Effect of the Helium Background Gas Pressure on the Structural and Optoelectronic Properties of Pulsed-Laser-Deposited PbS Thin Films
Abstract
1. Introduction
2. Materials and Methods
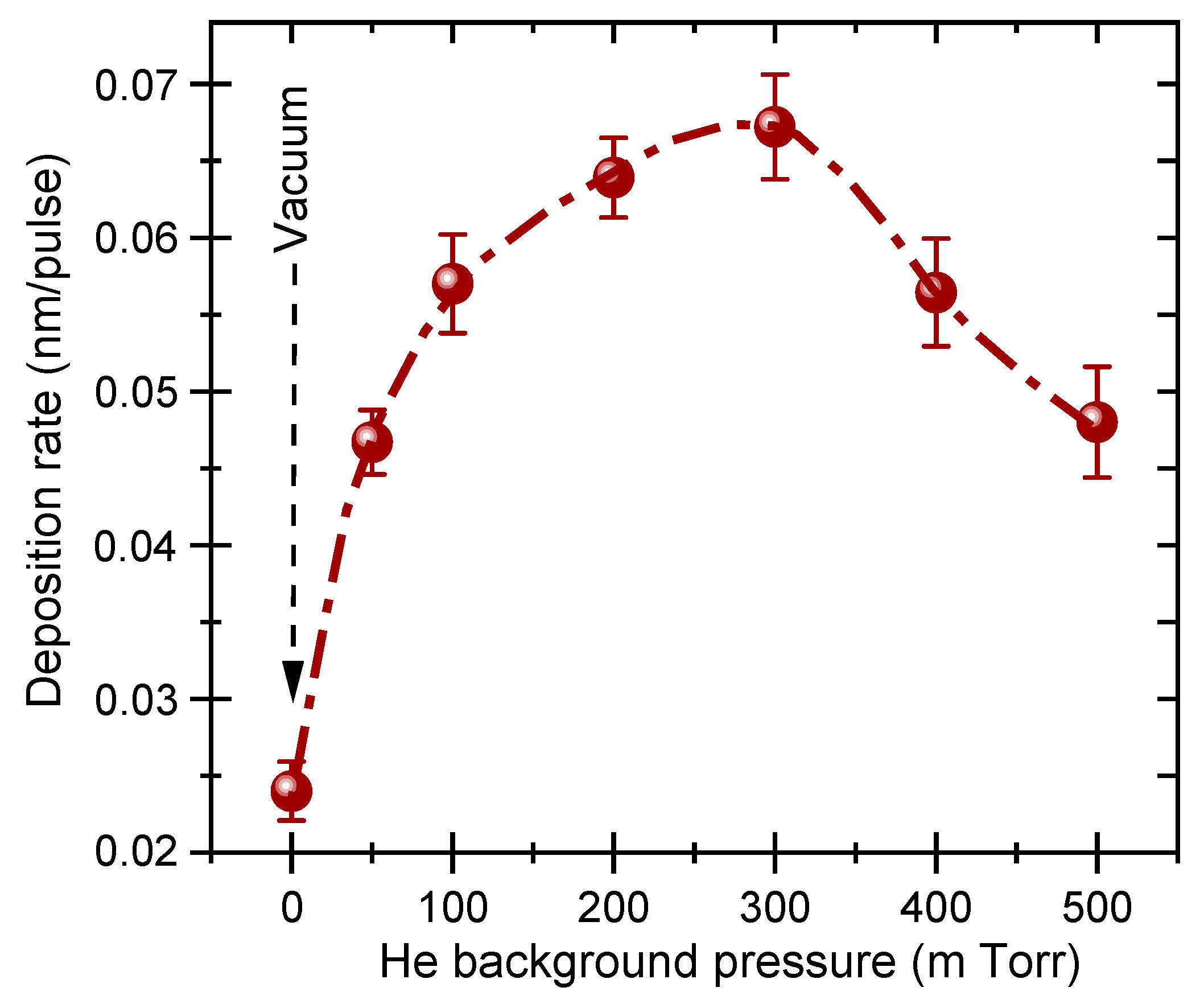
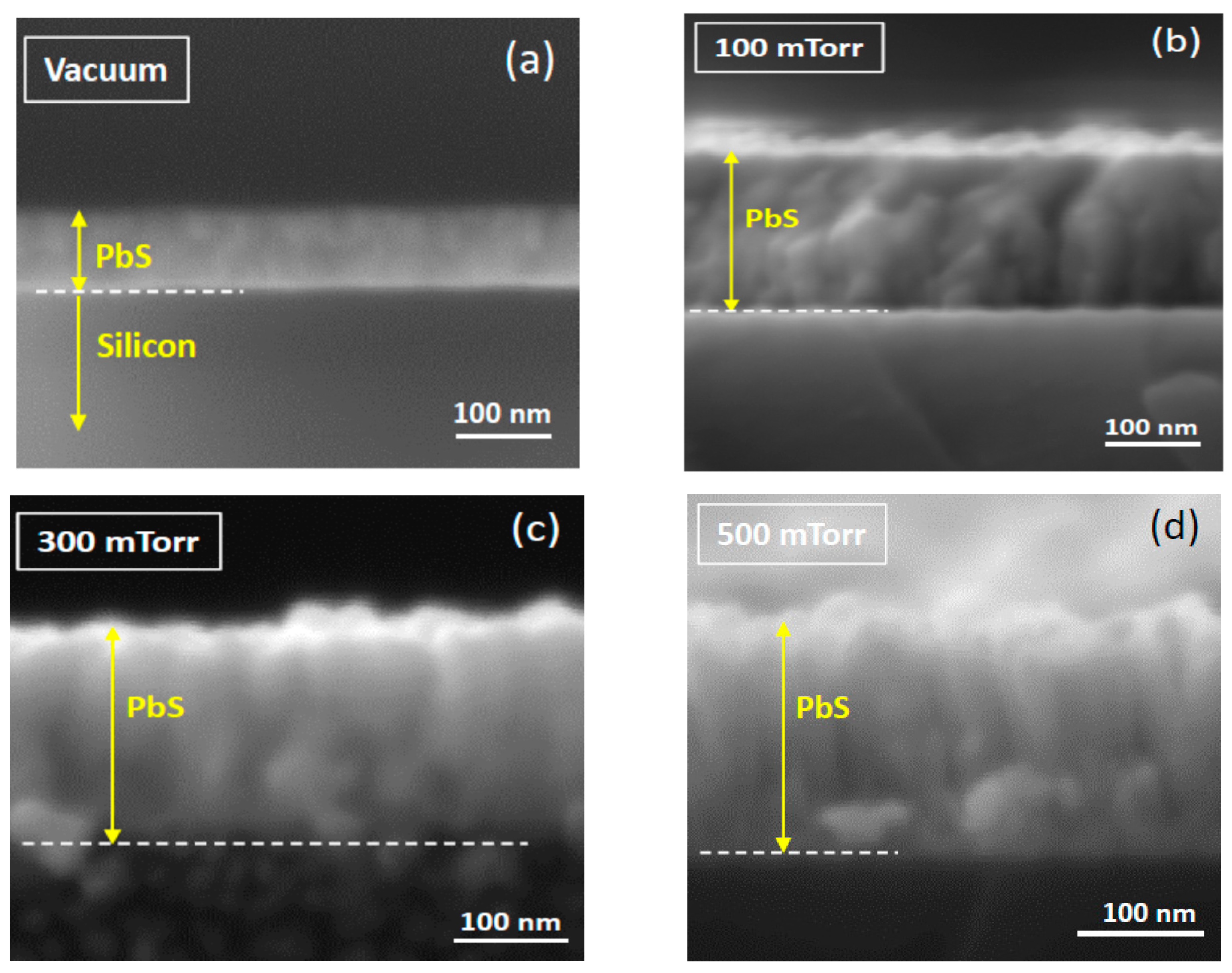
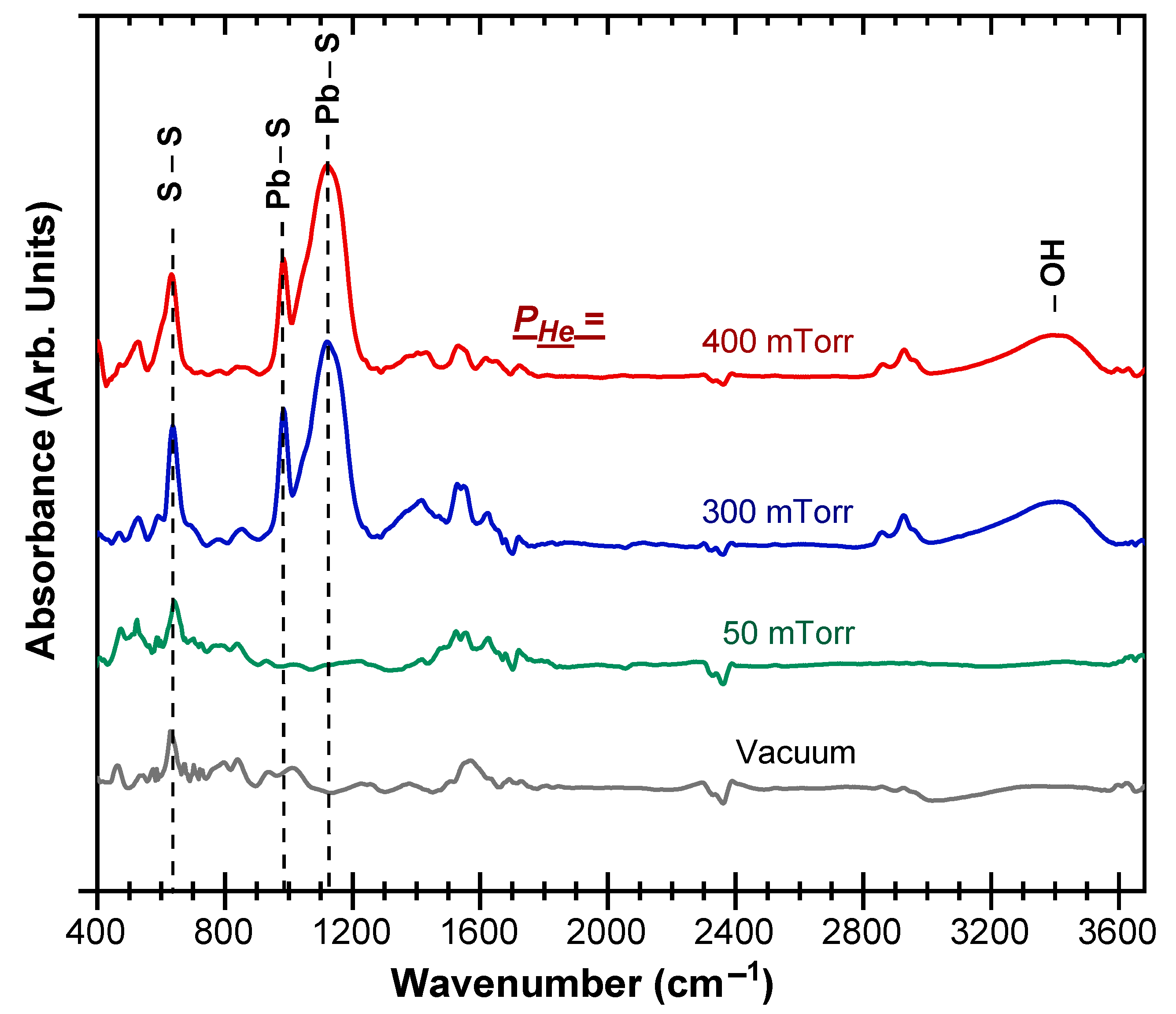
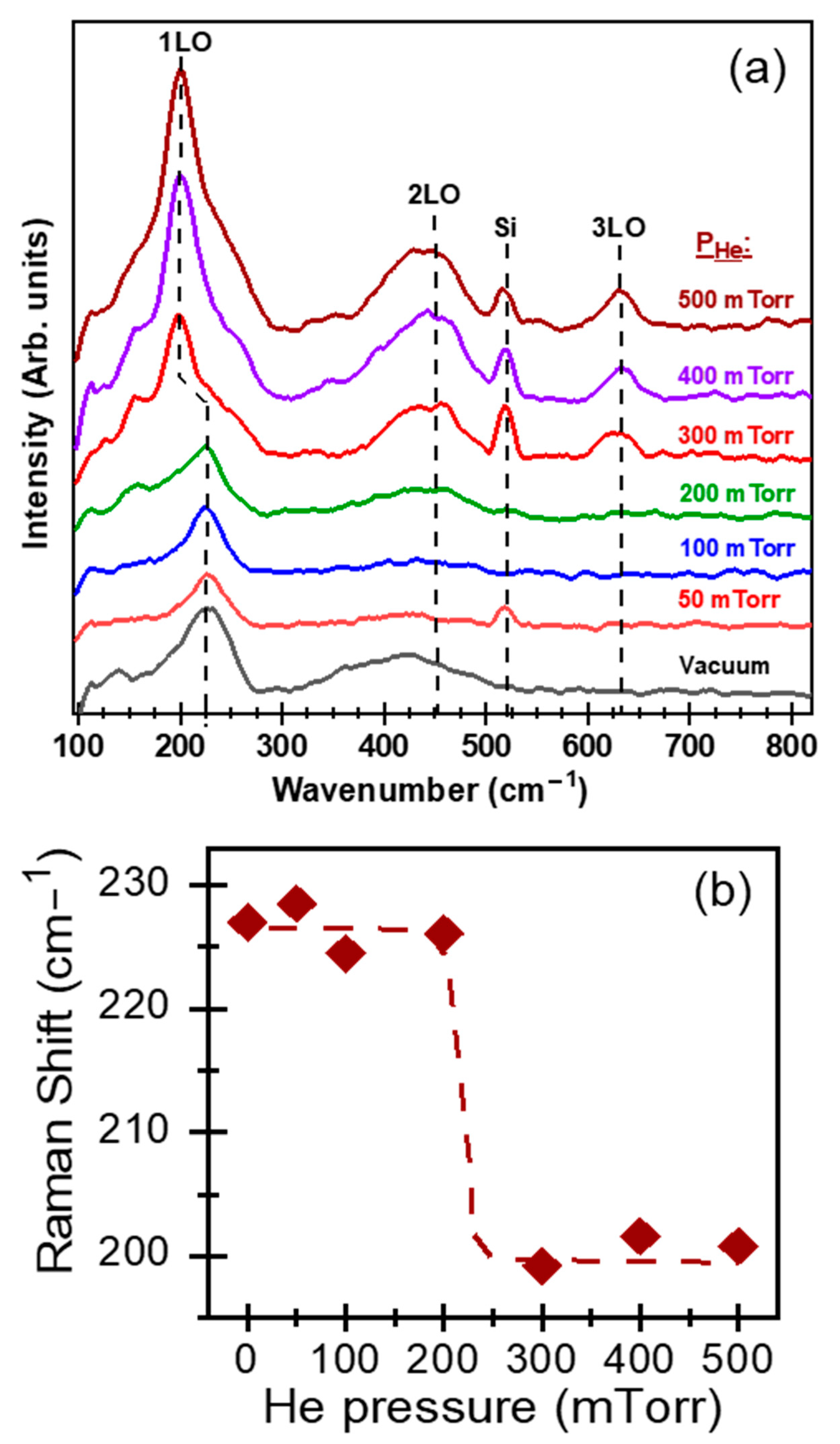
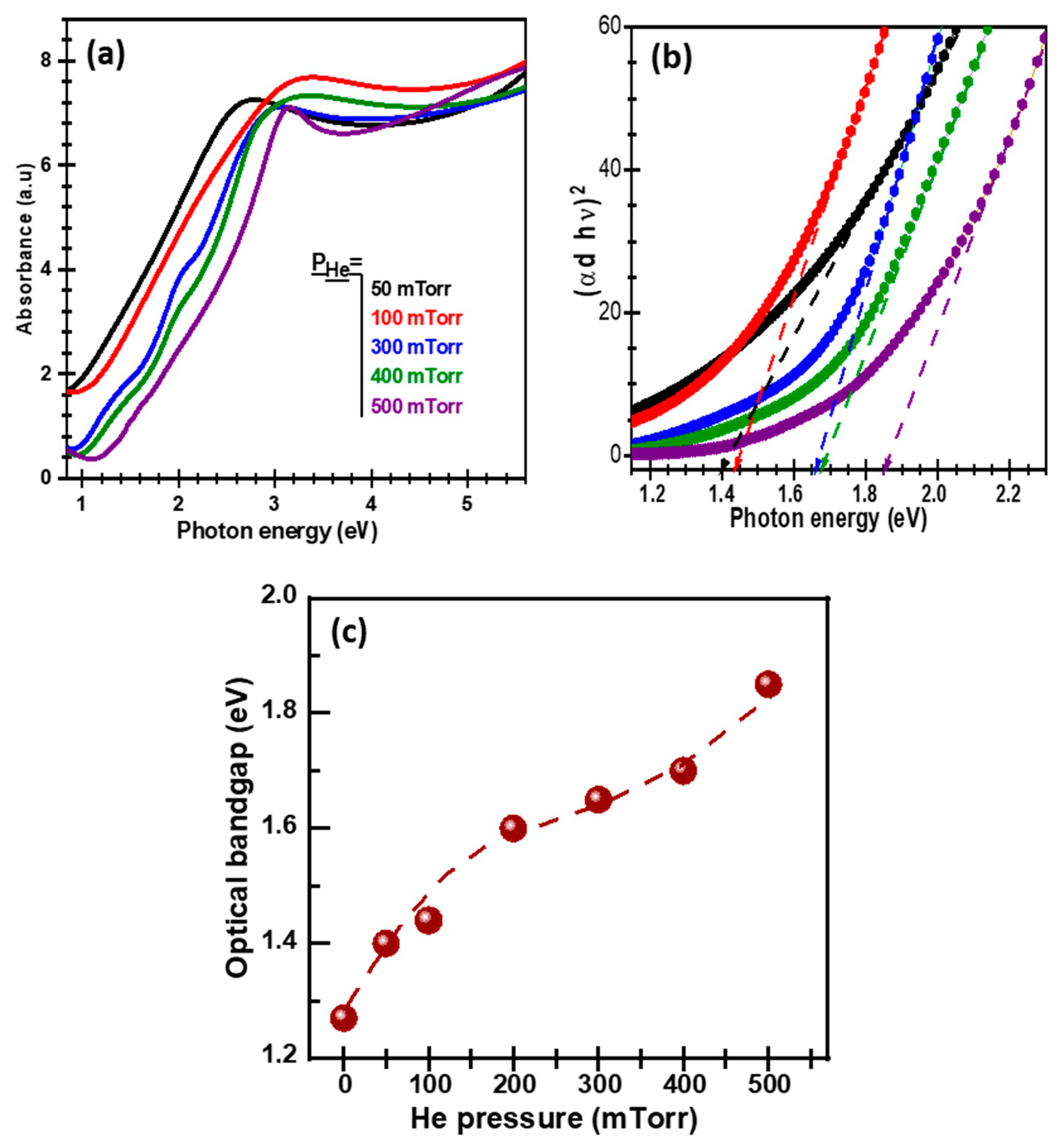
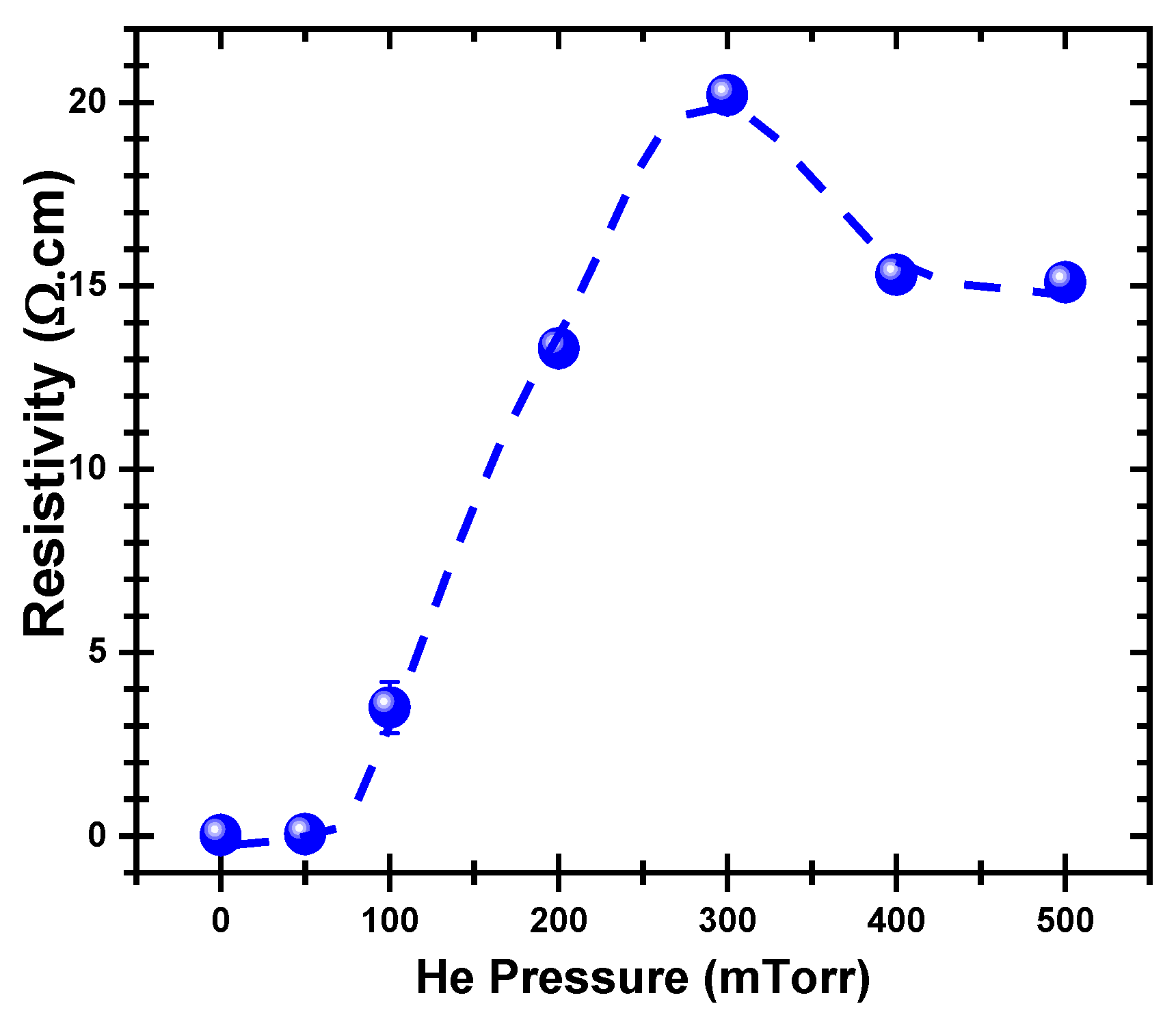
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madelung, O. Semiconductors: Data Handbook, 3rd ed.; Springer: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Touati, B.; Gassoumi, A.; AlFaify, S.; Kamoun-Turki, N. Optical, morphological and electrical studies of Zn: PbS thin films. Mater. Sci. Semicond. Process. 2015, 34, 82–87. [Google Scholar] [CrossRef]
- Sukhovatkin, V.; Hinds, S.; Brzozowski, L.; Sargent, E.H. Colloidal Quantum-Dot Photodetectors Exploiting Multiexciton Generation. Science 2009, 324, 1542–1544. [Google Scholar] [CrossRef]
- Maraghechi, P.; Labelle, A.J.; Kirmani, A.R.; Lan, X.; Adachi, M.M.; Thon, S.M.; Sargent, E.H. The Donor–Supply Electrode Enhances Performance in Colloidal Quantum Dot Solar Cells. ACS Nano 2013, 7, 6111–6116. [Google Scholar] [CrossRef]
- Rosario, S.R.; Kulandaisamy, I.; Arulanantham, A.M.S.; Kumar, K.D.A.; Valanarasu, S.; Shkir, M.; AlFaify, S. Fabrication and characterization of lead sulfide (PbS) thin film based heterostructure (FTO/CdS/PbS/Ag) solar cell by nebulizer spray method. Mater. Res. Express 2019, 6, 056416. [Google Scholar] [CrossRef]
- Moon, D.G.; Rehan, S.; Yeon, D.H.; Lee, S.M.; Park, S.J.; Ahn, S.; Cho, Y.S. A review on binary metal sulfide heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2019, 200, 109963. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M. Studies on PbS and PbSe Detectors for IR System. Int. J. Infrared Millim. Waves 2000, 21, 1697–1701. [Google Scholar] [CrossRef]
