Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications
Abstract
1. Introduction
2. Properties of Nanoparticles
2.1. Hardness or Elastic Modulus of Nanoparticles
2.2. Adhesion or Frictional Effects in Nanoparticles
2.3. Effect of Size
2.4. Effect of Surface Charge or pKa
2.5. Effect of Ligand Chemistry
2.6. Plasmonic Nanoparticles
2.7. Magnetic Nanoparticles
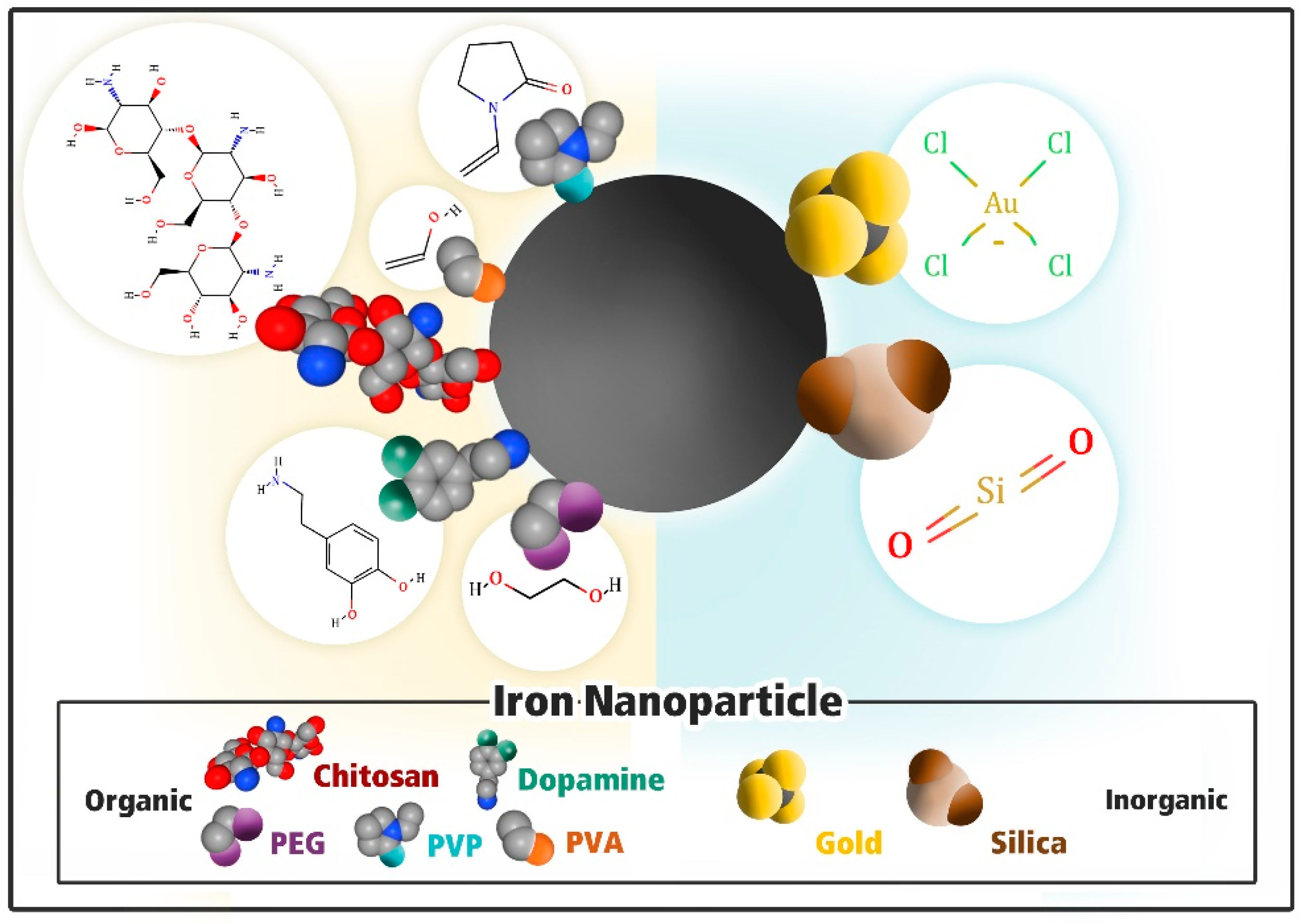
3. Iron Oxide Nanoparticle Functionalization
- Due to their nanometer size, surface modification is required in order to reduce the surface energy.
- Ligands bind inefficiently, and drug delivery fails if the surface of Fe3O4 NPs is not functionalized.
- Uncoated Fe3O4 NPs can form free radicals.
- To use Fe3O4 for biological applications that are inside the cells and proteins, it is appropriate to bind them with a suitable ligand for selective targeting [47].
