Mechanical and Thermal Evaluation of Aluminum Hybrid Nanocomposite Reinforced with Alumina and Graphene Oxide
Abstract
1. Introduction
2. Materials
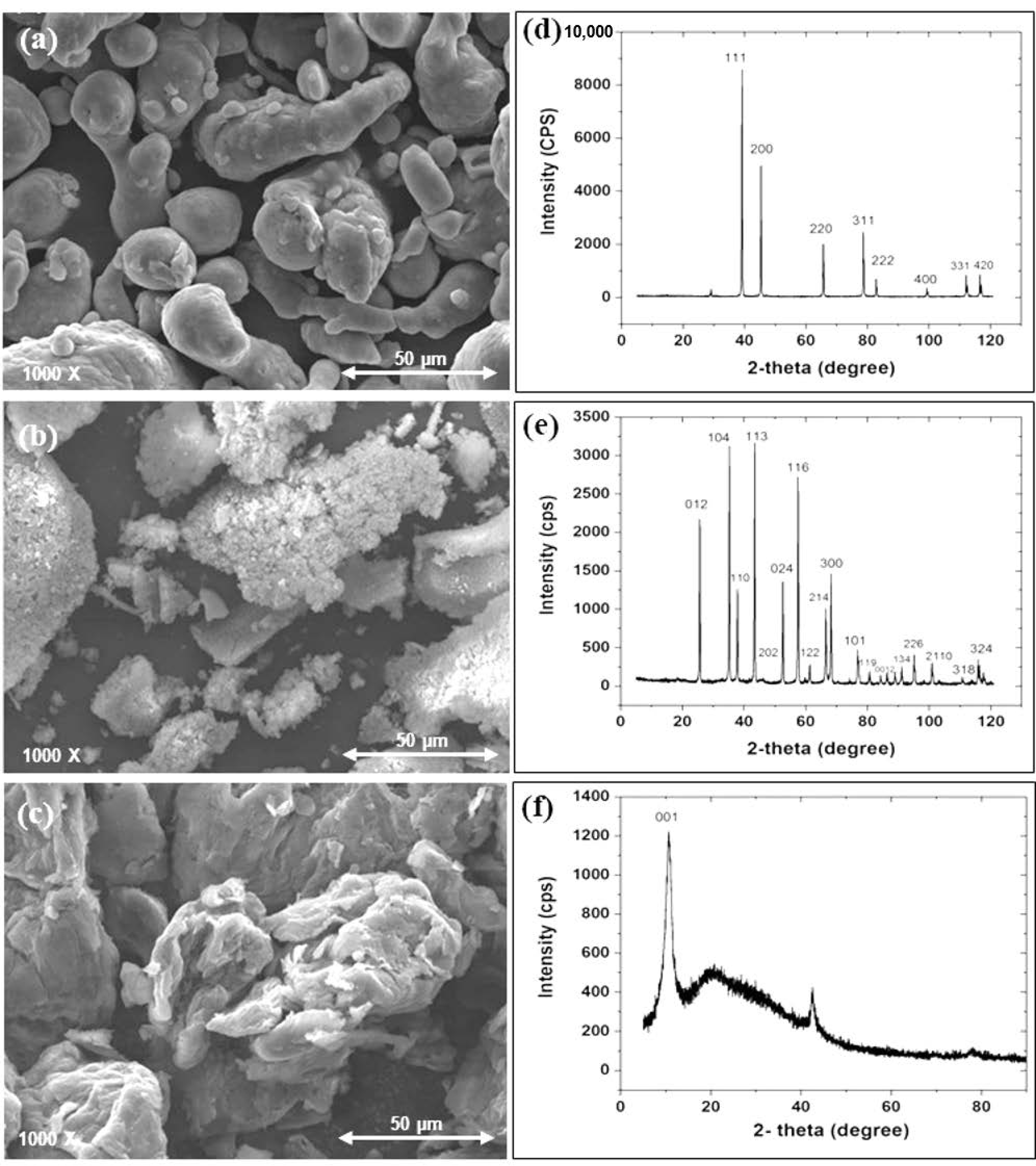
SEM and XRD Analysis of the as Received Powders
3. Experimental Procedure
3.1. Ultrasonication of Al2O3 and GO Powders
3.2. Ball-Milling Procedure
3.3. Spark Plasma Sintering Procedure
3.4. Densification, Mechanical, Thermal and Thermomechanical Analyses
4. Results and Discussion
4.1. Results of Phase I-Morphology and Mechanical Characterization of Al-X vol% Al2O3 Nanocomposites
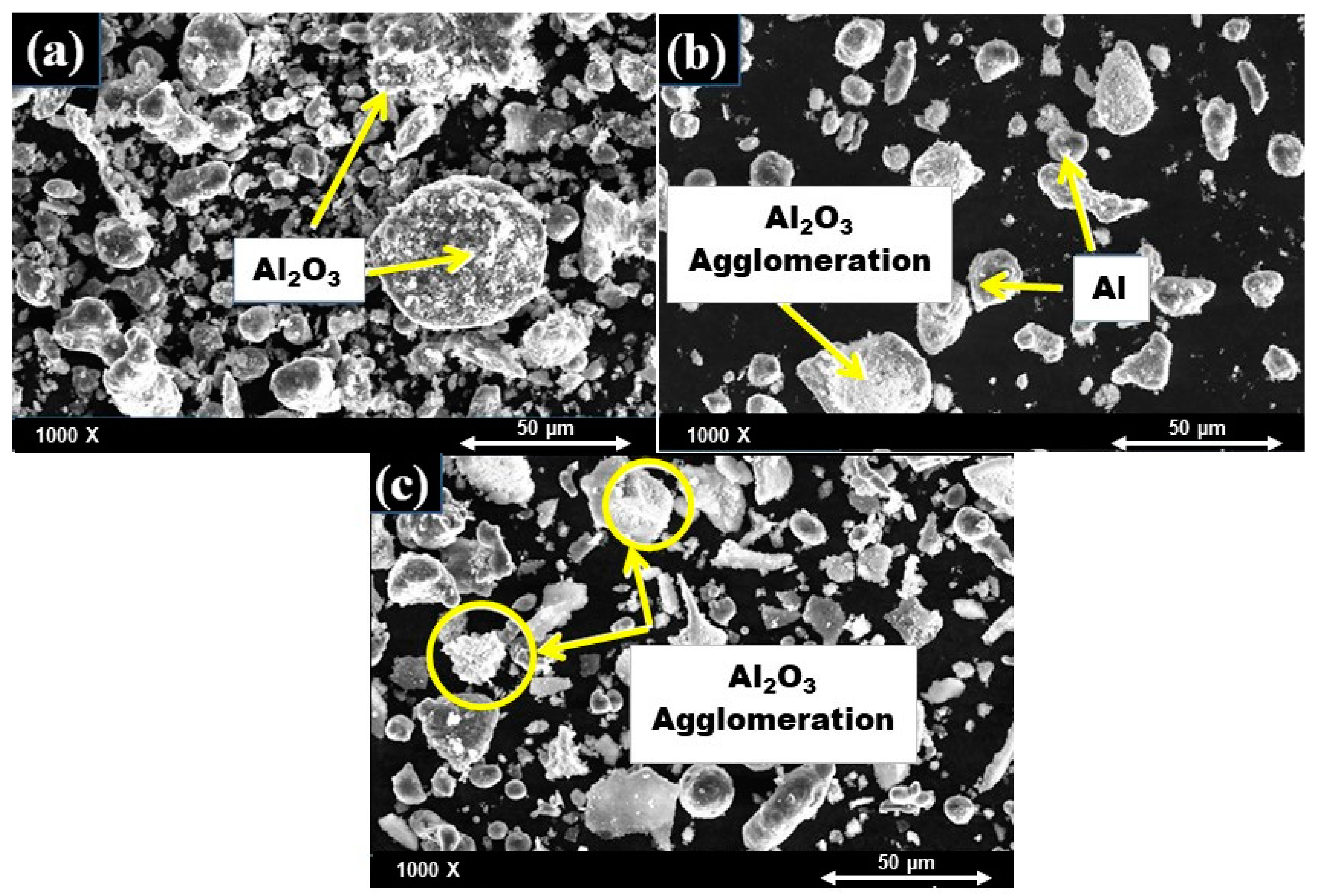
4.1.1. SEM Analysis of Al-X%Al2O3 Nanocomposite Powders after Mixing
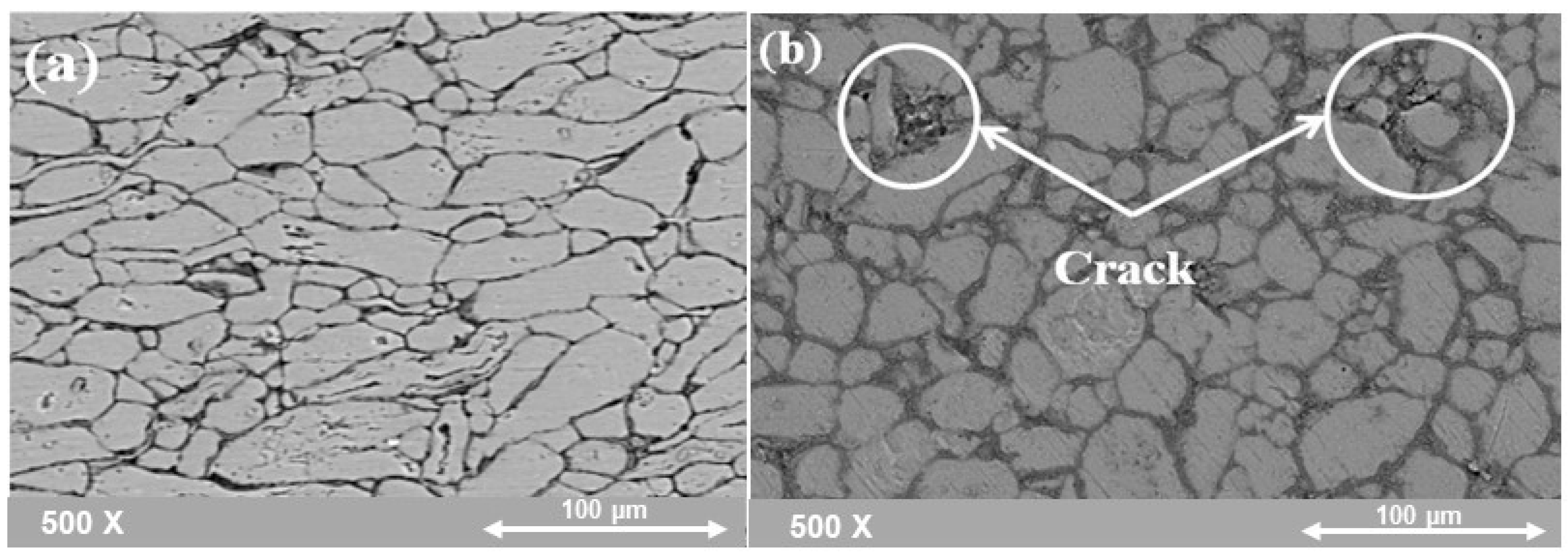
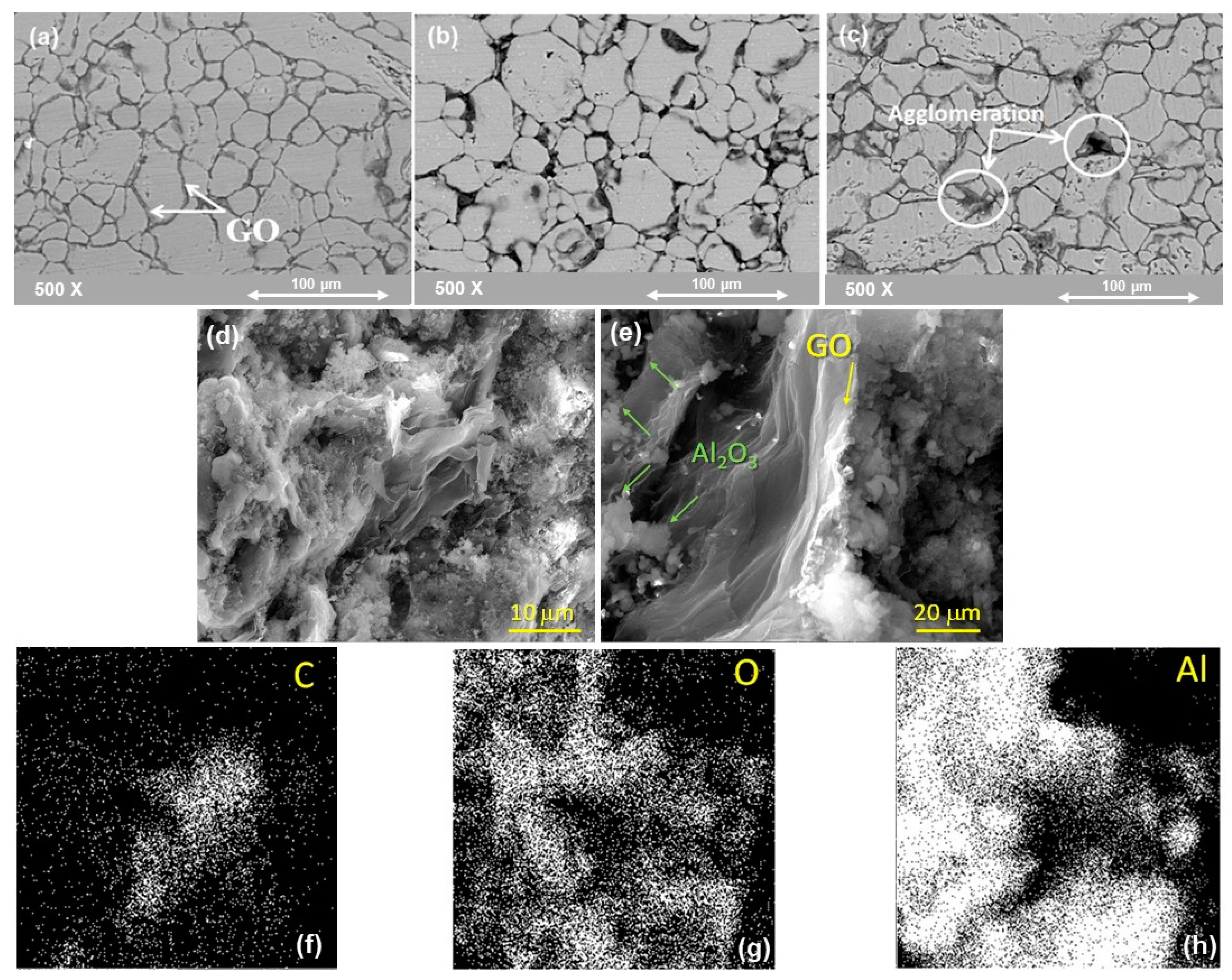
4.1.2. Microstructure of (Al-X%Al2O3) Nanocomposite Samples after SPS
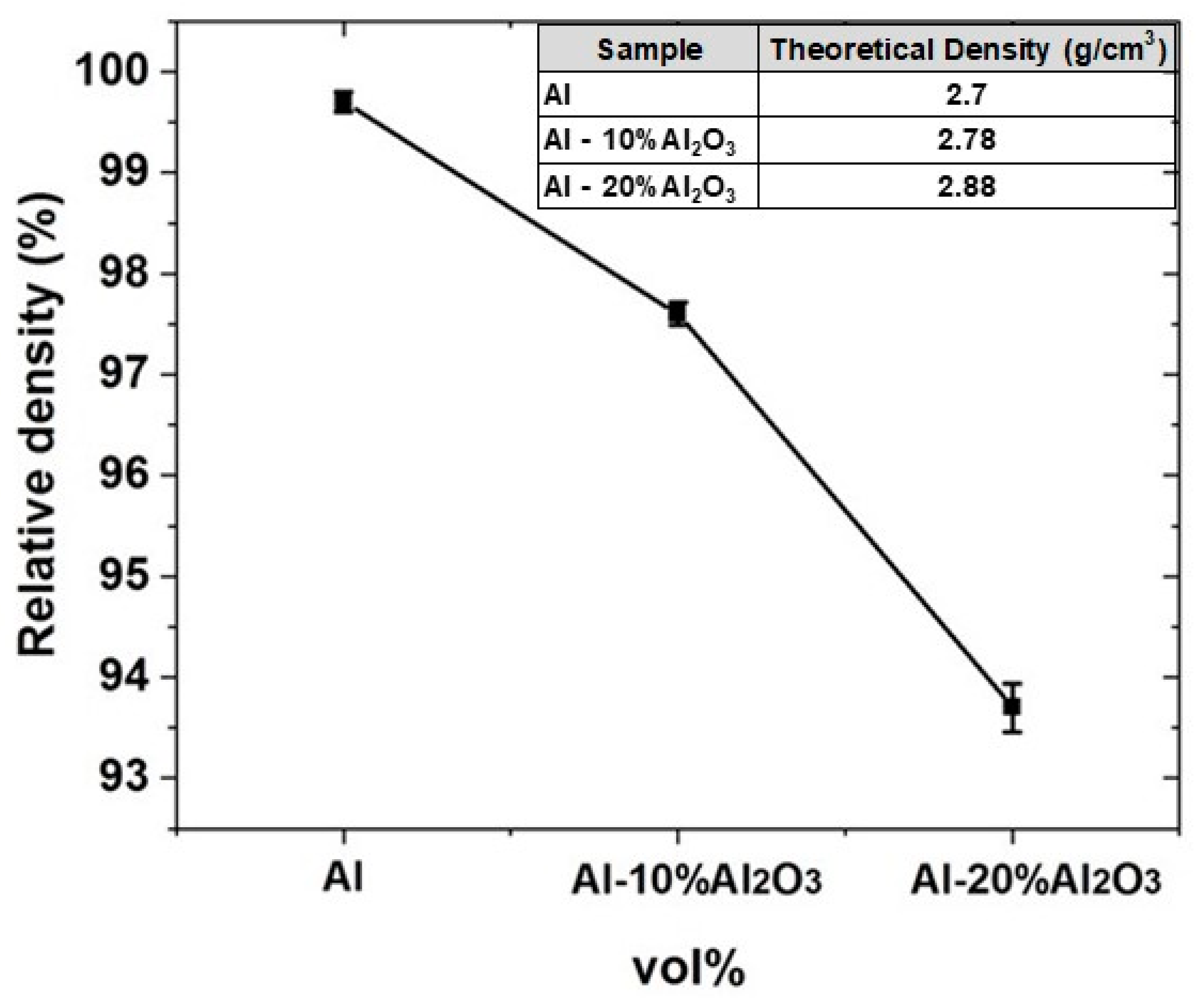
4.1.3. Density of Al-X vol% Al2O3 Nanocomposites
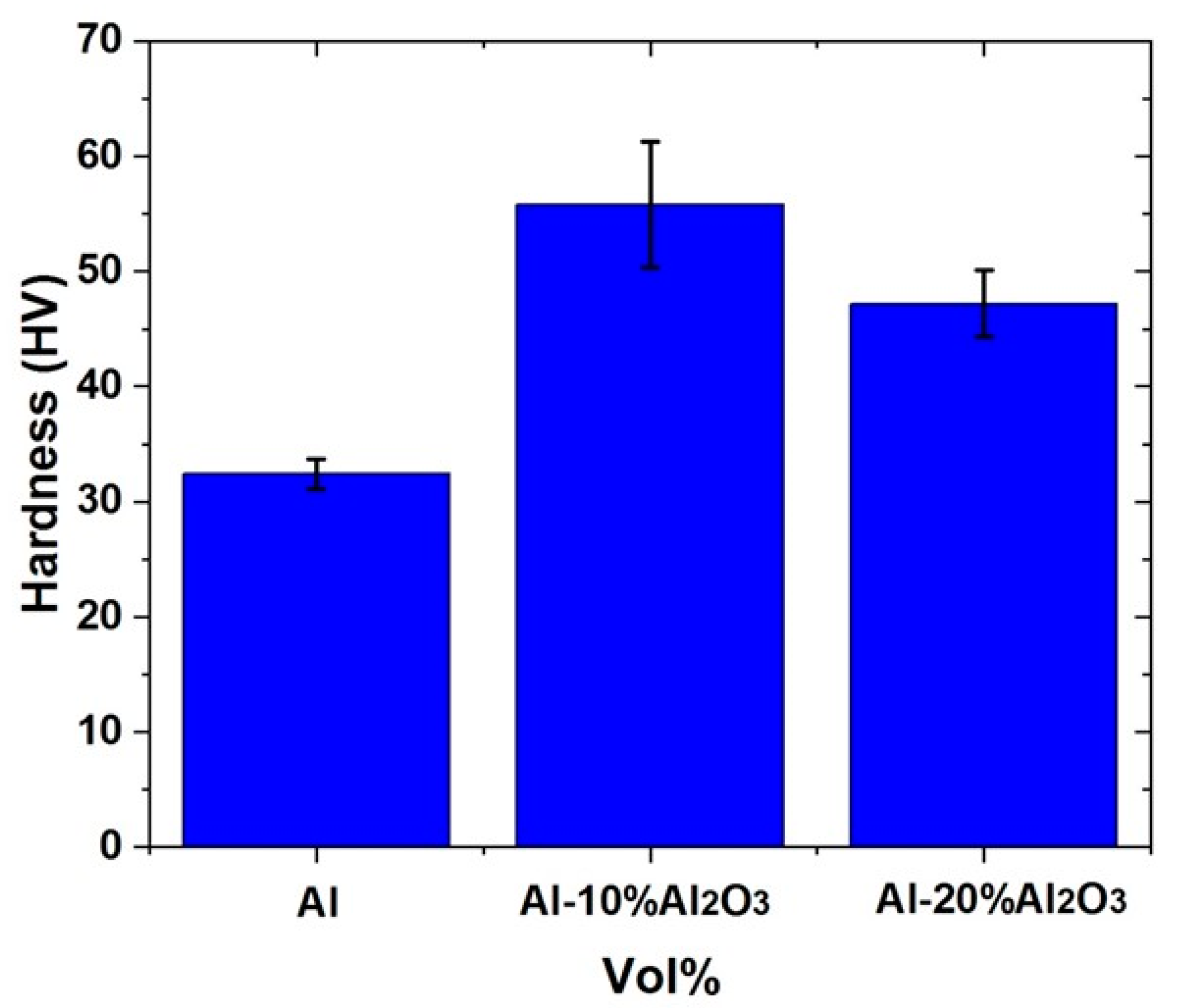
4.1.4. Hardness of Al-X vol% Al2O3 Nanocomposites
4.1.5. Summary of Phase I
4.2. Results of Phase II-Morphology and Mechanical Characterization of Al-10 vol% Al2O3 –Y wt.% GO Nanocomposites