- Safrani, T.; Kumar, T.A.; Klebanov, M.; Arad-Vosk, N.; Beach, R.; Sa’Ar, A.; Golan, Y. Chemically deposited PbS thin film photo-conducting layers for optically addressed spatial light modulators. J. Mater. Chem. C 2014, 2, 9132–9140. [Google Scholar] [CrossRef]
- Cheragizade, M.; Yousefi, R.; Jamali-Sheini, F.; Mahmoudian, M.R.; Sa, A.; Huang, N.M. Synthesis and characterization of PbS mesostructures as an IR detector grown by hydrogen-assisted thermal evaporation. Mater. Sci. Semicond. Process. 2014, 26, 704–709. [Google Scholar] [CrossRef]
- Aadim, K.; Ibrahim, A.; Marie, J. Structural and optical properties of PbS thin films deposited by pulsed laser deposited (PLD) technique at different annealing temperature. Int. J. Phy. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Barrios-Salgado, E.; Rodríguez-Lazcano, Y.; Pérez-Orozco, J.P.; Colin, J.; Altuzar, P.; Campos, J.; Quesada, D. Effect of Deposition Time on the Optoelectronics Properties of PbS Thin Films Obtained by Microwave-Assisted Chemical Bath Deposition. Adv. Condens. Matter Phys. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Göde, F.; Güneri, E.; Emen, F.M.; Kafadar, V.E.; Ünlü, S. Synthesis, structural, optical, electrical and thermoluminescence properties of chemically deposited PbS thin films. J. Lumin. 2014, 147, 41–48. [Google Scholar] [CrossRef]
- Bai, R.; Kumar, D.; Chaudhary, S.; Pandya, D.K. Highly crystalline p-PbS thin films with tunable optical and hole transport parameters by chemical bath deposition. Acta Mater. 2017, 131, 11–21. [Google Scholar] [CrossRef]