3.1. Modification with Inorganic Material
3.1.1. Gold (Au)
3.1.2. Silica (SiO2)
3.2. Modification with Organic Materials
3.2.1. Polydopamine
3.2.2. Poly Vinyl Pyrrolidone (PVP)
3.2.3. Chitosan
3.2.4. Polyethylene Glycol (PEG)
3.2.5. Polyvinyl Alcohol (PVA)
4. Iron–Gold Bifunctional Nanoparticles
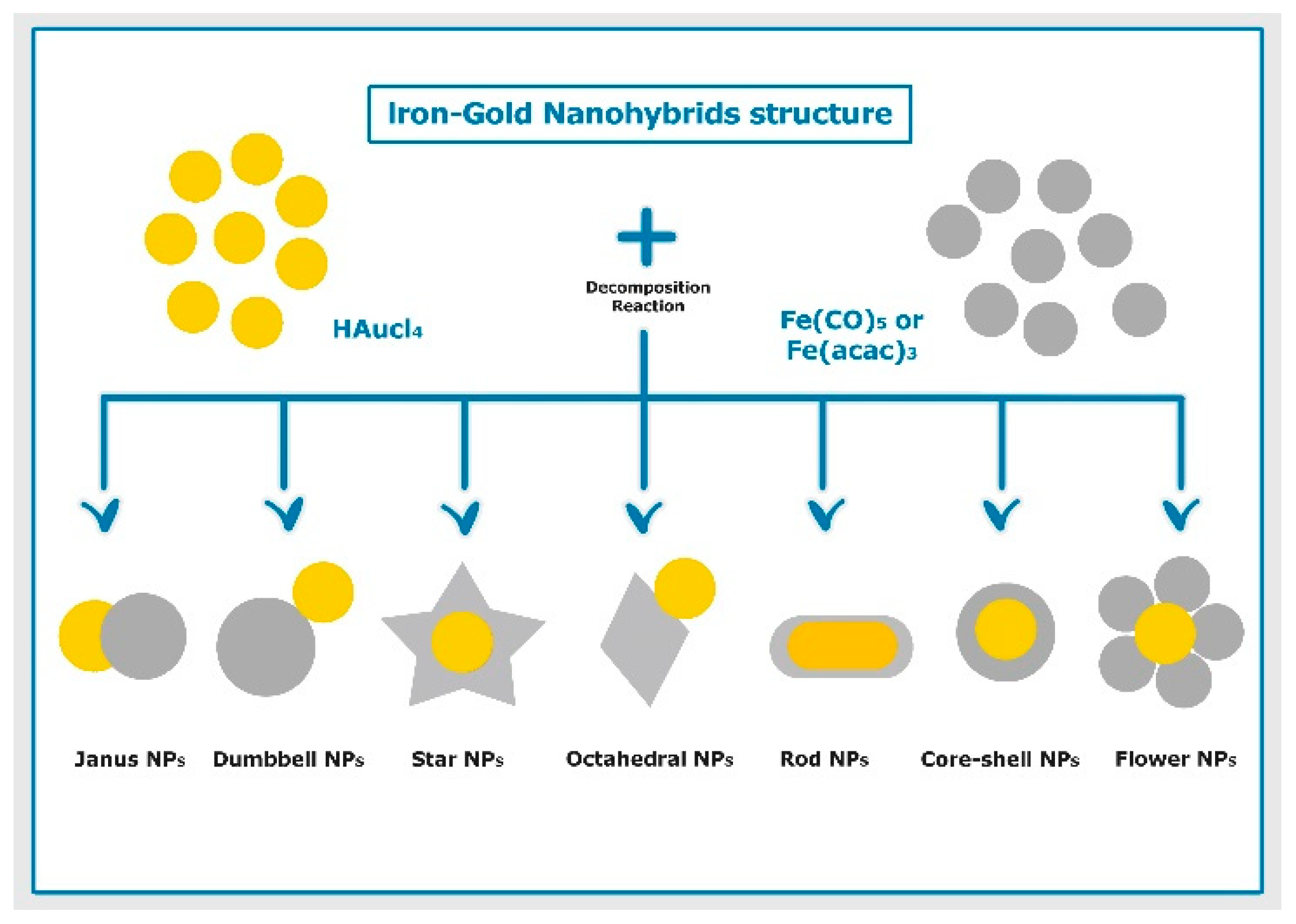
4.1. Structure of Iron–Gold Nanohybrids
4.1.1. Core–Shell Shape
4.1.2. Dumbbell Shape
4.1.3. Janus Shape
4.1.4. Flower-Shape
4.1.5. Star Shape
4.1.6. Octahedral Shape
4.1.7. Rod Shape
4.2. Particle Synthesis of Iron–Gold Nanohybrids
4.3. Biomedical Applications of Iron–Gold Nanohybrids
4.3.1. Iron–Gold Nanohybrids as Delivery Carriers
4.3.2. Iron–Gold Nanohybrids for Hyperthermia Applications
4.3.3. Iron–Gold Nanohybrids for Bioimaging
4.3.4. Iron–Gold Nanohybrids as Biosensors
5. Toxicity Assessment
5.1. In Vitro Toxicity Assessment Methods
5.1.1. Proliferation Assay
5.1.2. Apoptosis Assay
5.1.3. Necrosis Assay
5.1.4. Oxidative Stress Assay
5.2. In Vivo Toxicity Assessment Methods
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| Au | Gold |
| Au−Fe3O4 | Gold iron |
| DCFDA | 5-(and -6)-Carboxy-2,7-dichlorodihydrofluorescein diacetate |
| DHE | Dihydroethidium |
| DMF | Dimethylformamide |
| DNA | Deoxyribonucleic acid |
| DOPA | Dihydroxy-L-phenylalanine |
| ELISA | Enzymatic-linked immunosorbent assay |
| EPR | Electron paramagnetic resonance |
| Fe(CO)5 | Iron pentacarbonyl |
| Fe3O4 | Iron magnetite |
| (ɤ-Fe2O3) | Maghemite |
| HAuCl4 | Chloroauric acid |
| HEK 293T | Human embryonic kidney 293 cells |
| HER2 | Human epithelial growth factor receptor 2 |
| LDI-MS | Laser desorption and ionization mass spectrometry |
| LDH | Lactate dehydrogenase |
| MRI | Magnetic resonance imaging |
| MTT | 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAD | Nicotinamide-adenine dinucleotide |
| NHTP | Nitrosothioproline |
| NO | Nitric oxide |
| NPs | Nanoparticles |
| NIR | Near-infrared |
| PDA | Polydopamine |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PVA | Polyvinyl alcohol |
| PVP | Poly vinyl pyrrolidone |
| ROS | Reactive oxygen species |
| SERS | Surface-enhanced Raman spectroscopy |
| SiO2 | Silica |
| SPIONs | Superparamagnetic iron oxide nanoparticle |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| TEMP | Tetramethylpiperidine |
| UV | Ultraviolet |
| VDW | Van der Waals |
References
- Nikalje, A.P. Nanotechnology and its Applications in Medicine. Med. Chem. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.; Xue, Z.; Wang, T. Effect of structure: A new insight into nanoparticle assemblies from inanimate to animate. Sci. Adv. 2020, 6, eaba1321. [Google Scholar] [CrossRef]
- Rehan, F.; Ahemad, N.; Gupta, M. Casein nanomicelle as an emerging biomaterial: A comprehensive review. Colloids Surf. B Biointerfaces 2019, 179, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Nho, R. Pathological effects of nano-sized particles on the respiratory system. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102242. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Doxorubicin loaded magnetic gold nanoparticles for in vivo targeted drug delivery. Int. J. Pharm. 2015, 490, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Son, S.; Son, S.; Joo, M.K.; Kwon, I.C. The EPR Effect in Cancer Therapy. In Cancer Targeted Drug Delivery; Springer: New York, NY, USA, 2013; pp. 621–632. [Google Scholar]
- Nikolova, M.; Slavchov, R. Nanotechnology in Medicine. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Springer International Publishing: Cham, Switzerland, 2020; pp. 533–546. [Google Scholar]
- Fatima, H.; Kim, K.-S. Iron-based magnetic nanoparticles for magnetic resonance imaging. Adv. Powder Technol. 2018, 29, 2678–2685. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Dabbagh, A.; Abnisa, F.; Daud, W.M.A.W. Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancer. Eur. Polym. J. 2020, 133, 109789. [Google Scholar] [CrossRef]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and Gold-Coated Magnetic Nanoparticles as a DNA Sensor. Anal. Chem. 2006, 78, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; De Aberasturi, D.J.; Henriksen-Lacey, M.; Langer, J.; Espinosa, A.; Szczupak, B.; Wilhelm, C.; Liz-Marzán, L.M. Janus plasmonic–magnetic gold–iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale 2017, 9, 9467–9480. [Google Scholar] [CrossRef]