4.2.1. SEM Analysis of Al-10 vol%Al2O3-Y wt.%GO Hybrid Nanocomposite Powders after Ball Milling
4.2.2. Microstructure of Al-10 vol%Al2O3-Y wt.%GO Hybrid Nanocomposites after SPS
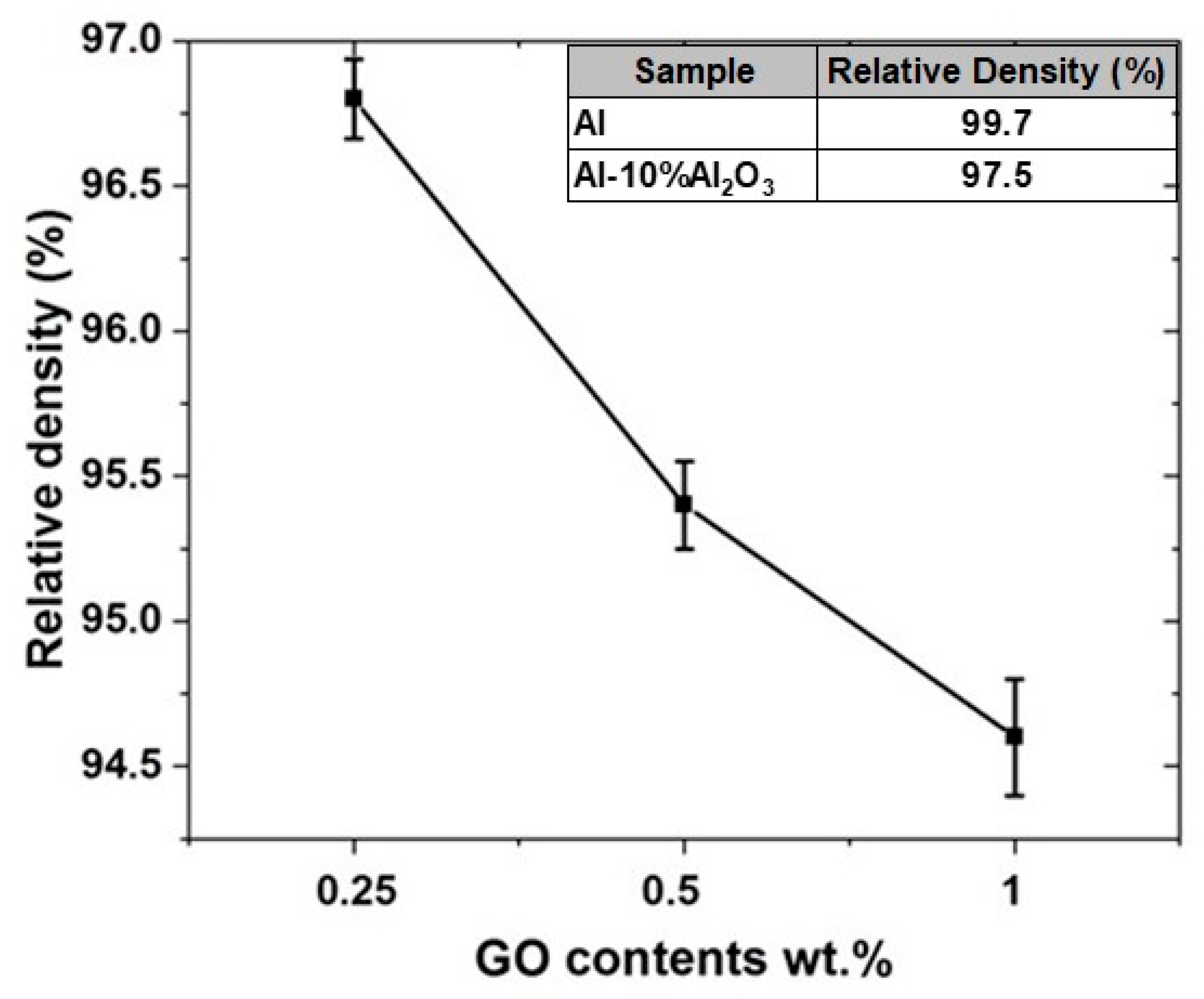
4.2.3. Density Measurement of Al-10% Al2O3-Y% GO Hybrid Nanocomposites
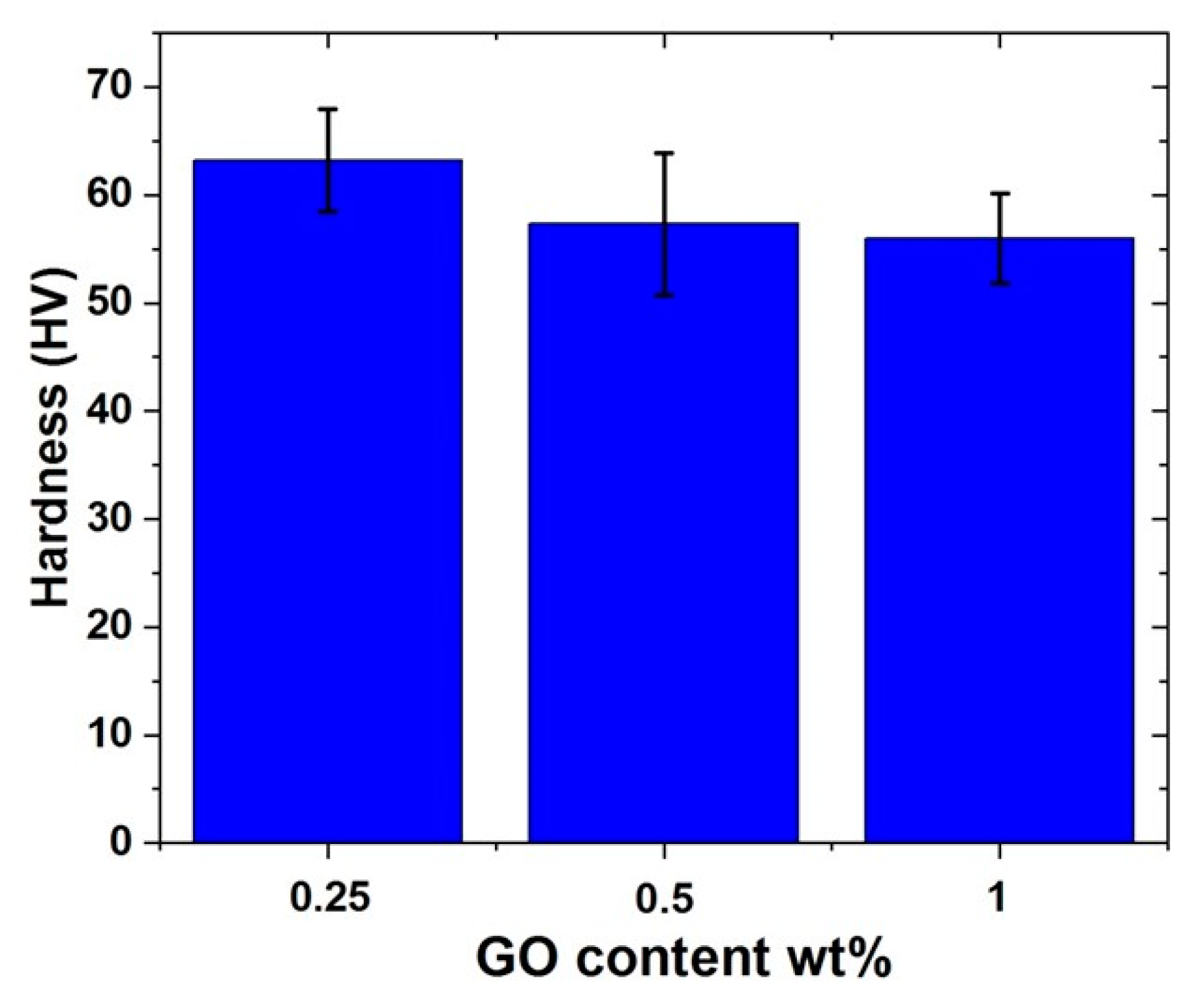
4.2.4. Hardness Measurement of Al-10% Al2O3-Y% GO Hybrid Nanocomposites
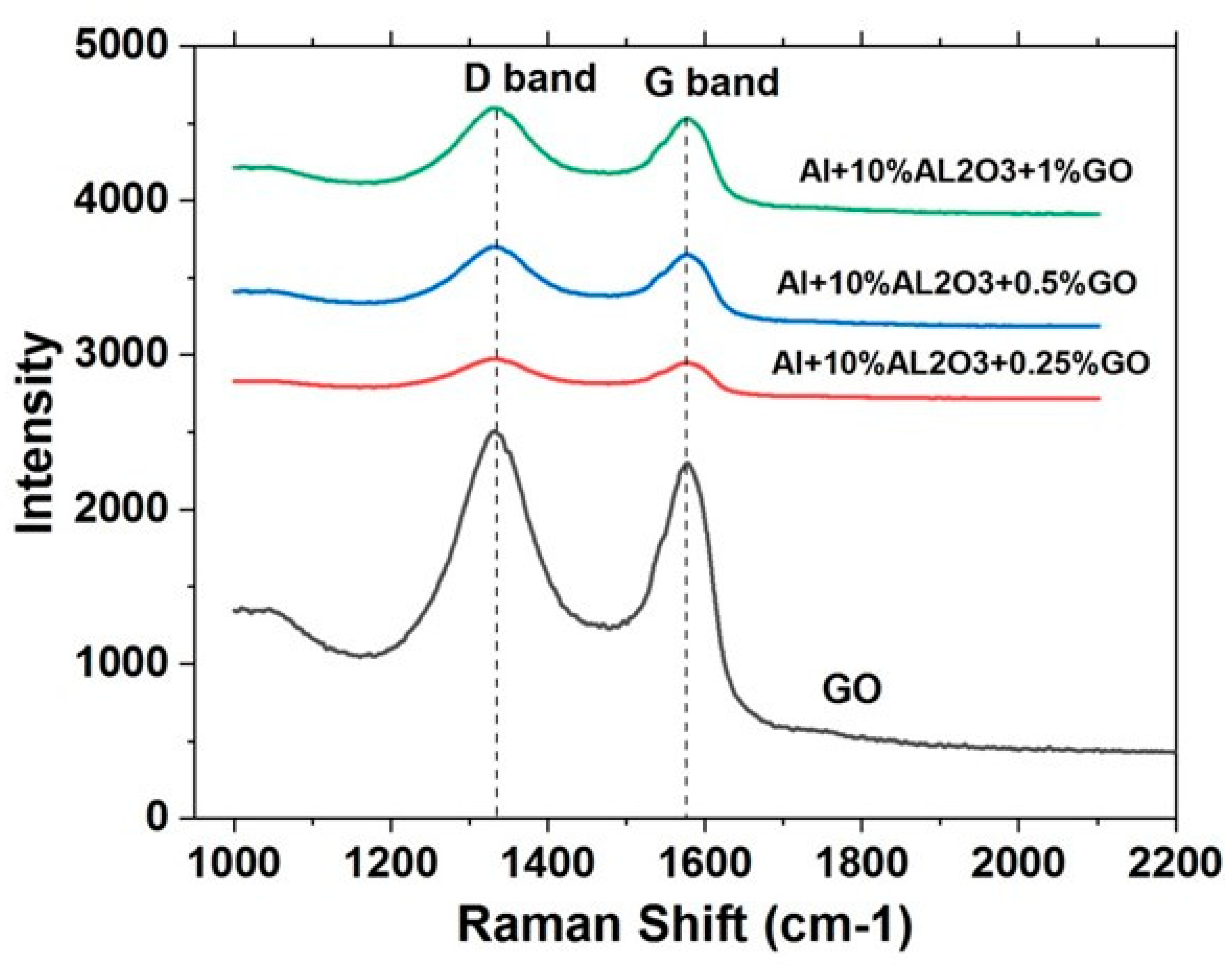
4.2.5. Raman Spectroscopy of Al-10% Al2O3-Y% GO Hybrid Nanocomposite Powders
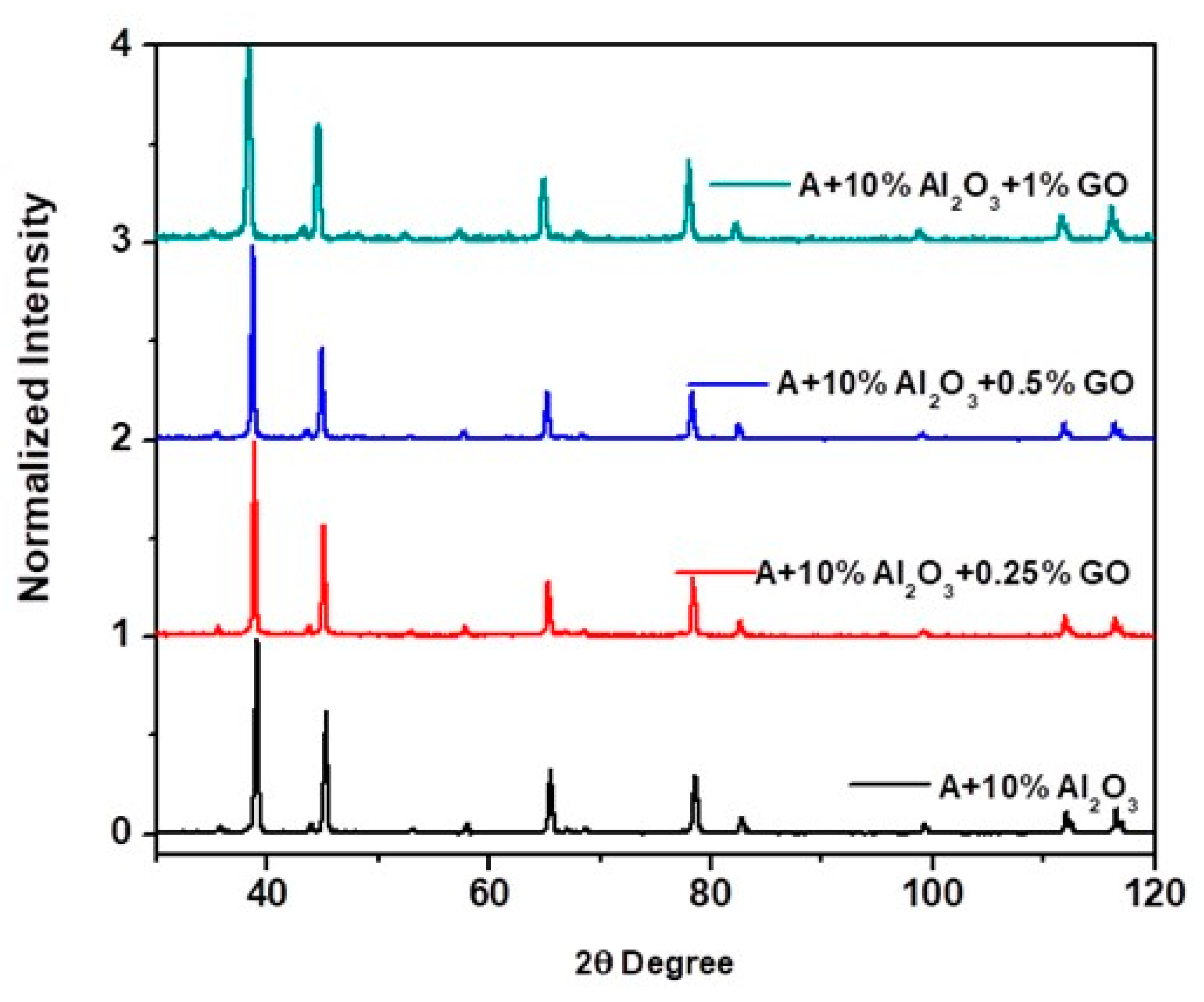
4.2.6. XRD Analysis of the Nanocomposite and Hybrid Nanocomposite Samples
4.2.7. Summary of Phase II
4.3. Results of Phase III-Mechanical and Thermal Characterization of Al-10 vol% Al2O3–0.25 wt.% GO Hybrid Nanocomposites
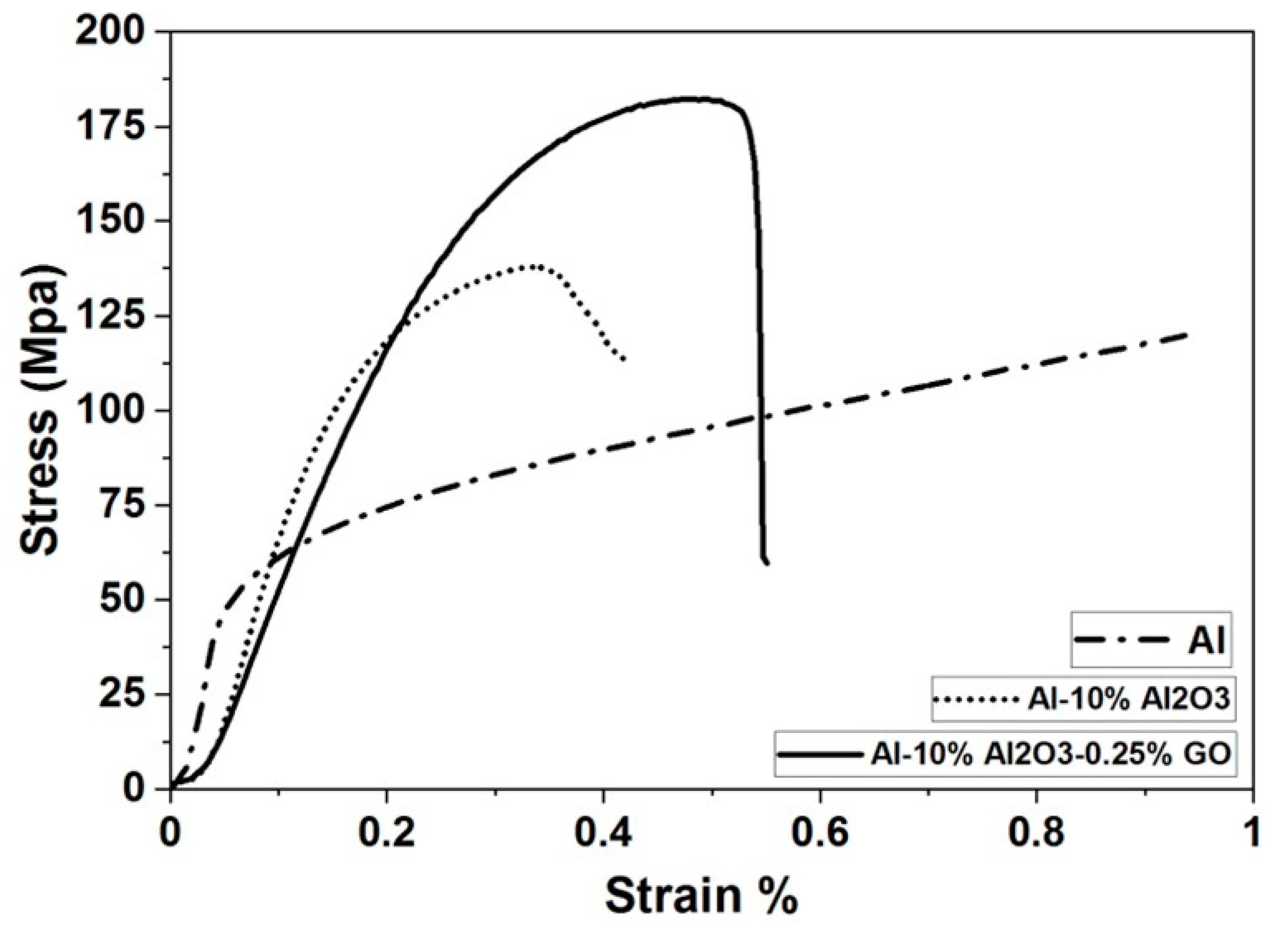
4.3.1. Evaluation of Compressive Strength for Al, (Al-10% Al2O3) and (Al-10% Al2O3-0.25%GO) Nanocomposites
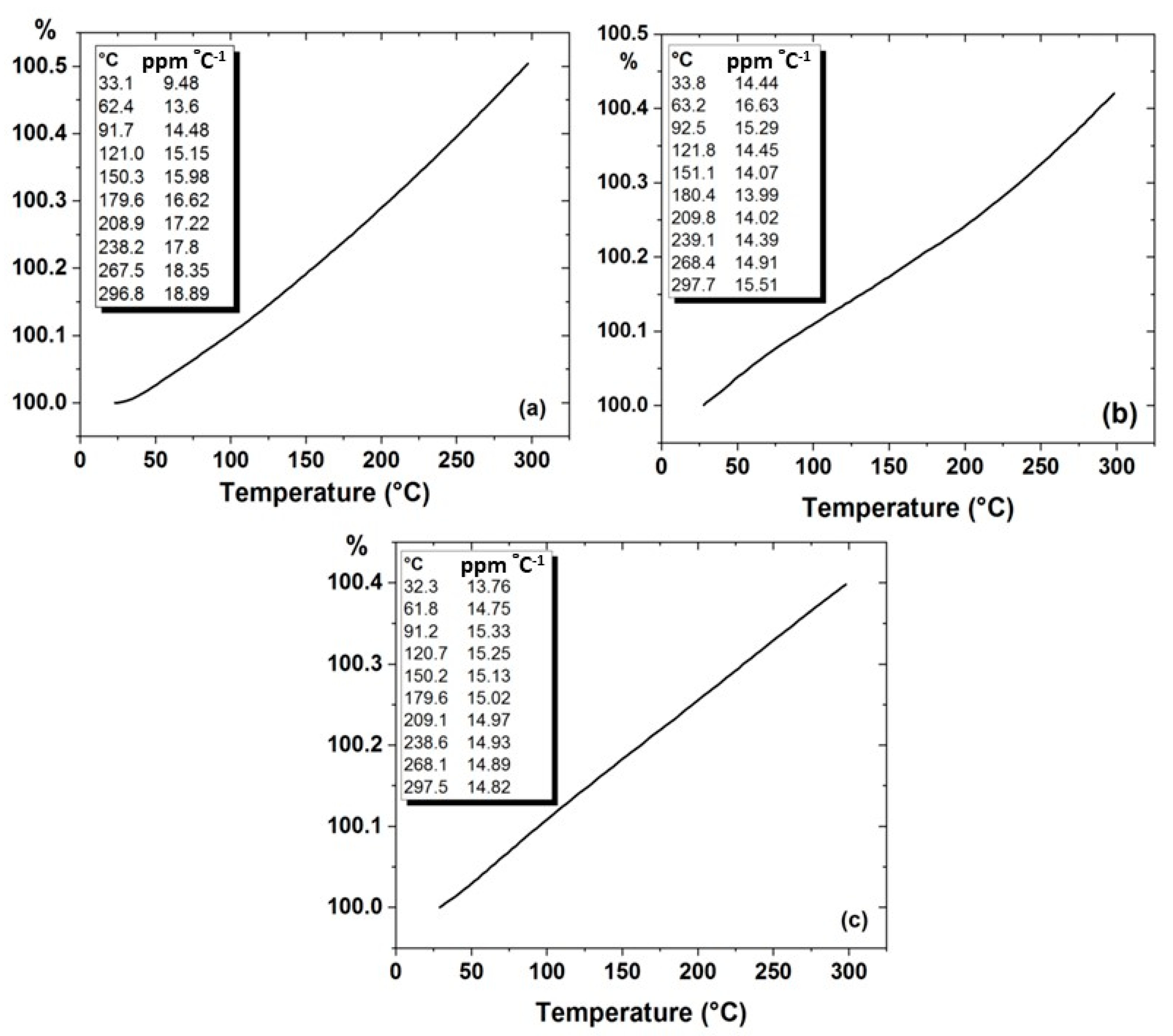
4.3.2. Thermal Expansion Measurement for Al, (Al-10% Al2O3) and (Al-10% Al2O3-0.25%GO) Nanocomposites