- Seghaier, S.; Kamoun, N.; Brini, R.; Amara, A.B. Structural and optical properties of PbS thin films deposited by chemical bath deposition. Mater. Chem. Phys. 2006, 97, 71–80. [Google Scholar] [CrossRef]
- Kanniainen, T.; Lindroos, S.; Ihanus, J.; Leskelä, M. Growth of strongly orientated lead sulfide thin films by successive ionic layer adsorption and reaction (SILAR) technique. J. Mater. Chem. 1996, 6, 161–164. [Google Scholar] [CrossRef]
- Alanyalıoğlu, M.; Bayrakçeken, F.; Demir, Ü. Preparation of PbS thin films: A new electrochemical route for underpotential deposition. Electrochim. Acta 2009, 54, 6554–6559. [Google Scholar] [CrossRef]
- Thiagarajan, R.; Beevi, M.M.; Anusuya, M.; Ramesh, T. Influence of reactant concentration on nano crystalline PbS thin films prepared by Chemical Spray Pyrolysis. Optoelectro. Adv. Mater. Rapid Commun. 2012, 6, 132–135. [Google Scholar]
- Sukharevska, N.; Bederak, D.; Goossens, V.M.; Momand, J.; Duim, H.; Dirin, D.N.; Loi, M.A. Scalable PbS Quantum Dot Solar Cell Production by Blade Coating from Stable Inks. ACS Appl. Mater. Interfaces 2021, 13, 5195–5207. [Google Scholar] [CrossRef]
- Rohom, A.B.; Londhe, P.U.; Jadhav, P.R.; Bhand, G.R.; Chaure, N.B. Studies on chemically synthesized PbS thin films for IR detector application. J. Mater. Sci. Mater. Electron. 2017, 28, 17107–17113. [Google Scholar] [CrossRef]
- Guozheng, M.; Binshi, X.; Haidou, W.; Shuying, C.; Zhiguo, X. Excellent Vacuum Tribological Properties of Pb/PbS Film Deposited by Rf Magnetron Sputtering and Ion Sulfurizing. ACS Appl. Mater. Interfaces 2014, 6, 532–538. [Google Scholar] [CrossRef]
