Figure 4 to further examine electronic structures. For the VB of bare Al
2O
3 nanosheets, two broad features were seen around 9 eV and 6 eV, attributed bonding 2pσ (mixed with Al 3s, Al 3p, and Al 3d) and antibonding 2pπ of the oxygen [
40]. For VB spectra of M-Al
2O
3 nanosheets, the density of states (DOS) was observed to be closer to the Fermi level. Especially, Rh, Pd, Ir, and Pt showed more clearly new features near 2 eV below the Fermi level, attributed to the Rh 4d, Pd 4d, Ir 5d, and Pt 5d, respectively. This could be related to the higher CO oxidation activities for these metals, discussed below. However, the DOS profiles showed no explicit relationship with the photocatalytic CO
2 reduction activity. The detailed roles of the overlayer elements could be understood with the aid of density functional theory.
Temperature-programmed CO oxidation profiles (Supporting Information,
Figure S6) were obtained to examine thermal CO oxidation catalytic activities for bare Al
2O
3 and M-Al
2O
3 nanosheets. To evaluate the catalytic activities of the catalysts,
Figure 5a,b display the CO oxidation onset temperatures for the first and the second runs, respectively.
Table 1 summarizes the onset temperatures (T
M-Al2O3,onset) and the temperature difference (T
M-Al2O3,2nd − T
M-Al2O3,1st) between the first and the second runs. The onset temperatures of Ir-, Pt-, Pd-, and Rh-loaded Al
2O
3 nanosheets were observed to be much lower than those of Au-, Ag-, Cu-, Co-, and Ni-loped Al
2O
3 nanosheets. The group 11 (Au, Ag, and Cu) and the period 4 (Co, Ni, and Cu) elements showed much poor catalytic activity on the Al
2O
3 support. Additionally, the onset temperatures of Au and Ag-loaded Al
2O
3 nanosheets were unexpectedly even higher than expected [
6,
10,
15]. In other words, the Au- and Ag-loaded Al
2O
3 nanosheets showed poorer CO oxidation activity. In the first run, the Rh-Al
2O
3 nanosheets showed the lowest onset of 135 °C, while the Ni-Al
2O
3 nanosheets showed the highest onset of 490 °C. The temperature difference between the two samples was estimated to be 335 °C. In the second run, the Rh-Al
2O
3 nanosheets also showed the lowest onset of 172 °C while the Ni-Al
2O
3 nanosheets showed the highest onset of 480 °C. The temperature difference was estimated to be 308 °C. Pd, Ir, and Pt showed the CO oxidation onsets at 207 °C, 217 °C, and 216 °C, respectively, in the second run. For highly dispersed (or single atom state) 0.2 wt % Pt on mesoporous Al
2O
3 support, Zhang et al. reported CO oxidation onset at ~200 °C, which was in good coincidence with the present result [
5]. These results clearly indicated that the CO oxidation activity was highly influenced by the nature of overlayer metal species.
Figure 5c shows the CO oxidation profiles for the first and the second runs of the selected samples (bare Al
2O
3, Ni-Al
2O
3, and Rh-Al
2O
3 catalysts). As seen in the
Figure 5, the CO oxidation onset of Rh-Al
2O
3 occurred much earlier than that of bare Al
2O
3. The onsets of Rh-Al
2O
3 in the first and the second runs were observed to be 251 °C and 201 °C lower than those of bare Al
2O
3, respectively. However, the onset temperatures became much higher upon loading Ni.
To examine the difference in catalytic activity between the first and the second runs,
Figure 5d plots the temperature differences (T
M-Al2O3,2nd − T
M-Al2O3,1st) in the CO oxidation onsets between the first and the second runs. In the first run, the CO oxidation reactions were performed with the as-prepared samples. In the second run, the CO oxidation reactions were performed with samples, which were already participated in the first run. Therefore, the surface states (or the catalytic-active sites) were expected to be different for the samples in the first and the second runs. The values (T
M-Al2O3,2nd − T
M-Al2O3,1st) are summarized in
Table 1. The positive value (
Figure 5d) indicated that the CO oxidation started at a higher temperature in the second run. In other words, the CO oxidation catalytic activity became lower in the second run.
For Co- and Ni-Al2O3 nanosheets, the onset temperatures in the second run were observed to be slightly lower than those in the first run. However, the other samples commonly showed higher onset temperatures in the second run, compared with the first run. This indicated that, for the latter, the catalytic activity became somewhat lower after the first run. The lower catalytic activity appeared to be mainly due to a change in crystallinity and lower catalytic-active sites.