4.3.3. Summary of Phase III
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Sevik, H.; Kurnaz, S.C. Properties of alumina particulate reinforced aluminum alloy produced by pressure die casting. Mater. Des. 2006, 27, 676–683. [Google Scholar] [CrossRef]
- Martins Freitas, B.J.; Jorge Junior, A.M.; Zepon, G.; Kiminami, C.S.; Botta, W.J.; Bolfarini, C. Outstanding Tensile Ductility in High Iron-Containing Al-Si-Cu Alloys. Met. Mater. Trans. A 2020, 51, 2703–2710. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Ringer, S.P. Precipitation processes in Al-Cu-Mg alloys microalloyed with Si. Met. Mater. Trans. A 2000, 31, 2721–2733. [Google Scholar] [CrossRef]
- Dev Srivyas, P.; Charoo, M.S. Aluminum metal matrix composites a review of reinforcement; mechanical and tribological behavior. Int. J. Eng. Technol. 2018, 7, 117. [Google Scholar] [CrossRef]
- Tjong, S.C. Processing and Deformation Characteristics of Metals Reinforced with Ceramic Nanoparticles, 2nd ed.; Elsevier: Oxford, UK, 2014; Chapter 8; pp. 269–304. [Google Scholar]
- Tham, L.M.; Gupta, M.; Cheng, L. Effect of limited matrix—Reinforcement interfacial reaction on enhancing the mechanical properties of aluminium—Silicon carbide composites. Acta Mater. 2001, 49, 3243–3253. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Soni, V.K.; Das, S. Characterization of mechanical properties and microstructure of aluminium alloy-SiC composites. Mater. Today Proc. 2015, 2, 1149–1156. [Google Scholar] [CrossRef]
- Song, M. Effects of volume fraction of SiC particles on mechanical properties of SiC/Al composites. Trans. Nonferrous Met. Soc. China 2009, 19, 1400–1404. [Google Scholar] [CrossRef]
- Kumar, C.A.V.; Rajadurai, J.S. Influence of rutile (TiO2) content on wear and microhardness characteristics of aluminium-based hybrid composites synthesized by powder metallurgy. Trans. Nonferrous Met. Soc. China 2016, 26, 63–73. [Google Scholar] [CrossRef]
- Ezatpour, H.R.; Torabi-parizi, M.; Sajjadi, S.A. Microstructure and mechanical properties of extruded Al/Al2O3 composites fabricated by stir-casting process. Trans. Nonferrous Met. Soc. China 2013, 23, 1262–1268. [Google Scholar] [CrossRef]
