Mechanical and Water Uptake Properties of Epoxy Nanocomposites with Surfactant-Modified Functionalized Multiwalled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
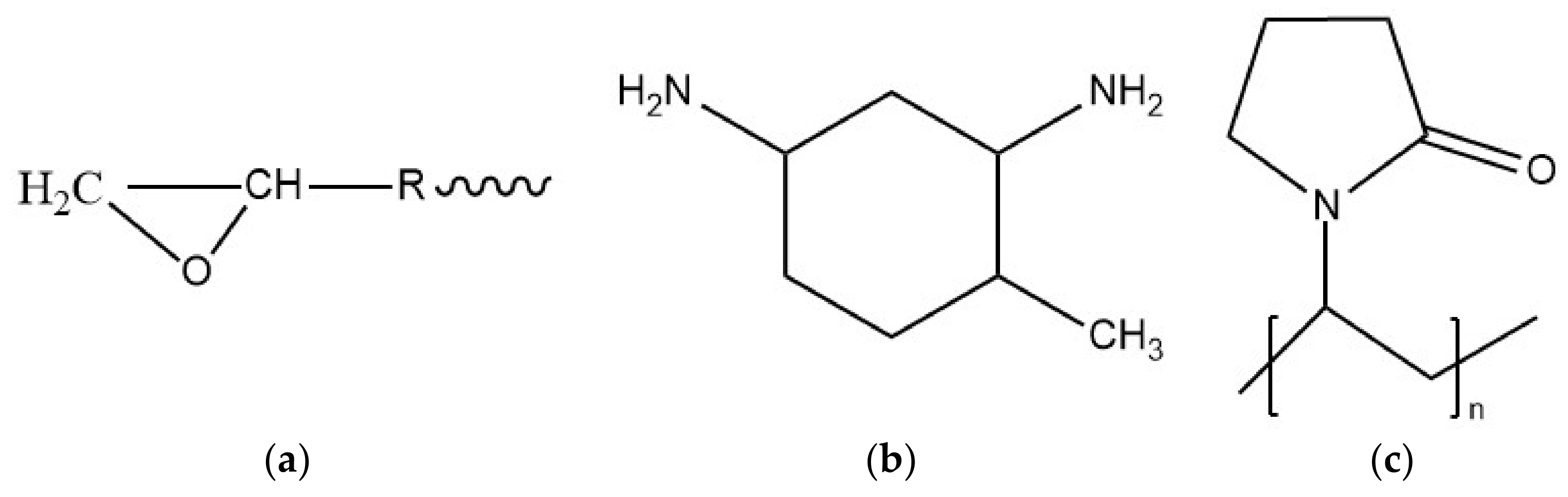
2.1. Raw Materials
2.2. Methods
2.2.1. Preparation of Neat Epoxy Specimens
2.2.2. Preparation of Epoxy/CNT Nanocomposites