- Da Silva Filho, J.M.C.; Marques, F.C. Structural and optical temperature-dependent properties of PbS thin films deposited by radio frequency sputtering. Mater. Sci. Semicond. Process. 2019, 91, 188–193. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, T.P.; Zulfequar, M.; Husain, M. Characterization of vacuum evaporated PbS thin films. Phys. B Condens. Matter 2003, 325, 8–16. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Lee, W.; Prinz, F.B. Atomic Layer Deposition of Lead Sulfide Thin Films for Quantum Confinement. Chem. Mater. 2009, 21, 3973–3978. [Google Scholar] [CrossRef]
- Ka, I.; Ma, D.; El Khakani, M.A. Tailoring the photoluminescence of PbS-nanoparticles layers deposited by means of the pulsed laser ablation technique. J. Nanopart. Res. 2011, 13, 2269–2274. [Google Scholar] [CrossRef]
- Atwa, D.M.M.; Azzouz, I.M.; Badr, Y. Optical, structural and optoelectronic properties of pulsed laser deposition PbS thin film. Appl. Phys. B 2011, 103, 161–164. [Google Scholar] [CrossRef]
- Beatriceveena, T.V.; Prabhu, E.; Jayaraman, V.; Gnanasekar, K.I. X-ray photoelectron and Hall studies on nanostructured thin films of PbS grown by pulsed laser deposition. Mater. Lett. 2019, 238, 324–327. [Google Scholar] [CrossRef]
- El Khakani, M.A.; Chaker, M. Reactive pulsed laser deposition of iridium oxide thin films. Thin Solid Films 1998, 335, 6–12. [Google Scholar] [CrossRef]
- Ka, I.; Le Borgne, V.; Ma, D.; El Khakani, M.A. Pulsed Laser Ablation based Direct Synthesis of Single-Wall Carbon Nanotube/PbS Quantum Dot Nanohybrids Exhibiting Strong, Spectrally Wide and Fast Photoresponse. Adv. Mater. 2012, 24, 6289–6294. [Google Scholar] [CrossRef]
- Ka, I.; Gonfa, B.; Le Borgne, V.; Ma, D.; El Khakani, M.A. Pulsed Laser Ablation Based Synthesis of PbS-Quantum Dots-Decorated One-Dimensional Nanostructures and Their Direct Integration into Highly Efficient Nanohybrid Heterojunction-Based Solar Cells. Adv. Funct. Mater. 2014, 24, 4042–4050. [Google Scholar] [CrossRef]