- Zahid, G.H.; Azhar, T.; Musaddiq, M.; Rizvi, S.S.; Ashraf, M.; Hussain, N.; Iqbal, M. In situ processing and aging behaviour of an aluminium/Al2O3 composite. Mater. Des. 2011, 32, 1630–1635. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Yazdani-Rad, R.; Manafi, S.A. Effect of volume fraction and particle size of alumina reinforcement on compaction and densification behavior of Al–Al2O3 nanocomposites. Mater. Sci. Eng. A 2011, 528, 1105–1110. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, N.; Zhu, D. Friction and wear properties of in-situ synthesized Al2O3 reinforced aluminum composites. Trans. Nonferrous Met. Soc. China 2014, 24, 2352–2358. [Google Scholar] [CrossRef]
- Rahimian, M.; Parvin, N.; Ehsani, N. The effect of production parameters on microstructure and wear resistance of powder metallurgy Al–Al2O3 composite. Mater. Des. 2011, 32, 1031–1038. [Google Scholar] [CrossRef]
- Shuvho, M.B.A.; Chowdhury, M.A.; Kchaou, M.; Rahman, A.; Islam, M.A. Surface characterization and mechanical behavior of aluminum based metal matrix composite reinforced with nano Al2O3, SiC, TiO2 particles. Chem. Data Collect. 2020, 100442. [Google Scholar] [CrossRef]