- Tian, Y.; Qiang, S.; Wang, L. Gold Nanomaterials for Imaging-Guided Near-Infrared in vivo Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 398. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted Hyperthermia Using Metal Nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Ding, X.; Li, D.; Jiang, J. Gold-based Inorganic Nanohybrids for Nanomedicine Applications. Theranostics 2020, 10, 8061–8079. [Google Scholar] [CrossRef]
- Larsen, G.K.; Farr, W.; Murph, S.E.H. Multifunctional Fe2O3–Au Nanoparticles with Different Shapes: Enhanced Catalysis, Photothermal Effects, and Magnetic Recyclability. J. Phys. Chem. C 2016, 120, 15162–15172. [Google Scholar] [CrossRef]
- Das, P.; Fatehbasharzad, P.; Colombo, M.; Fiandra, L.; Prosperi, D. Multifunctional Magnetic Gold Nanomaterials for Cancer. Trends Biotechnol. 2019, 37, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Ocsoy, I.; Gulbakan, B.; Shukoor, M.I.; Xiong, X.; Chen, T.; Powell, D.H.; Tan, W. Aptamer-Conjugated Multifunctional Nanoflowers as a Platform for Targeting, Capture, and Detection in Laser Desorption Ionization Mass Spectrometry. ACS Nano 2013, 7, 417–427. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef]
- Ibarra, M.R.; Khlebtsov, G.N. Magnetic and Plasmonic Nanoparticles for Biomedical Devices. J. Appl. Phys. 2019, 126, 170401. [Google Scholar] [CrossRef]
- Karamipour, S.; Sadjadi, M.; Farhadyar, N. Fabrication and spectroscopic studies of folic acid-conjugated Fe3O4@Au core–shell for targeted drug delivery application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mammeri, F.; Ammar, S. Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review. Nanomaterials 2018, 8, 149. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Wang, X.B.; Liu, W.M. Nanoparticle-Based Lubricant Additives. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013; pp. 2369–2376. [Google Scholar]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R. Polymer Matrix Nanocomposites, Processing, Manufacturing and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Ramos, M.; Ortiz-Jordan, L.; Hurtado-Macias, A.; Flores, S.; Elizalde-Galindo, J.T.; Rocha, C.; Torres, B.; Zarei-Chaleshtori, M.; Chianelli, R.R. Hardness and Elastic Modulus on Six-Fold Symmetry Gold Nanoparticles. Materials 2013, 6, 198–205. [Google Scholar] [CrossRef]
- Ilie, F. Models of nanoparticles movement, collision, and friction in chemical mechanical polishing (CMP). J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Mougin, K.; Gnecco, E.; Rao, A.; Cuberes, T.; Jayaraman, S.; McFarland, E.W.; Haidara, H.; Meyer, E. Manipulation of Gold Nanoparticles: Influence of Surface Chemistry, Temperature, and Environment (Vacuum Versus Ambient Atmosphere). Langmuir 2008, 24, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, D.; Bhushan, B. Effect of spherical Au nanoparticles on nanofriction and wear reduction in dry and liquid environments. Beilstein J. Nanotechnol. 2012, 3, 759–772. [Google Scholar] [CrossRef]
- Sitti, M. Atomic Force Microcope Probe Based Controlled Pushing for Nanotribo-Logical Characterization. IEEE/ASME Trans. Mechatron. 2004, 9, 343–349. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Buckley, A.; Hodgson, A.; Warren, J.; Guo, C.; Smith, R. Size-dependent deposition of inhaled nanoparticles in the rat respiratory tract using a new nose-only exposure system. Aerosol Sci. Technol. 2015, 50, 1–10. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Bao, G.; Zhang, S. Variable Nanoparticle-Cell Adhesion Strength Regulates Cellular Uptake. Phys. Rev. Lett. 2010, 105, 138101. [Google Scholar] [CrossRef]
- Lin, J.; Miao, L.; Zhong, G.; Lin, C.H.; Dargazangy, R.; Alexander-Katz, A. Understanding the synergistic effect of physicochemical properties of nanoparticles and their cellular entry pathways. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Lehn, R.C.; Atukorale, P.U.; Carney, R.P.; Yang, Y.S.; Stellacci, F.; Irvine, D.J.; Alexander-Katz, A. Effect of Particle Diameter and Surface Composition on the Spontaneous Fusion of Monolayer-Protected Gold Nanoparticles with Lipid Bilayers. Nano Lett. 2013, 13, 4060–4067. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Chadha, T.S.; Haddad, K.; Biswas, P. Perspective on Nanoparticle Technology for Biomedical Use. Curr. Pharm. Des. 2016, 22, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kafshgari, M.H.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical Properties and Biomedical Applications of Plasmonic Nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Morales, I.; De La Presa, P.; Mille, N.; Carrey, J.; Garcia, M.A.; Hernando, A.; Herrasti, P. Hybrid nanoparticles for magnetic and plasmonic hyperthermia. Phys. Chem. Chem. Phys. 2018, 20, 24065–24073. [Google Scholar] [CrossRef]