- Gaidi, M.; Hajjaji, A.; Smirani, R.; Bessaïs, B.; El Khakani, M.A. Structure and photoluminescence of ultrathin films of SnO2 nanoparticles synthesized by means of pulsed laser deposition. J. Appl. Phys. 2010, 108, 63537. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G.K. Pulsed Laser Deposition of Thin Films; Wiley Inter-Science Publications: Hoboken, NJ, USA, 1994. [Google Scholar]
- Popescu, A.C.; Stan, G.E.; Duta, L.; Nita, C.; Popescu, C.; Surdu, V.A.; Craciun, V. The Role of Ambient Gas and Pressure on the Structuring of Hard Diamond-Like Carbon Films Synthesized by Pulsed Laser Deposition. Materials 2015, 8, 3284–3305. [Google Scholar] [CrossRef]
- Dhlamini, M.S.; Terblans, J.J.; Ntwaeaborwa, O.M.; Ngaruiya, J.M.; Hillie, K.T.; Botha, J.R.; Swart, H.C. Photoluminescence properties of powder and pulsed laser-deposited PbS nanoparticles in SiO2. J. Lumin. 2008, 128, 1997–2003. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, R. Influence of laser flux density on the surface morphology of lead sulphide thin films. Chalcogenide Lett. 2009, 6. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.M.; Zhang, S.; Zeng, H.R. Oxygen-pressure dependence of the crystallinity of MgO films grown on Si(100) by PLD. J. Cryst. Growth 2004, 270, 553–559. [Google Scholar] [CrossRef]
- Riabinina, D.; Chaker, M.; Rosei, F. Correlation between plasma dynamics and porosity of Ge films synthesized by pulsed laser deposition. Appl. Phys. Lett. 2006, 89, 131501. [Google Scholar] [CrossRef]
- Dolbec, R.; El Khakani, M.A.; Serventi, A.M.; Saint-Jacques, R.G. Influence of the nanostructural characteristics on the gas sensing properties of pulsed laser deposited tin oxide thin films. Sens. Actuators B Chem. 2003, 93, 566–571. [Google Scholar] [CrossRef]