- Aybarc, U.; Dispinar, D.; Seydibeyoglu, M.O. Aluminum metal matrix composites with SiC, Al2O3 and graphene—Review. Arch. Foundry Eng. 2018, 18, 5–10. [Google Scholar]
- Ozben, T.; Kilickap, E.; Çakır, O. Investigation of mechanical and machinability properties of SiC particle reinforced Al-MMC. Mater. J. Process. Technol. 2008, 198, 220–225. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, Q.; Zhang, D.; Zhu, J.; Li, R.; Yu, H. Carbon materials reinforced aluminum composites: A review. Acta Met. Sin. Engl. Lett. 2014, 27, 775–786. [Google Scholar] [CrossRef]
- Kumar, N.; Chittappa, H.C.; Ezhil Vannan, S. Development of aluminium-nickel coated short carbon fiber metal matrix composites. Mater. Today Proc. 2018, 5, 11336–11345. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Sun, P.; Cui, Y.; Wu, G. Microstructure and flexural properties of Z-pinned carbon fiber-reinforced aluminum matrix composites. Materials 2019, 12, 174. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Khanduja, D. Effect of graphite reinforcement on physical and mechanical properties of aluminum metal matrix composites. Part. Sci. Technol. 2016, 34, 17–22. [Google Scholar] [CrossRef]