- Lukianova-Hleb, E.; Hu, Y.; Latterini, L.; Tarpani, L.; Lee, S.; Drezek, R.A.; Hafner, J.H.; Lapotko, D.O. Plasmonic Nanobubbles as Transient Vapor Nanobubbles Generated around Plasmonic Nanoparticles. ACS Nano 2010, 4, 2109–2123. [Google Scholar] [CrossRef]
- Karkan, S.F.; Mohammadhosseini, M.; Panahi, Y.; Milani, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, E.; Hosseini, A.; Davaran, S. Magnetic nanoparticles in cancer diagnosis and treatment: A review. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–5. [Google Scholar] [CrossRef]
- Akbulut, M. Nanoparticle-Based Lubrication Systems. J. Powder Met. Min. 2012, e101. [Google Scholar] [CrossRef]
- Yu, P.; Yao, Y.; Wu, J.; Niu, X.; Rogach, A.L.; Wang, Z. Effects of Plasmonic Metal Core -Dielectric Shell Nanoparticles on the Broadband Light Absorption Enhancement in Thin Film Solar Cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Abu Noqta, O.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Sahoo, B.; Devi, K.S.P.; Dutta, S.; Maiti, T.K.; Pramanik, P.; Dhara, D. Biocompatible Mesoporous Silica-Coated Superparamagnetic Manganese Ferrite Nanoparticles for Targeted Drug Delivery and Mr Imaging Applications. J. Colloid Interface Sci. 2014, 431, 31–41. [Google Scholar] [CrossRef]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Chen, H.; Tang, J.; Nie, L. Sonochemical synthesis, structure and magnetic properties of air-stable Fe3O4/Au nanoparticles. Nanotechnology 2007, 18, 145609. [Google Scholar] [CrossRef]
- Xu, Z.; Yanglong, H.; Shouheng, S. Magnetic Core/Shell Fe3o4/Au and Fe3o4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed Core−Shell Fe3o4@Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Zhu, Y.; Da, H.; Yang, X.; Hu, Y. Preparation and characterization of core-shell monodispersed magnetic silica microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2003, 231, 123–129. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Tang, X.; Liu, W. Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II). Dye. Pigment. 2011, 91, 26–32. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles Modified by Polydopamine: Working as “Drug” Carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Zheng, W.; Fan, H.; Wang, L.; Jin, Z. Oxidative Self-Polymerization of Dopamine in an Acidic Environment. Langmuir 2015, 31, 11671–11677. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone): A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.H.; Xu, C.; Xie, J.; Lee, J.H.; Wu, B.; Koh, A.L.; Wang, X.; Sinclair, R.; Wang, S.X.; et al. Synthesis and characterization of PVP-coated large core iron oxide nanoparticles as an MRI contrast agent. Nanotechnology 2008, 19, 165101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.Y.; Ma, S.; Zhang, Y.J.; Zhao, X.; Zhang, X.D.; Zhang, Z.D. Synthesis of PVP-coated ultra-small Fe3O4 nanoparticles as a MRI contrast agent. J. Mater. Sci. Mater. Electron. 2010, 21, 1205–1210. [Google Scholar] [CrossRef]
- Huang, J.; Bu, L.; Xie, J.; Chen, K.; Cheng, Z.; Li, X.; Chen, X. Effects of Nanoparticle Size on Cellular Uptake and Liver MRI with Polyvinylpyrrolidone-Coated Iron Oxide Nanoparticles. ACS Nano 2010, 4, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Kas, H.S. Chitosan: Properties, preparations and application to microparticulate systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Wu, Y.; Jin, J.; Gao, W.; Xie, Q.; Wang, S.; Zhang, Y.; Deng, H. Conjugation of catecholamines on magnetic nanoparticles coated with sulfonated chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 169–174. [Google Scholar] [CrossRef]
- Kohler, N.; Fryxell, G.E.; Zhang, M. A Bifunctional Poly(ethylene glycol) Silane Immobilized on Metallic Oxide-Based Nanoparticles for Conjugation with Cell Targeting Agents. J. Am. Chem. Soc. 2004, 126, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Mengersen, F.; Bunjes, H. PEGylation of supercooled smectic cholesteryl myristate nanoparticles. Eur. J. Pharm. Biopharm. 2012, 81, 409–417. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, A.; Iván, B.; Tombácz, E. Pegylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-Peg Brushes for Contrast Enhancement in Mri Diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef]
- Adoor, G.S.; Manjeshwar, L.D.; Naidu, B.V.K.; Sairam, M.; Aminabhavi, T. Poly(Vinyl Alcohol)/Poly(Methyl Methacrylate) Blend Membranes for Pervaporation Separation of Water+Isopropanol and Water+1,4-Dioxane Mixtures. J. Membr. Sci. 2006, 280, 594–602. [Google Scholar] [CrossRef]