To evaluate the roles of the transition metals in catalytic activities, compared with bare Al
2O
3,
Figure 5e,f show the relative CO oxidation onsets (T
Al2O3 − T
M-Al2O3), compared with those of the first and the second runs of the bare Al
2O
3, respectively. The values are summarized in
Table 2. In the first runs, the T
Al2O3,1st − T
M-Al2O3,1st values of Co and Ni showed positive, and others showed negative values. In the second runs, Co, Ni, Cu, Ag, and Au showed positive, and others showed negative values. On the basis of
Figure 5e,f, the catalytic activity became poorer upon loading Co and Ni, compared with bare Al
2O
3. Unexpectedly, the Au, Ag, and Cu (group 11) showed somewhat higher activities in the first run but showed poorer catalytic activity in the second run, compared with the bare Al
2O
3 nanosheet. The Rh, Pd, Ir, and Pt showed much higher (with lowering of onset temperatures between 156 °C and 261 °C) CO oxidation activity in the first and second runs. Conclusively, the CO oxidation activity showed the order of Ni < Co < Au < Cu < Ag < Pd < Pt < Ir < Rh in the first run, and Ni < Au < Ag < Cu < Co < Ir < Pt ≈ Pd < Rh in the second run.
For CO oxidation, a simplified mechanism is described below;
The CO oxidation mechanism was explained by the Langmuir-Hinshelwood mechanism [
12,
13] and the non-Langmuir-Hinshelwood mechanism [
4,
11], depending on the overlayer transition metals. In reaction (1), CO was adsorbed on metal site, and in reaction (2), oxygen was dissociatively adsorbed on the surface. In reaction (3), gaseous CO and surface O reacted to release CO
2 [
12,
13]. When moisture was present in the reaction, the surface OH group was plausibly formed and CO might also react with the surface metal hydroxide to form the CO
2 in reaction (4) [
15]. On the basis of the FT-IR spectra (Supporting Information,
Figure S7), surface OH groups were observed in the as-prepared samples. Therefore, reaction (4) was likely involved in the first run of CO
2 formation. If H was not desorbed as H
2O, the surface H was recycled as shown in reaction (4). Otherwise, the H was removed from the surface as gaseous H
2O, and, thus, the reaction (4) was diminished in the second run. Reaction (5) was also reported for surfaces such as Pt/Al
2O
3 [
4,
11]. In reaction (5), CO was adsorbed on oxygenated metal atoms to initially form carbonate. Then, the carbonate dissociated to generate CO
2 in reaction (6).
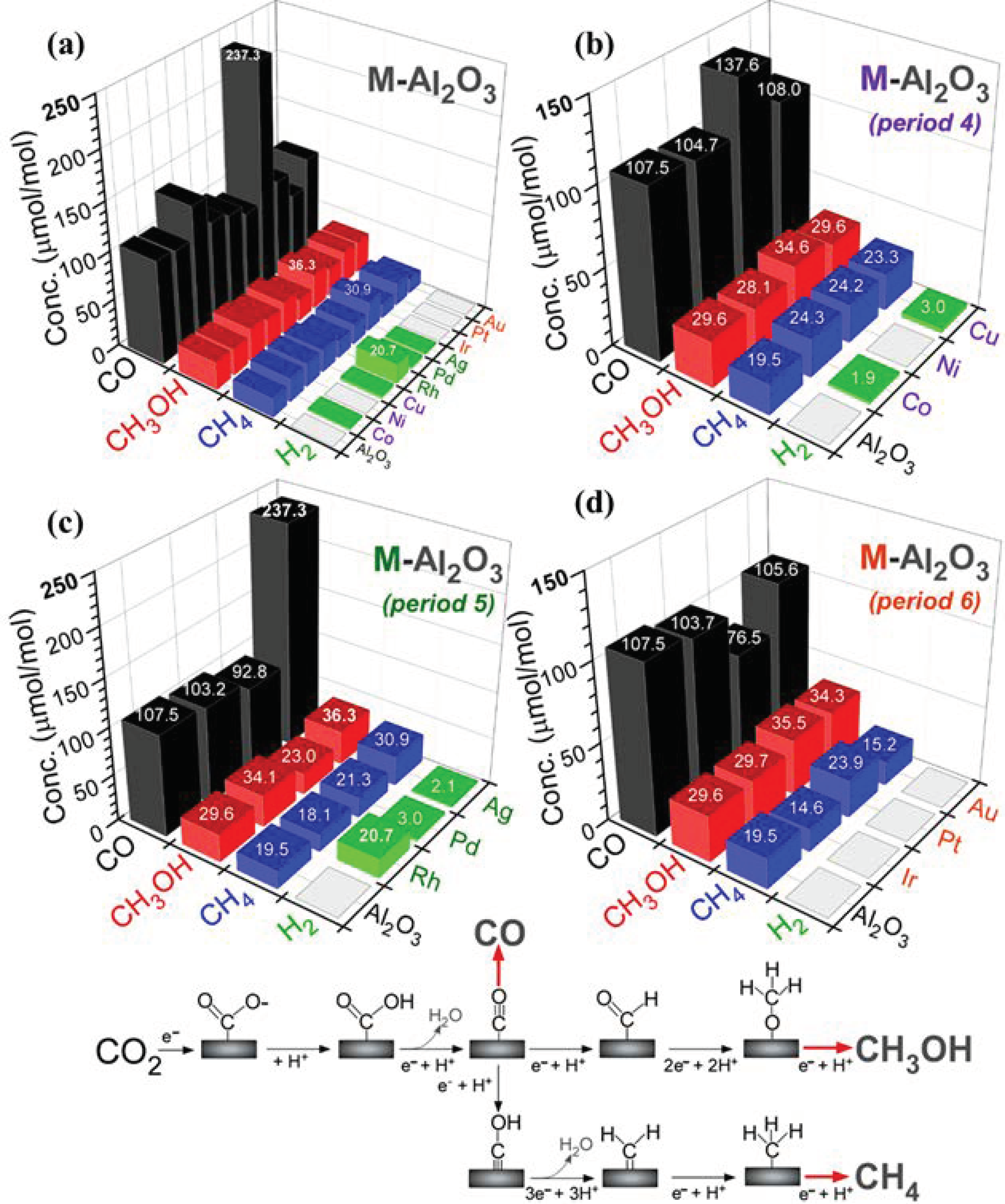
Photocatalytic CO
2 reduction products were examined for bare Al
2O
3 and M-Al
2O
3 nanosheets and are displayed in
Figure 6 [
39,
41,
42]. Major CO
2 reduction products were observed to be carbon monoxide (CO), methanol (CH
3OH), and methane (CH
4) with an order: CH
4 < CH
3OH < CO. CO was the most dominantly produced species. CH
3OH showed a higher production amount compared with CH
4. Hydrogen (H
2) was additionally observed as a photocatalytic water splitting product during CO
2 reduction.