- Moshier, W.C. Nonequilibrium alloying of graphite-reinforced aluminum metal matrix composites. In Corrosion; NACE International: Houston, TX, USA, 1993; Volume 49, Number 10. [Google Scholar]
- Jagannatham, M.; Chandran, P.; Sankaran, S.; Haridoss, P.; Nayan, N.; Bakshi, S.R. Tensile properties of carbon nanotubes reinforced aluminum matrix composites: A review. Carbon N. Y. 2020, 160, 14–44. [Google Scholar] [CrossRef]
- Sridhar, I.; Narayanan, K.R. Processing and characterization of MWCNT reinforced aluminum matrix composites. J. Mater. Sci. 2009, 44, 1750–1756. [Google Scholar] [CrossRef]
- Munir, K.; Wen, C.; Li, Y. Graphene nanoplatelets-reinforced magnesium metal matrix nanocomposites with superior mechanical and corrosion performance for biomedical applications. J. Magnes. Alloy. 2020, 8, 269–290. [Google Scholar] [CrossRef]
- Seyed Pourmand, N.; Asgharzadeh, H. aluminum matrix composites reinforced with graphene: A review on production, microstructure, and properties. Crit. Rev. Solid State Mater. Sci. 2019, 1–49. [Google Scholar] [CrossRef]