- Caramori, S.S.; Fernandes, K.F.; Junior, L.B.D.C. Immobilized Horseradish Peroxidase on Discs of Polyvinyl Alcohol-Glutaraldehyde Coated with Polyaniline. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, N.; Rabiee, M.; Abdolrahim, M.; Tahriri, M.; Vashaee, D.; Tayebi, L. A novel electrochemical biosensor based on Fe 3 O 4 nanoparticles-polyvinyl alcohol composite for sensitive detection of glucose. Anal. Biochem. 2017, 519, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef]
- Gan, N.; Jin, H.; Li, T.; Zheng, L. Fe3o4/Au Magnetic Nanoparticle Amplifcation Strategies for Ultrasensitive Electrochemical Immunoassay of Alfa-Fetoprotein. Int. J. Nanomed. 2011, 6, 3259–3269. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Shams, S.F.; Ghazanfari, M.R.; Schmitz-Antoniak, C. Magnetic-Plasmonic Heterodimer Nanoparticles: Designing Contemporarily Features for Emerging Biomedical Diagnosis and Treatments. Nanomaterials 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, J.; Liu, F.; Xing, R.; Zhang, C.; Yang, C.; Yin, H.; Hou, Y. Controlled synthesis of FePt–Au hybrid nanoparticles triggered by reaction atmosphere and FePt seeds. Nanoscale 2013, 5, 9141–9149. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Liu, D.; Guo, D.; Yang, F.; Pang, X.; Che, R.; Zhou, N.; Xie, J.; Sun, J.; Huang, Z.; et al. Shape-controlled fabrication of magnetite silver hybrid nanoparticles with high performance magnetic hyperthermia. Biomaterials 2017, 124, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.H.; Sarkar, T.; Kemp, B.A. Tunable and large plasmonic field enhancement in core-shell heterodimer/trimer. J. Electromagn. Waves Appl. 2019, 33, 2423–2433. [Google Scholar] [CrossRef]
- Ahrberg, C.D.; Choi, J.W.; Chung, B.G. Automated droplet reactor for the synthesis of iron oxide/gold core-shell nanoparticles. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Tancredi, P.; Da Costa, L.S.; Calderon, S.; Moscoso-Londoño, O.; Socolovsky, L.M.; Ferreira, P.J.; Muraca, D.; Zanchet, D.; Knobel, M. Exploring the synthesis conditions to control the morphology of gold-iron oxide heterostructures. Nano Res. 2019, 12, 1781–1788. [Google Scholar] [CrossRef]
- Mahdavi, Z.; Rezvani, H.; Moraveji, M.K. Core–shell nanoparticles used in drug delivery-microfluidics: A review. RSC Adv. 2020, 10, 18280–18295. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; Alothman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, Synthesis and Applications of Core–Shell, Hollow Core, and Nanorattle Multifunctional Nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef] [PubMed]
- Lukosi, M.; Zhu, H.; Dai, S. Recent advances in gold-metal oxide core-shell nanoparticles: Synthesis, characterization, and their application for heterogeneous catalysis. Front. Chem. Sci. Eng. 2016, 10, 39–56. [Google Scholar] [CrossRef]
- Woodard, L.E.; Dennis, C.L.; Borchers, J.A.; Attaluri, A.; Velarde, E.; Dawidczyk, C.; Searson, P.C.; Pomper, M.G.; Ivkov, R. Nanoparticle Architecture Preserves Magnetic Properties During Coating to Enable Robust Multi-Modal Functionality. Sci. Rep. 2018, 8, 12706. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-Like Bifunctional Au-Fe3O4 Nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Nurmikko, A.V.; Sun, S. Enhanced Magnetooptical Response in Dumbbell-like Ag−CoFe2O4 Nanoparticle Pairs. Nano Lett. 2005, 5, 1689–1692. [Google Scholar] [CrossRef]
- Xu, C.; Xie, J.; Ho, D.; Wang, C.; Kohler, N.; Walsh, E.G.; Morgan, J.R.; Chin, Y.E.; Sun, S. Au–Fe3O4 Dumbbell Nanoparticles as Dual-Functional Probes. Angew. Chem. Int. Ed. 2007, 47, 173–176. [Google Scholar] [CrossRef]
- Najafishirtari, S.; Guardia, P.; Scarpellini, A.; Prato, M.; Marras, S.; Manna, L.; Colombo, M. The effect of Au domain size on the CO oxidation catalytic activity of colloidal Au–FeOx dumbbell-like heterodimers. J. Catal. 2016, 338, 115–123. [Google Scholar] [CrossRef]