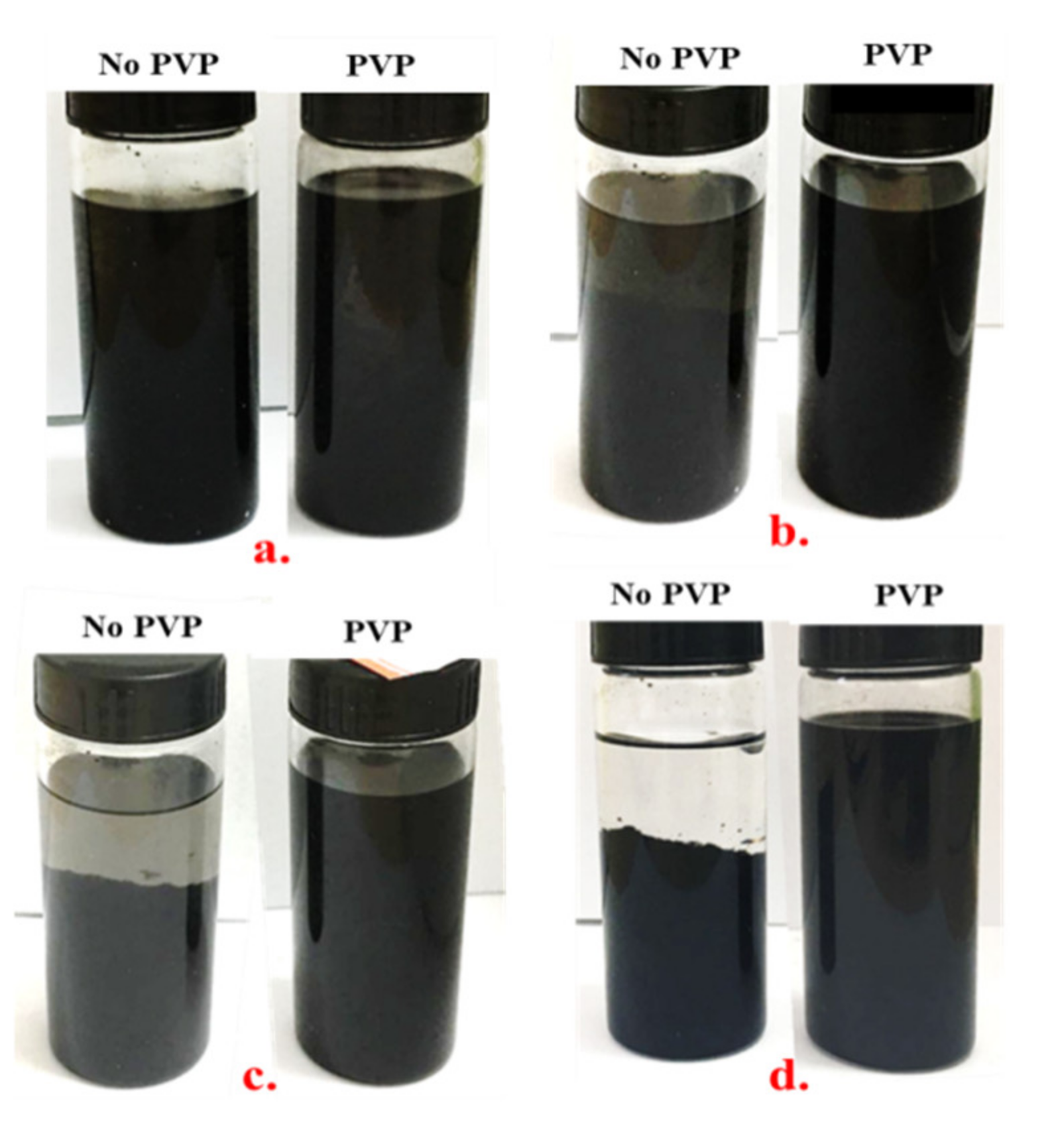
2.3. CNT Dispersion with PVP
2.4. Mechanical Properties Tests
2.5. Dynamic Mechanical Analysis (DMA)
2.6. Morphological Analysis
2.7. Contact Angle Tests
2.8. Water Uptake Tests
3. Results and Discussion
3.1. Mechanical Properties
3.1.1. Flexural Properties