- Hamoudi, Z.; El Khakani, M.A.; Mohamedi, M. Influence of Pressure on the Structural and Electrocatalytic Properties of Pt Nanoparticles Grown by Pulsed Laser Ablation onto Carbon Paper Substrate. Int. J. Electrochem. Sci. 2012, 7, 1666–1676. [Google Scholar]
- Geohegan, D.B.; Puretzky, A.A. Dynamics of laser ablation plume penetration through low pressure background gases. Appl. Phys. Lett. 1995, 67, 197–199. [Google Scholar] [CrossRef]
- Farid, N.; Harilal, S.S.; Ding, H.; Hassanein, A. Emission features and expansion dynamics of nanosecond laser ablation plumes at different ambient pressures. J. Appl. Phys. 2014, 115, 033107. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Sylvestre, J.P.; Patskovsky, S.; Meunier, M. Correlation between photoluminescence properties and morphology of laser-ablated Si/SiOx nanostructured. J. Appl. Phys. 2002, 91, 3248–3254. [Google Scholar] [CrossRef]
- Brassard, D.; El Khakani, M.A. Pulsed-laser deposition of high-k titanium silicate thin films. J. Appl. Phys. 2005, 98, 54912. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Thakur, P.; Sachar, S.; Kaur, G.; Banipal, T.S.; Possmayer, F.; Petersen, N.O. Aqueous Phase Surfactant Selective Shape Controlled Synthesis of Lead Sulfide Nanocrystals. J. Phys. Chem. C 2007, 111, 18087–18098. [Google Scholar] [CrossRef]
- Nafees, M.; Ikram, M.; Ali, S. Thermal stability of lead sulfide and lead oxide nano-crystalline materials. Appl. Nanosci. 2017, 7, 399–406. [Google Scholar] [CrossRef]
- Giribabu, K.; Suresh, R.; Manigandan, R.; Vijayalakshmi, L.; Stephen, A.; Narayanan, V. Hydrothermal Synthesis of Lead Sulphide Nanoparticles and their Electrochemical Sensing Property. Adv. Mater. Res. 2012, 584, 276–279. [Google Scholar] [CrossRef]
- Qi, J.; White, J.M.; Belcher, A.M.; Masumoto, Y. Optical spectroscopy of silicon nanowires. Chem. Phys. Lett. 2003, 372, 763–766. [Google Scholar] [CrossRef]