- Landgraf, L.; Christner, C.; Storck, W.; Schick, I.; Krumbein, I.; Dähring, H.; Haedicke, K.; Heinz-Herrmann, K.; Teichgräber, U.; Reichenbach, J.R.; et al. A Plasma Protein Corona Enhances the Biocompatibility of Au@Fe3o4 Janus Particles. Biomaterials 2015, 68, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Jishkariani, D.; Wu, Y.; Wang, D.; Liu, Y.; van Blaaderen, A.; Murray, C.M. Preparation and Self-Assembly of Dendronized Janus Fe3O4–Pt and Fe3O4–Au Heterodimers. ACS Nano 2017, 11, 7958–7966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; Zhou, L.; Han, Q.; Chen, X.; Li, S.; Li, L.; Su, Z.; Wang, C. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials 2018, 181, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Li, S.; Chen, X.; Zhang, M.; Wang, T.; Li, L.; Wang, C. Designed Synthesis of Au/Fe3O4 @C Janus Nanoparticles for Dual-Modal Imaging and Actively Targeted Chemo-Photothermal Synergistic Therapy of Cancer Cells. Chemistry 2017, 23, 17242–17248. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, F.; Aronova, M.; Zhu, L.; Lin, X.; Quan, Q.; Liu, G.; Zhang, G.; Choi, K.Y.; Kim, K.; et al. Manipulating the Power of an Additional Phase: A Flower-like Au−Fe3O4 Optical Nanosensor for Imaging Protease Expressions In vivo. ACS Nano 2011, 5, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.; Osório, I.; Dória, G.; Carvalho, P.A.; Pereira, A.; Langer, J.; Araújo, P.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Franco, R.; et al. Star-Shaped Magnetite@Gold Nanoparticles for Protein Magnetic Separation and Sers Detection. RSC Adv. 2014, 4, 3659–3667. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lau-Truong, S.; Mammeri, F.; Ammar, S. Star-Shaped Fe3-xO4-Au Core-Shell Nanoparticles: From Synthesis to SERS Application. Nanomaterials 2020, 10, 294. [Google Scholar] [CrossRef]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 1–19. [Google Scholar] [CrossRef]
- Kozenkova, E.; Levada, K.; Efremova, M.V.; Omelyanchik, A.; Nalench, Y.A.; Garanina, A.S.; Pshenichnikov, S.; Zhukov, D.G.; Lunov, O.; Lunova, M.; et al. Multifunctional Fe3o4-Au Nanoparticles for the Mri Diagnosis and Potential Treatment of Liver Cancer. Nanomaterials 2020, 10, 1646. [Google Scholar] [CrossRef]
- Gole, A.; Stone, J.W.; Gemmill, W.R.; zur Loye, H.C.; Murphy, C.J. Iron Oxide Coated Gold Nanorods: Synthesis, Characterization, and Magnetic Manipulation. Langmuir 2008, 24, 6232–6237. [Google Scholar] [CrossRef]
- Chapman, B.S.; Wu, W.C.; Li, Q.; Holten-Andersen, N.; Tracy, J.B. Heteroaggregation Approach for Depositing Magnetite Nanoparticles onto Silica-Overcoated Gold Nanorods. Chem. Mater. 2017, 29, 10362–10368. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Chaffin, E.; Shen, X.; Chen, J.; Zou, Q.; Wu, Z.; Gai, Z.; Bhana, S.; O’Connor, R.; Wang, L.; et al. Size- and Shape-Controlled Synthesis and Properties of Magnetic–Plasmonic Core–Shell Nanoparticles. J. Phys. Chem. C 2016, 120, 10530–10546. [Google Scholar] [CrossRef]
- Guardia, P.; Riedinger, A.; Nitti, S.; Pugliese, G.; Marras, S.; Genovese, A.; Materia, M.E.; Lefevre, C.; Manna, L.; Pellegrino, T. One pot synthesis of monodisperse water soluble iron oxide nanocrystals with high values of the specific absorption rate. J. Mater. Chem. B 2014, 2, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Javed, Y.; Hussain, M.I.; Yaseen, M.; Asif, M. Gold–Iron Oxide Nanohybrids: Characterization and Biomedical Applications; Taylor & Francis: Milton, UK, 2019. [Google Scholar]
- Yin, H.; Wang, C.; Zhu, H.; Overbury, S.H.; Sun, S.; Dai, S. Colloidal deposition synthesis of supported gold nanocatalysts based on Au–Fe3O4 dumbbell nanoparticles. Chem. Commun. 2008, 4357–4359. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, H.; Dai, S.; Sun, S. A General Approach to Noble Metal−Metal Oxide Dumbbell Nanoparticles and Their Catalytic Application for CO Oxidation. Chem. Mater. 2010, 22, 3277–3282. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, K.; Liu, Y.; Qin, Y.; Wang, X.; Qi, L.; Shi, D. Photo-controlled aptamers delivery by dual surface gold-magnetic nanoparticles for targeted cancer therapy. Mater. Sci. Eng. C 2017, 80, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Ledari, R.; Zhang, W.; Radmanesh, M.; Mirmohammadi, S.S.; Maleki, A.; Cathcart, N.; Kitaev, V. Multi-Stimuli Nanocomposite Therapeutic: Docetaxel Targeted Delivery and Synergies in Treatment of Human Breast Cancer Tumor. Small 2020, 16, 2002733. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, X.; Wei, C.; Xu, Z.J.; Sim, S.S.W.; Liu, L.; Xu, C. Micro-optical coherence tomography tracking of magnetic gene transfection via Au–Fe3O4dumbbell nanoparticles. Nanoscale 2015, 7, 17249–17253. [Google Scholar] [CrossRef]
- Sood, A.; Varun, A.; Shah, J.; Kotnala, R.K.; Jain, T.K. Multifunctional Gold Coated Iron Oxide Core-Shell Nanoparticles Stabilized Using Thiolated Sodium Alginate for Biomedical Applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef]