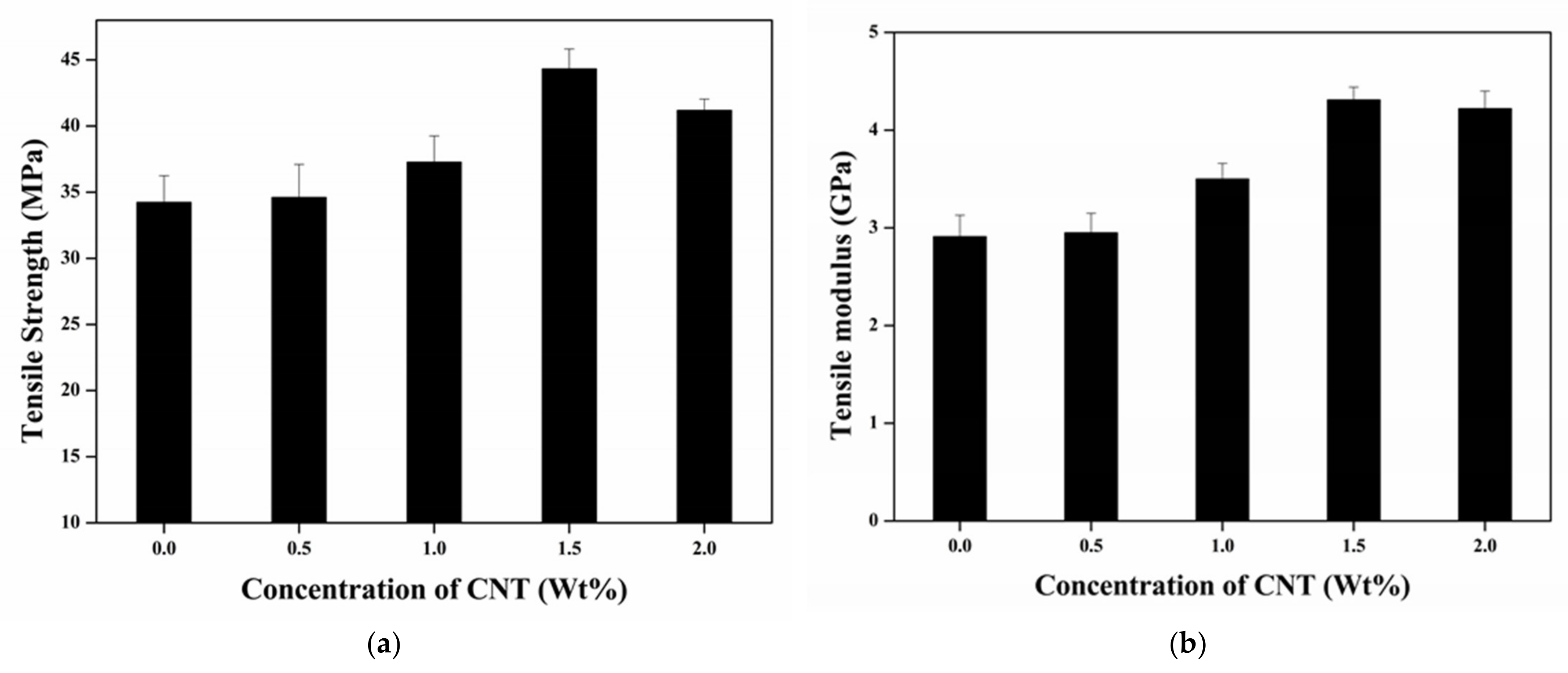
3.1.2. Tensile Properties
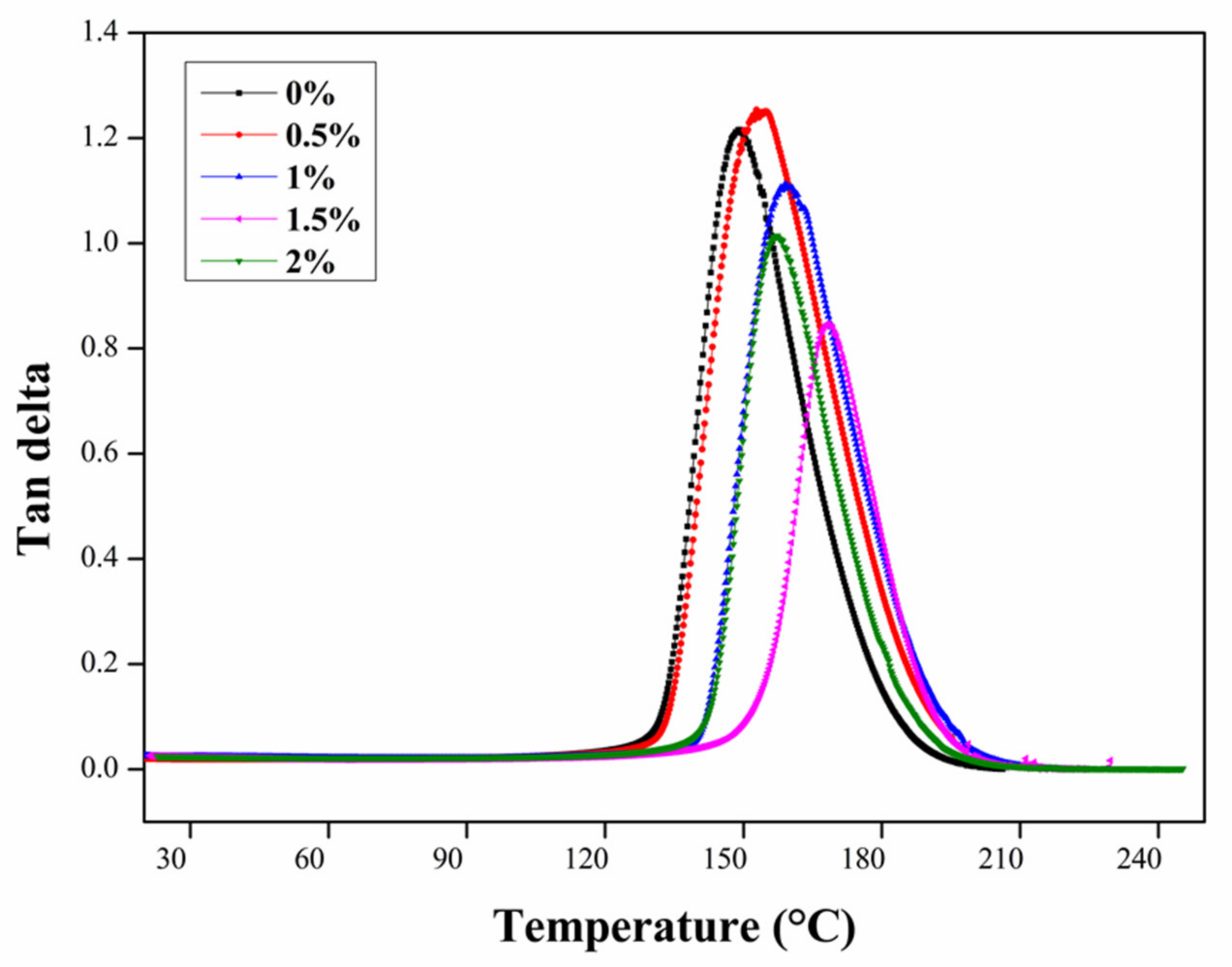
3.2. Dynamic Mechanical Analysis (DMA)
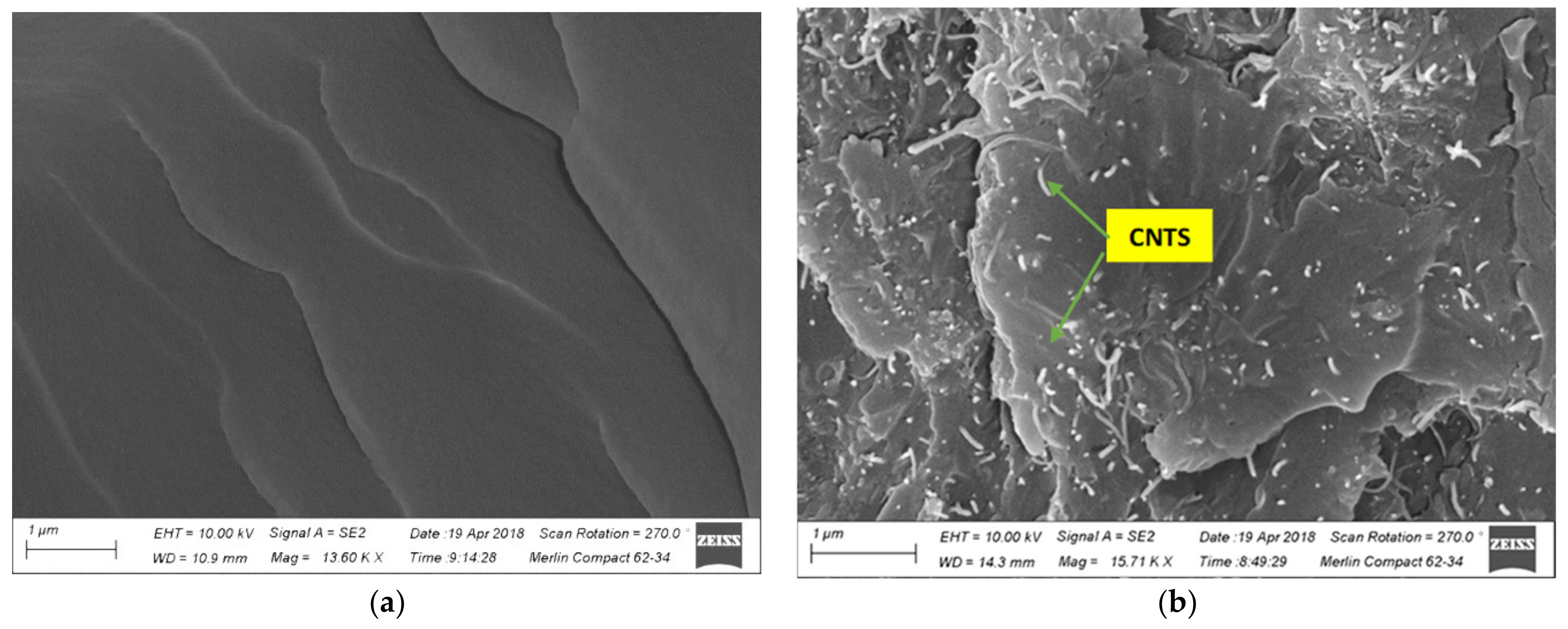
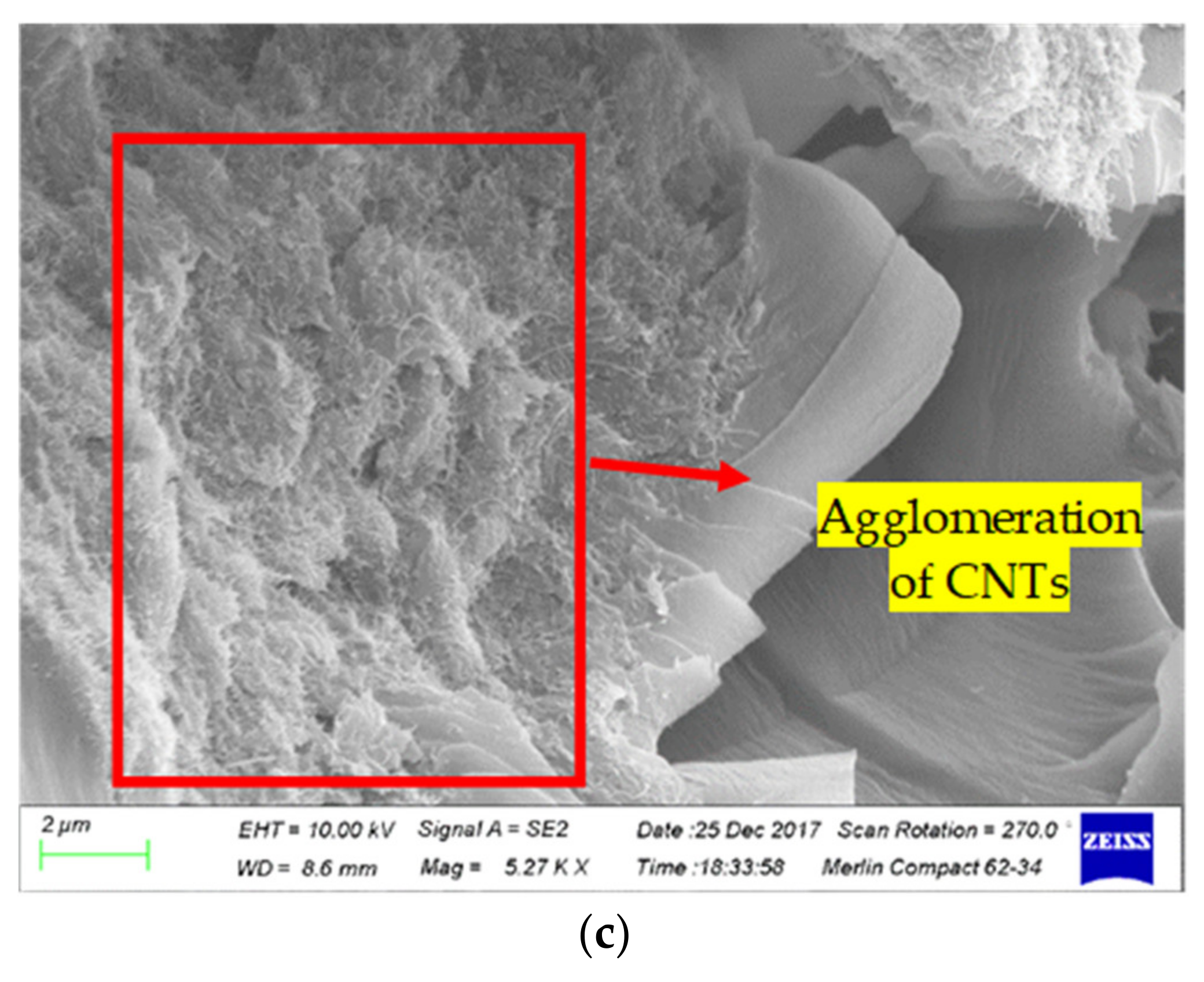
3.3. Morphological Analysis
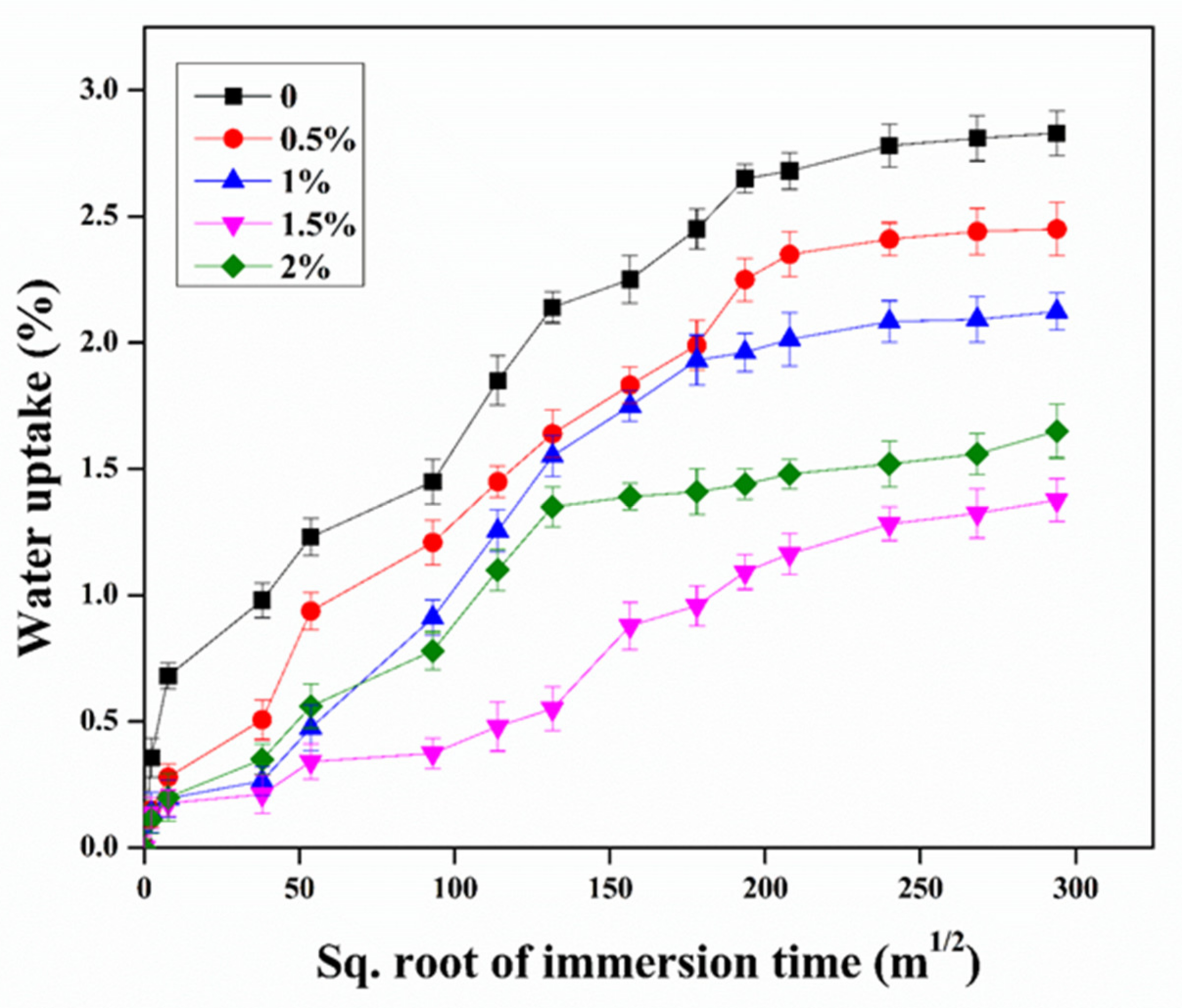
3.4. Water Uptake Resistance