Figure 6a plots all of the product amounts (μmol/mol = ppm) for bare Al
2O
3 and M-Al
2O
3 nanosheets. As a quick glance, Ag-Al
2O
3 nanosheets showed the highest amounts of CO
2 reduction products: 237.3 ppm for CO, 36.3 ppm for CH
3OH, and 30.9 ppm for CH
4, and Rh-Al
2O
3 nanosheets showed the highest H
2 production (20.7 ppm). For the bare Al
2O
3 nanosheets in
Figure 6b, CO, CH
3OH, and CH
4 were observed to be 107.5 ppm, 29.6 ppm, and 19.5 ppm, respectively. No H
2 was detected. CO reduction yields (μmol/mol) in different groups of 9, 10, and 11, and with different units (μmol/g), are provided in the Supporting Information,
Figures S8 and S9, respectively.
For bare Al
2O
3, the selectivities for CO, CH
3OH, and CH
4 were estimated to be 68.6%, 18.9%, and 12.5%, respectively. Upon Co- and Cu-loading, CH
4 and H
2 showed meaningful (>25%) enhancements. However, the amounts of CO and CH
3OH showed no critical change. CO, CH
3OH, and CH
4 productions were enhanced by 28%, 17%, and 24% upon Ni-loading. CO was increased by 2.2× upon loading Ag in
Figure 6c. CH
3OH and CH
4 were also increased by 1.23× and 1.58×, respectively, upon loading Ag. Rh and Pd-loadings had a smaller effect on the CO production relative to the bare support. CH
3OH and CH
4 productions were not meaningfully enhanced by Rh- and Pd-loadings. Instead, interestingly the H
2 production was commonly observed in these metal-loadings. For Ir, Pt, and Au elements in period 6, CO productions were all decreased by metal-loadings. CH
3OH productions were somewhat increased by 19% and 16% upon loading of Pt and Au, respectively. The CH
4 production was only increased upon loading Pt relative to the bare substrate.
For H2 production, Ag, Pd, and Rh (in period 5) metals commonly showed H2 productions with amounts of 2.1 ppm, 3.0 ppm, and 20.7 ppm, respectively. For the metals of Co, Ni, and Cu (in period 4), the H2 production amounts were observed to be 1.9 ppm, 0 ppm, and 3.0 ppm, respectively. That is, Ni showed no H2 production. The metals of Au, Pt, and Ir in period 6 commonly showed no H2 production at all. The Rh-Al2O3 nanosheets predominantly showed the highest H2 production with an amount of 20.7 ppm.
The photocatalytic CO
2 reduction mechanism is generally written as
xCO
2 +
yH
+ +
ze
− → C
aH
bO
c products +
dH
2O [
41,
42]. Electrons (e
−) and holes (h
+) were generated under UVC irradiation in reaction (7). H
+ ion was generated via the reactions in (8)–(11). The generation of electrons was an important factor for the multielectron processes. The mechanisms for the productions of CO (in reaction (12)), CH
3OH (in reaction (13)), and CH
4 (in reaction (14)) are written as below and shown in
Figure 6 [
38,
39].
These reaction channels were closely spaced in free energy change, and, thus, the hydrogen production channel (H+ + e− → 1/2H2, −0.42 V vs. SHE) occurred competitively. In the mechanism, CO2 was initially adsorbed to form COOH. The COOH was then attacked by H+ and e− to generate gaseous CO. The CO production channel was only enhanced by loading Ag or Ni on Al2O3 support. CH3OH production was likely formed when surface COad underwent step-wise hydrogenation. This production was enhanced by loading Ni, Rh, Ag, Pt, or Au on Al2O3 support. CH4 production was formed via C–O bond scission of hydrogenated ≡C‒OH and new C‒H bond formation. This production was somewhat enhanced by loading Co, Ni, Cu, Ag, or Pt. The present pre-screening tests need further investigations to understand the detailed roles of the overlayer elements, with the aid of density functional theory.