- Ravichandran, M.; Velumani, S.; Ramirez, J.T.; Vera, A.; Leija, L. Biofunctionalized Mnfe2o4@Au Core–Shell Nanoparticles for Ph-Responsive Drug Delivery and Hyperthermal Agent for Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, S993–S1003. [Google Scholar]
- Wagstaff, A.J.; Brown, S.D.; Holden, M.R.; Craig, G.E.; Plumb, J.A.; Brown, R.E.; Schreiter, N.; Chrzanowski, W.; Wheate, N.J. Cisplatin Drug Delivery Using Gold-Coated Iron Oxide Nanoparticles for Enhanced Tumour Targeting with External Magnetic Fields. Inorg. Chim. Acta 2012, 393, 328–333. [Google Scholar] [CrossRef]
- Arora, V.; Sood, A.; Kumari, S.; Kumaran, S.S.; Jain, T.K. Hydrophobically modified sodium alginate conjugated plasmonic magnetic nanocomposites for drug delivery & magnetic resonance imaging. Mater. Today Commun. 2020, 25, 101470. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xu, M.; Dhawan, U.; Liu, W.C.; Wu, K.T.; Liu, X.R.; Lin, C.; Zhao, G.; Wu, Y.C.; Chung, R.J. Iron–gold alloy nanoparticles serve as a cornerstone in hyperthermia-mediated controlled drug release for cancer therapy. Int. J. Nanomed. 2018, 13, 5499–5509. [Google Scholar] [CrossRef]
- Singh, N.; Patel, K.; Sahoo, S.K.; Kumar, R. Human nitric oxide biomarker as potential NO donor in conjunction with superparamagnetic iron oxide @ gold core shell nanoparticles for cancer therapeutics. Colloids Surf. B Biointerfaces 2018, 163, 246–256. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Sun, S. Dumbbell-like Au−Fe3O4 Nanoparticles for Target-Specific Platin Delivery. J. Am. Chem. Soc. 2009, 131, 4216–4217. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, G.; Szigeti, G.P.; Szász, A. Hyperthermia versus Oncothermia: Cellular Effects in Complementary Cancer Therapy. Evid. Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of Magnetic Nanoparticles for Applications in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Moise, S.; Byrne, J.M.; El Haj, A.J.; Telling, N.D. The Potential of Magnetic Hyperthermia for Triggering the Differentiation of Cancer Cells. Nanoscale 2018, 10, 20519–20525. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Dennis, C.; Ivkov, R. Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Martínez, F.P.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-Mediated Photothermal Therapy: A Comparative Study of Heating for Different Particle Types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Bogart, L.K.; Southern, P.; Olivo, M.; Pankhurst, Q.A.; Parkin, I.P. Enhancing the Magnetic Heating Capacity of Iron Oxide Nanoparticles through Their Postproduction Incorporation into Iron Oxide–Gold Nanocomposites. Eur. J. Inorg. Chem. 2017, 18, 2386–2395. [Google Scholar] [CrossRef]
- Espinosa, A.; Bugnet, M.; Radtke, G.; Neveu, S.; Botton, G.A.; Wilhelm, C.; Abou-Hassan, A. Can magneto-plasmonic nanohybrids efficiently combine photothermia with magnetic hyperthermia? Nanoscale 2015, 7, 18872–18877. [Google Scholar] [CrossRef]
- Espinosa, A.; Reguera, J.; Curcio, A.; Muñoz-Noval, Á.; Kuttner, C.; Van De Walle, A.; Liz-Marzán, L.M.; Wilhelm, C. Janus Magnetic-Plasmonic Nanoparticles for Magnetically Guided and Thermally Activated Cancer Therapy. Small 2020, 16, e1904960. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic therapy: Apoptosis, paraptosis and beyond. Apoptosis 2020, 25, 611–615. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Health Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Cho, S.; Han, J.; Shin, H.; Na, K.; Lee, B.; Kim, D.H. Advanced smart-photosensitizers for more effective cancer treatment. Biomater. Sci. 2018, 6, 79–90. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-Infrared-Responsive Cancer Photothermal and Photodynamic Therapy Using Gold Nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Dou, Q.Q.; Ye, E.; Teng, C.P. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Li, Y.; Wang, K.; Zhu, J. Synergistic chemo-photodynamic therapy mediated by light-activated ROS-degradable nanocarriers. J. Mater. Chem. B 2019, 7, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Xie, Z. Metal–Organic Frameworks for Photodynamic Therapy: Emerging Synergistic Cancer Therapy. Biotechnol. J. 2021, 16, e1900382. [Google Scholar] [CrossRef]
- Bhana, S.; Lin, G.; Wang, L.; Starring, H.; Mishra, S.R.; Liu, G.; Huang, X. Near-Infrared-Absorbing Gold Nanopopcorns with Iron Oxide Cluster Core for Magnetically Amplified Photothermal and Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 11637–11647. [Google Scholar] [CrossRef]
- National Cancer Institute. Hyperthermia in Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types/surgery/hyperthermia-fact-sheet (accessed on 14 March 2021).