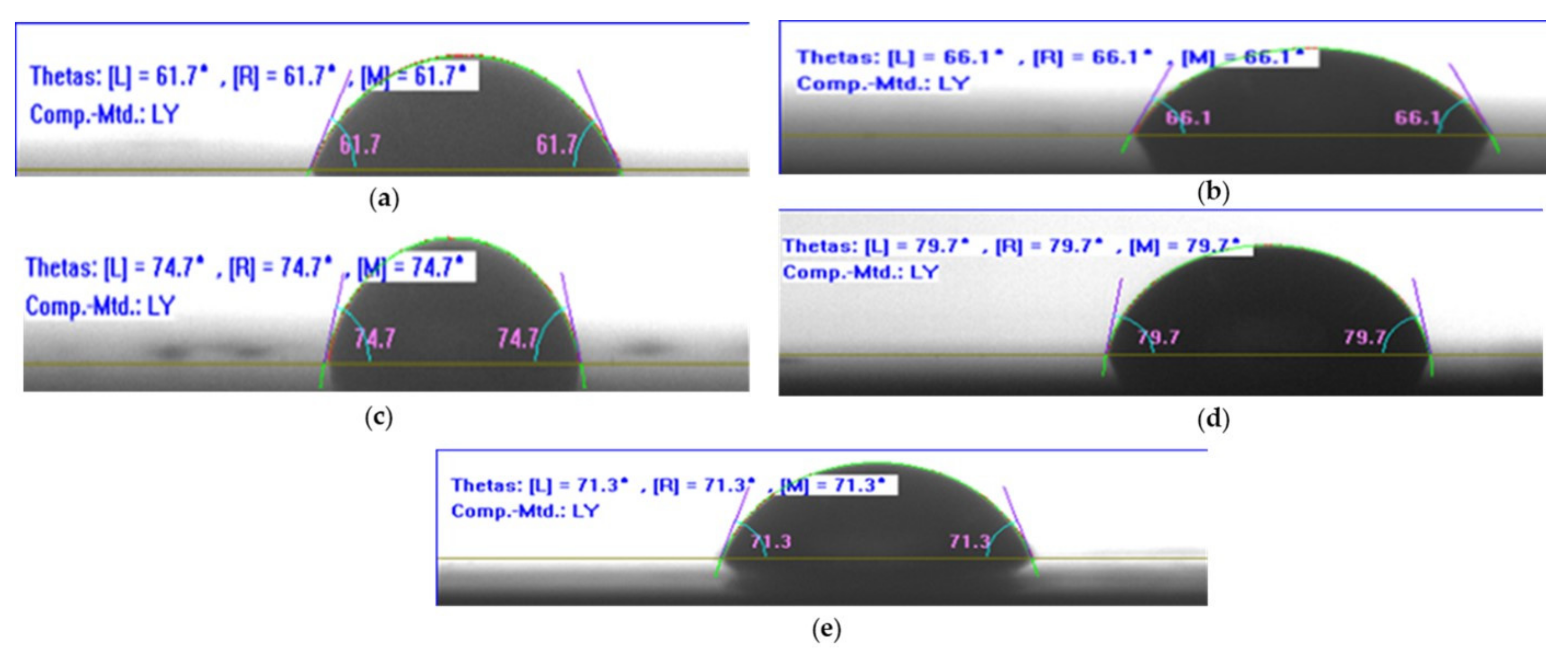
3.4.1. Contact Angle Measurement
3.4.2. Water Absorption Behavior
4. Conclusions
- (1)
- The addition of CNTs into the epoxy caused a maximum increase percentage of 52.9% (flexural strength) and 25.5 % (flexural modulus), 29.5% (tensile strength) and 48.1% (tensile modulus) for the 1.5 wt.% of the CNTs. The improvement mechanism was attributed to strong interfacial bonding and an efficient load transfer between the epoxy and the CNTs. However, the mechanical properties of the epoxy/CNT nanocomposite decreased or saturated owing to the agglomeration effect when the weight concentration of the CNTs was 2.0%.