- Koao, L.F.; Hone, F.G.; Dejene, F.B. Synthesis and characterization of PbS nanowires doped with Tb3+ ions by using chemical bath deposition method. J. Nanostruct. Chem. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Adu, K.W.; Gutiérrez, H.R.; Kim, U.J.; Sumanasekera, G.U.; Eklund, P.C. Confined Phonons in Si Nanowires. Nano Lett. 2005, 5, 409–414. [Google Scholar] [CrossRef]
- Nanda, K.K.; Sahu, S.N.; Soni, R.K.; Tripathy, S. Raman spectroscopy of PbS nanocrystalline semiconductors. Phys. Rev. B 1998, 58, 405. [Google Scholar] [CrossRef]
- Baranov, A.V.; Bogdanov, K.V.; Ushakova, E.V.; Cherevkov, S.A.; Fedorov, A.V.; Tscharntke, S. Comparative analysis of Raman spectra of PbS macro- and nanocrystals. Opt. Spectrosc. 2010, 109, 268–271. [Google Scholar] [CrossRef]
- Prasad, N.; Karthikeyan, B. Resonant and Off-Resonant Phonon Properties of Wurtzite ZnS: Effect of Morphology on Fröhlich Coupling and Phonon Lifetime. J. Phys. Chem. C 2018, 122, 18117–18123. [Google Scholar] [CrossRef]
- Habinshuti, J.; Kilian, O.; Cristini-Robbe, O.; Sashchiuk, A.; Addad, A.; Turrell, S.; Wirtz, L. Anomalous quantum confinment of the longitudinal optical phonon mode in PbSe quantum dots. Phys. Rev. B 2013, 88, 115313. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P., III. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; Smeets, D.; De Muynck, D.; Nollet, T.; Martins, J.C.; Hens, Z. Size-Dependent Optical Properties of Colloidal PbS Quantum Dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S.; Fabbri, F.; Cannizzaro, A.; Agati, M.; Dolbec, R.; Drera, G.; Castrucci, P. High-temperature nitrogen annealing induced bonding states and photoluminescence changes in inductively coupled plasma torch synthesized silicon nanostructures. J. Appl. Phys. 2020, 128, 024302. [Google Scholar] [CrossRef]