- Liu, D.; Li, X.; Chen, C.; Li, C.; Zhou, C.; Zhang, W.; Zhao, J.; Fan, J.; Cheng, K.; Chen, L. Target-Specific Delivery of Oxaliplatin to Her2-Positive Gastric Cancer Cells in Vivo Using Oxaliplatin-Au-Fe3o4-Herceptin Nanoparticles. Oncol. Lett. 2018, 15, 8079–8087. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2011, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; De Cuyper, M. Assessing Iron Oxide Nanoparticle Toxicity in Vitro: Current Status and Future Prospects. Nanomedicine 2010, 5, 1261–1275. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahmad, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A. Concentration-Dependent Toxicity of Iron Oxide Nanoparticles Mediated by Increased Oxidative Stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef]
- Gupta, A.K.; Guptab, M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing Toxicity of Fine and Nanoparticles: Comparing In Vitro Measurements to In Vivo Pulmonary Toxicity Profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef]
- Saber, H.; Frazier, J. Cellular Toxicity of Hydrazine in Primary Rat Hepatocytes. Toxicol. Sci. 2002, 69, 424–432. [Google Scholar]
- Casey, A.; Herzog, E.; Davoren, M.; Lyng, F.; Byrne, H.; Chambers, G. Spectroscopic Analysis Confirms the Interaction between Swcnt and Various Dyes Commonly Used to Assess Cytotoxicity. Carbon 2007, 45, 1425–1432. [Google Scholar] [CrossRef]
- Tian, F.; Cui, D.; Schwarz, H.; Estrada, G.G.; Kobayashi, H. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol. Vitr. 2006, 20, 1202–1212. [Google Scholar] [CrossRef]
- Hu, K.G.N.; Cen, L.; Kang, E.T. Cellular Response to Magnetic Nanoparticles “PEGylated” via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2006, 7, 809–816. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. 2020, 203, 111778. [Google Scholar] [CrossRef]
- Savage, D.T.; Zach, J.H.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. In Nanotoxicity: Methods and Protocols; Zhang, Q., Ed.; Springer: New York, NY, USA, 2019; pp. 1–29. [Google Scholar]
- Suman, S.; Pandey, A.; Chandna, S. An improved non-enzymatic “DNA ladder assay” for more sensitive and early detection of apoptosis. Cytotechnology 2011, 64, 9–14. [Google Scholar] [CrossRef]
- Hu, W.; Culloty, S.; Darmody, G.; Lynch, S.; Davenport, J.; Ramirez-Garcia, S.; Dawson, K.; Lynch, I.; Doyle, H.; Sheehan, D. Neutral red retention time assay in determination of toxicity of nanoparticles. Mar. Environ. Res. 2015, 111, 158–161. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Bin Lee, S.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med. Cell. Longev. 2019, 2019, 1–29. [Google Scholar] [CrossRef]
- Li, J.J.; Zou, L.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y.L. Gold Nanoparticles Induce Oxidative Damage in Lung Fibroblasts in Vitro. Adv. Mater. 2008, 20, 138–142. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y.L. Autophagy and Oxidative Stress Associated with Gold Nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au−ZnO Hybrid Nanopyramids and Their Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.T.; Feng, W.Y.; Wang, B.; Wang, T.C.; Gu, Y.Q.; Wang, M.; Wang, Y.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F. Comparative study of pulmonary responses to nano-and submicron-sized ferric oxide in rats. Toxicology 2008, 247, 102–111. [Google Scholar] [CrossRef]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H.; et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16–24. [Google Scholar] [CrossRef] [PubMed]
| Properties | Implication | References |
|---|---|---|
| Hardness or elastic module | Helps to better accomplish versatile goals and control action mechanisms. | [45] |
| Adhesion or frictional effects | Determines the colloidal stability, lubrication, nanofabrication, nanodevice design and drug delivery capabilities. | [29] |
| Nanoparticle size | Increasing the size enhances the physical difficulty to cross the lipid membrane of the cell and adversely affects the particle uptake through receptor-mediated endocytosis. | [36] |
| Surface charge or pKa | Increasing the surface charge/ionization increases the driving force of NPs and makes them translocate the cell membrane. It serves as opposition to the influence of enhanced size. | [37,38] |
| Ligand chemistry | AuNPs processing with hydrophobic ligands enhances targeted delivery and facilitates various diagnostic and therapeutic applications. The hydrophobic nature of the particle helps in trapping it inside the membrane by enhancing enthalpic reactions between the membrane and the ligand. NPs with high hydrophobic ligands possess high free energy gains as compared to the NPs with less hydrophobic ligands when placed inside lipid membranes and would help entrap the NPs inside the membrane. | [37,38] |
| Plasmonic | Plasmonic properties of noble metallic NPs are exploited for biomedical applications such as hyperthermia and bioimaging. | [14,41,42,46] |
| Magnetic | High magnetic properties with functionally designed surface of iron oxide NPs can be exploited in hyperthermia and image-guided delivery. | [12,16] |
| Structure | SIZE (nm) | Properties | Application | Implication | References |
|---|---|---|---|---|---|
| Flower | 30–300 nm | Comprises three to five petals of iron oxide and a core Au | Targeted cancer, LDI-MS | Suitable for use as efficient matrices in LDI-MS. Use in biomedical applications, such as targeting cancer cells and capture of ATP molecules. Use in detection and analysis of metabolites from cancer cells and molecular imaging. | [20] |
| Star | ≈70–150 nm | Improved plasmonic and magnetic properties | X-ray, MRI | Suitable as contrast agents for different imaging techniques, such as X-ray computed tomography, MRI, optical microscopy, SERS detection of target molecules, and photoacoustic imaging. | [14,93,94] |
| Dumbbell | ≈10–60 nm | Optically active plasmonic and high magnetic properties | Photothermal, biocatalysis | High symmetry and large free surface areas make them desirable for use in the photo- and biocatalysis applications. | [87] |
| Core-shell | 70 to 250 nm | High stability and tenability | Catalysis and cancer therapy | Colloidal stability in dispersion ensures a better shelf life. | [99] |
| Octahedral | 25 nm | High magnetization High crystallinity, relaxometric efficiency | MRI | Superior in vitro and in vivo T2 relaxivity, suitable for use as contrast agents in MRI. Two functional surfaces make them suitable for theranostic applications. | [95,96] |
| Rod | 8 nm | Enhanced plasmonic properties | Photothermal therapy | Robust iron oxide–gold nanorods can be prepared using the heteroaggregation approach | [97,98] |
| Janus | ≈120 nm | Chemically different domains Anisotropic properties | Targeted cancer, simultaneous differential release of drugs | Suitable for multiple functionalities in one nanoplatform. | [90,91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarkistani, M.A.M.; Komalla, V.; Kayser, V. Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1227. https://doi.org/10.3390/nano11051227
Tarkistani MAM, Komalla V, Kayser V. Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications. Nanomaterials. 2021; 11(5):1227. https://doi.org/10.3390/nano11051227
Chicago/Turabian StyleTarkistani, Mariam Abdulaziz M., Varsha Komalla, and Veysel Kayser. 2021. "Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications" Nanomaterials 11, no. 5: 1227. https://doi.org/10.3390/nano11051227
APA StyleTarkistani, M. A. M., Komalla, V., & Kayser, V. (2021). Recent Advances in the Use of Iron–Gold Hybrid Nanoparticles for Biomedical Applications. Nanomaterials, 11(5), 1227. https://doi.org/10.3390/nano11051227