- (2)
- The maximum increase percentage (~9.5%) of the glass transition temperature (Tg) and the lower height of the tan delta peak of the nanocomposites were achieved for the 1.5 wt.% of the CNTs compared to the neat epoxy matrix. This can be attributed to the uniform dispersion of the CNTs in the epoxy effectively improving the crosslinking between the carbon nanotubes and the epoxy, and a reduced free space of the macromolecular segmental motion. The higher addition ratio (2%) of CNTs caused agglomeration, which acted as a plasticizer and decreased the crosslinking and interfacial bonding between the epoxy matrix and the CNTs.
- (3)
- The incorporation of the CNTs into the epoxy could impart a reduction in the wettability on the surface of the epoxy/CNT nanocomposite, leading to an increase in the contact angle and a reduction in the water uptake, which was significant to improve the durability of the epoxy. Moreover, a higher concentration (2%) of the CNTs showed a larger water uptake owing to the agglomeration possibly causing the formation of microcracks and microvoids in the epoxy.
- (4)
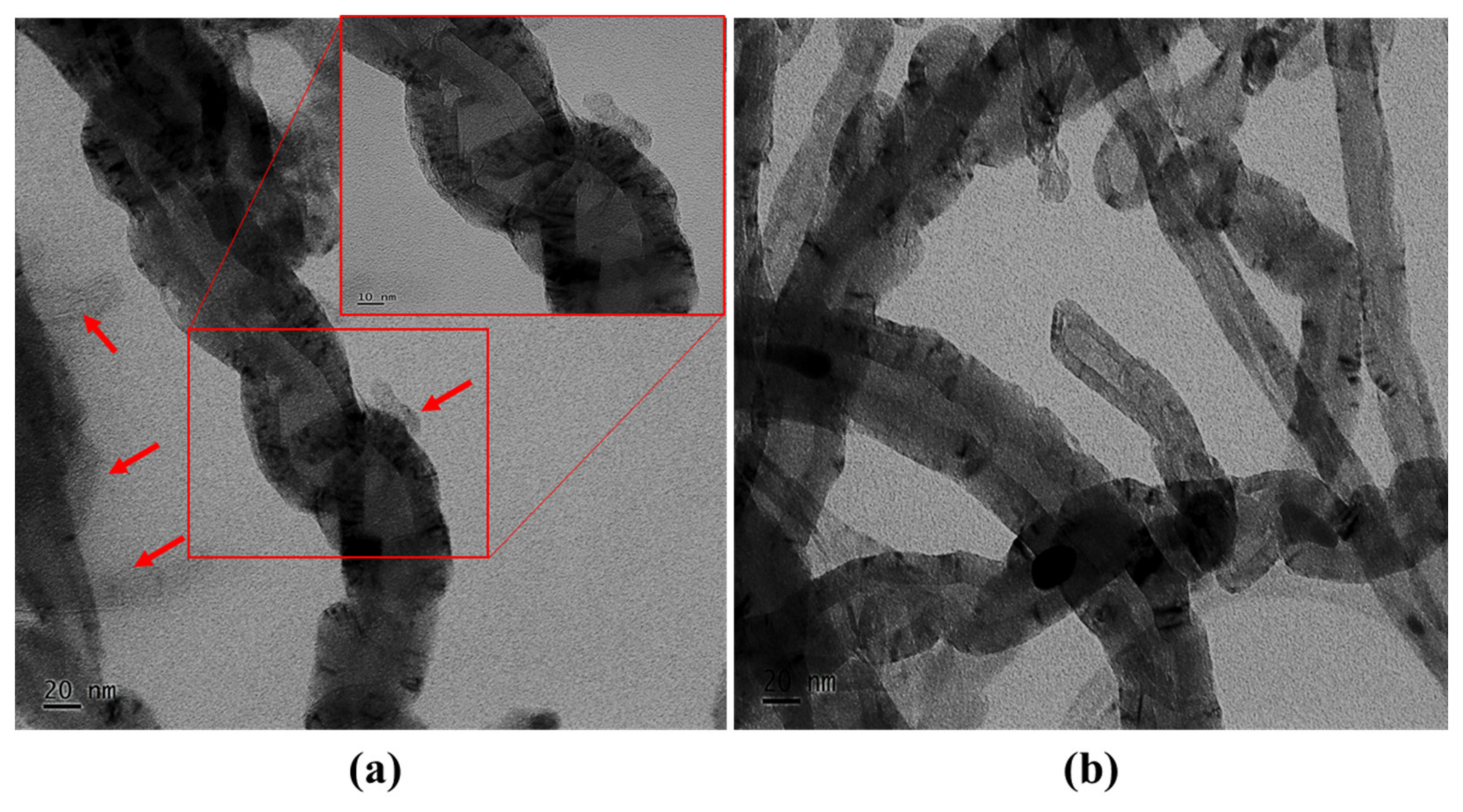
- The wrapping of PVP onto the surface of the CNTs was confirmed by TEM analysis. A surface morphology analysis showed a uniform distribution of the surfactant-modified CNTs in the epoxy matrix formed at the weight concentration of 1.5%. Meanwhile, the agglomeration of the CNTs in the epoxy matrix was observed at a higher weight concentration of 2%. It is recommended that the weight concentration of PVP/CNTs in epoxy does not exceed the optimal concentration of 1.5% to ensure enhanced mechanical properties and a longer service life. A further investigation is needed to analyze the effect of PVP modified CNTs on the epoxy nanocomposite at a higher concentration (> 2%).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 2011, 49, 1919–1930. [Google Scholar] [CrossRef]
- Qian, H.; Greenhalgh, E.S.; Shaffer, M.S.P.; Bismarck, A. Carbon nanotube-based hierarchical composites: A review. J. Mater. Chem. 2010, 20, 4751–4762. [Google Scholar] [CrossRef]
- Ashrafi, B.; Guan, J.; Mirjalili, V.; Zhang, Y.; Chun, L.; Hubert, P.; Simard, B.; Kingston, C.T.; Bourne, O.; Johnston, A. Enhancement of mechanical performance of epoxy/carbon fiber laminate composites using single-walled carbon nanotubes. Compos. Sci. Technol. 2011, 71, 1569–1578. [Google Scholar] [CrossRef]
- Shen, J.; Huang, W.; Wu, L.; Hu, Y.; Ye, M. Thermo-physical properties of epoxy nanocomposites reinforced with amino-functionalized multi-walled carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1331–1336. [Google Scholar] [CrossRef]
- Cha, J.; Jun, G.H.; Park, J.K.; Kim, J.C.; Ryu, H.J.; Hong, S.H. Improvement of modulus, strength and fracture toughness of CNT/Epoxy nanocomposites through the functionalization of carbon nanotubes. Compos. Part B Eng. 2017, 129, 169–179. [Google Scholar] [CrossRef]
- Lu, X.D.; Huang, Y.D.; Zhang, C.H. Curing behaviour of epoxy resin with a diamine containing heterocyclic rings. Polym. Polym. Compos. 2007, 15, 469–474. [Google Scholar] [CrossRef]
- Uthaman, A.; Xian, G.; Thomas, S.; Wang, Y.; Zheng, Q.; Liu, X. Durability of an epoxy matrix and its carbon fiber-reinforced polymer composite upon immersion in water, acidic, and alkaline solutions. Polymers 2020, 12, 614. [Google Scholar] [CrossRef]
- Allaoui, A.; Bai, S.; Cheng, H.M.; Bai, J.B. Mechanical and electrical properties of a MWNT/epoxy composite. Compos. Sci. Technol. 2002, 62, 1993–1998. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef]