- Ross, G.G.; Barba, D.; Martin, F. Structure and luminescence of silicon nanocrystals embedded in SiO2. Int. J. Nanotechnol. 2008, 5, 984–1017. [Google Scholar] [CrossRef]
- Veena, E.; Bangera, K.V.; Shivakumar, G.K. Effective role of thickness on structural, electrical and optical properties of lead sulphide thin films for photovoltaic applications. Mater. Sci. Eng. B 2017, 223, 64–69. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebhi, A.; Hajjaji, A.; Leblanc-Lavoie, J.; Aouida, S.; Gaidi, M.; Bessais, B.; El Khakani, M.A. Effect of the Helium Background Gas Pressure on the Structural and Optoelectronic Properties of Pulsed-Laser-Deposited PbS Thin Films. Nanomaterials 2021, 11, 1254. https://doi.org/10.3390/nano11051254
Rebhi A, Hajjaji A, Leblanc-Lavoie J, Aouida S, Gaidi M, Bessais B, El Khakani MA. Effect of the Helium Background Gas Pressure on the Structural and Optoelectronic Properties of Pulsed-Laser-Deposited PbS Thin Films. Nanomaterials. 2021; 11(5):1254. https://doi.org/10.3390/nano11051254
Chicago/Turabian StyleRebhi, Ameni, Anouar Hajjaji, Joël Leblanc-Lavoie, Salma Aouida, Mounir Gaidi, Brahim Bessais, and My Ali El Khakani. 2021. "Effect of the Helium Background Gas Pressure on the Structural and Optoelectronic Properties of Pulsed-Laser-Deposited PbS Thin Films" Nanomaterials 11, no. 5: 1254. https://doi.org/10.3390/nano11051254
APA StyleRebhi, A., Hajjaji, A., Leblanc-Lavoie, J., Aouida, S., Gaidi, M., Bessais, B., & El Khakani, M. A. (2021). Effect of the Helium Background Gas Pressure on the Structural and Optoelectronic Properties of Pulsed-Laser-Deposited PbS Thin Films. Nanomaterials, 11(5), 1254. https://doi.org/10.3390/nano11051254