- Lal, H.M.; Xian, G.; Thomas, S.; Zhang, L.; Zhang, Z.; Wang, H. Experimental study on the flexural creep behaviors of pultruded unidirectional carbon/glass fiber-reinforced hybrid bars. Materials 2020, 13, 976. [Google Scholar] [CrossRef]
- Georgakilas, V.; Gournis, D.; Tzitzios, V.; Pasquato, L.; Guldi, D.M.; Prato, M. Decorating carbon nanotubes with metal or semiconductor nanoparticles. J. Mater. Chem. 2007, 17, 2679–2694. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Plyhm, T.; Barbezat, M.; Necola, A.; Terrasi, G.P. Carbon nanotube (CNT)-epoxy nanocomposites: A systematic investigation of CNT dispersion. J. Nanoparticle Res. 2011, 13, 6493–6506. [Google Scholar] [CrossRef]
- Zheng, M.P.; Jin, Y.P.; Jin, G.L.; Gu, M.Y. Characterization of TiO2-PVP nanocomposites prepared by the sol-gel method. J. Mater. Sci. Lett. 2000, 19, 433–436. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Montazeri, A.; Chitsazzadeh, M. Effect of sonication parameters on the mechanical properties of multi-walled carbon nanotube/epoxy composites. Mater. Des. 2014, 56, 500–508. [Google Scholar] [CrossRef]
- Wernik, J.M.; Meguid, S.A. On the mechanical characterization of carbon nanotube reinforced epoxy adhesives. Mater. Des. 2014, 59, 19–32. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, A.G.; Dai, H.; Hafner, J.H.; Kelley Bradley, R.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. Solubilization of single-wall carbon nanotubes in water. Nano. Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Sreekumar, T.V.; Kumar, S.; Moore, V.C.; Hauge, R.H.; Smalley, R.E. Poly(vinyl alcohol)/SWNT composite film. Nano. Lett. 2003, 3, 1285–1288. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, M.Y.; Li, J.; Shi, X.M.; Kim, J.K. Effects of surfactant treatment on mechanical and electrical properties of CNT/epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1876–1883. [Google Scholar] [CrossRef]
- Moosa, A.A.; Ramazani, A.; Ibrahim, M.N. Effects of carbon-nanotubes on the mechanical and electrical properties of epoxy nanocomposites. Int. J. Curr. Eng. Technol. 2015, 5, 3253–3258. [Google Scholar]
- Zulfli, N.M.; Bakar, A.A.; Chow, W.S. Mechanical and water absorption behaviors of carbon nanotube reinforced epoxy/glass fiber laminates. J. Reinf. Plast. Compos. 2013, 32, 1715–1721. [Google Scholar] [CrossRef]
- So, H.H.; Cho, J.W.; Sahoo, N.G. Effect of carbon nanotubes on mechanical and electrical properties of polyimide/carbon nanotubes nanocomposites. Eur. Polym. J. 2007, 43, 3750–3756. [Google Scholar] [CrossRef]
- Cha, J.; Jin, S.; Shim, J.H.; Park, C.S.; Ryu, H.J.; Hong, S.H. Functionalization of carbon nanotubes for fabrication of CNT/epoxy nanocomposites. Mater. Des. 2016, 95, 1–8. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Montazeri, A. The effect of functionalization on the viscoelastic behavior of multi-wall carbon nanotube/epoxy composites. Mater. Des. 2013, 45, 510–517. [Google Scholar] [CrossRef]
- Montazeri, A.; Khavandi, A.; Javadpour, J.; Tcharkhtchi, A. Viscoelastic properties of multi-walled carbon nanotube/epoxy composites using two different curing cycles. Mater. Des. 2010, 31, 3383–3388. [Google Scholar] [CrossRef]
- Montazeri, A.; Javadpour, J.; Khavandi, A.; Tcharkhtchi, A.; Mohajeri, A. Mechanical properties of multi-walled carbon nanotube/epoxy composites. Mater. Des. 2010, 31, 4202–4208. [Google Scholar] [CrossRef]
- Zhou, Y.; Pervin, F.; Rangari, V.K.; Jeelani, S. Fabrication and evaluation of carbon nano fiber filled carbon/epoxy composite. Mater. Sci. Eng. A 2006, 426, 221–228. [Google Scholar] [CrossRef]
- Srivastava, V.K. Modeling and mechanical performance of carbon nanotube/epoxy resin composites. Mater. Des. 2012, 39, 432–436. [Google Scholar] [CrossRef]
- Korayem, A.H.; Li, C.Y.; Zhang, Q.H.; Zhao, X.L.; Duan, W.H. Effect of carbon nanotube modified epoxy adhesive on CFRP-to-steel interface. Compos. Part B Eng. 2015, 79, 95–104. [Google Scholar] [CrossRef]
- Armstrong, G.; Ruether, M.; Blighe, F.; Blau, W. Functionalised multi-walled carbon nanotubes for epoxy nanocomposites with improved performance. Polym. Int. 2009, 58, 1002–1009. [Google Scholar] [CrossRef]
- Gojny, F.H.; Schulte, K. Functionalisation effect on the thermo-mechanical behaviour of multi-wall carbon nanotube/epoxy-composites. Compos. Sci. Technol. 2004, 64, 2303–2308. [Google Scholar] [CrossRef]
- Gong, X.; Liu, J.; Baskaran, S.; Voise, R.D.; Young, J.S. Surfactant-assisted processing of carbon nanotube/polymer composites. Chem. Mater. 2000, 12, 1049–1052. [Google Scholar] [CrossRef]
- Khare, K.S.; Khare, R. Effect of carbon nanotube dispersion on glass transition in cross-linked epoxy-carbon nanotube nanocomposites: Role of interfacial interactions. J. Phys. Chem. B 2013, 117, 7444–7454. [Google Scholar] [CrossRef]
- Kim, S.H. Fabrication of superhydrophobic surfaces. J. Adhes. Sci. Technol. 2008, 22, 235–250. [Google Scholar] [CrossRef]
- Park, J.M.; Wang, Z.J.; Jang, J.H.; Gnidakoung, J.R.N.; Lee, W.; Il Park, J.K.; Devries, K.L. Interfacial and hydrophobic evaluation of glass fiber/CNT-epoxy nanocomposites using electro-micromechanical technique and wettability test. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1722–1731. [Google Scholar] [CrossRef]
- Liu, W.; Hoa, S.V.; Pugh, M. Fracture toughness and water uptake of high-performance epoxy/nanoclay nanocomposites. Compos. Sci. Technol. 2005, 65, 2364–2373. [Google Scholar] [CrossRef]
- Starkova, O.; Chandrasekaran, S.; Prado, A.; Tölle, F.; Mülhaupt, R.; Schulte, K. Hydrothermally resistant thermally reduced graphene oxide and multi-wall carbon nanotube based epoxy nanocomposites. Polym. Degrad. Stab. 2013, 98, 519–526. [Google Scholar] [CrossRef]




| Weight Concentration of CNTs (%) | Flexural Strength (%) | Flexural Modulus (%) |
|---|---|---|
| 0.0 | / | / |
| 0.5 | 11.1 | 5.6 |
| 1 | 30.3 | 10.5 |
| 1.5 | 52.9 | 25.5 |
| 2 | 42.8 | 25.8 |
| Weight of CNTs (%) | Tensile Strength (%) | Tensile Modulus (%) |
|---|---|---|
| Control | / | / |
| 0.5 | 1.1 | 1.4 |
| 1 | 8.9 | 20.3 |
| 1.5 | 29.5 | 48.1 |
| 2 | 20.3 | 45.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthaman, A.; Lal, H.M.; Li, C.; Xian, G.; Thomas, S. Mechanical and Water Uptake Properties of Epoxy Nanocomposites with Surfactant-Modified Functionalized Multiwalled Carbon Nanotubes. Nanomaterials 2021, 11, 1234. https://doi.org/10.3390/nano11051234
Uthaman A, Lal HM, Li C, Xian G, Thomas S. Mechanical and Water Uptake Properties of Epoxy Nanocomposites with Surfactant-Modified Functionalized Multiwalled Carbon Nanotubes. Nanomaterials. 2021; 11(5):1234. https://doi.org/10.3390/nano11051234
Chicago/Turabian StyleUthaman, Arya, Hiran Mayookh Lal, Chenggao Li, Guijun Xian, and Sabu Thomas. 2021. "Mechanical and Water Uptake Properties of Epoxy Nanocomposites with Surfactant-Modified Functionalized Multiwalled Carbon Nanotubes" Nanomaterials 11, no. 5: 1234. https://doi.org/10.3390/nano11051234
APA StyleUthaman, A., Lal, H. M., Li, C., Xian, G., & Thomas, S. (2021). Mechanical and Water Uptake Properties of Epoxy Nanocomposites with Surfactant-Modified Functionalized Multiwalled Carbon Nanotubes. Nanomaterials, 11(5), 1234. https://doi.org/10.3390/nano11051234