- Lian, W.; Mai, Y.; Wang, J.; Zhang, L.; Liu, C.; Jie, X. Fabrication of graphene oxide-Ti3AlC2 synergistically reinforced copper matrix composites with enhanced tribological performance. Ceram. Int. 2019, 45, 18592–18598. [Google Scholar] [CrossRef]
- Dasari, B.L.; Morshed, M.; Nouri, J.M.; Brabazon, D.; Naher, S. Mechanical properties of graphene oxide reinforced aluminium matrix composites. Compos. Part B Eng. 2018, 145, 136–144. [Google Scholar] [CrossRef]
- Khoshghadam-Pireyousefan, M.; Rahmanifard, R.; Orovcik, L.; Švec, P.; Klemm, V. Application of a novel method for fabrication of graphene reinforced aluminum matrix nanocomposites: Synthesis, microstructure, and mechanical properties. Mater. Sci. Eng. A 2020, 772, 138820. [Google Scholar] [CrossRef]
- Nyanor, P.; Hamada, K.; Nakamura, A.S.; Hassan, M.A.; El-Kady, O.; Yehia, H.M. Effect of carbon nanotube (cnt) content on the hardness, wear resistance and thermal expansion of in-situ reduced graphene oxide (rGO)-reinforced aluminum matrix composites. Met. Mater. Int. Jan. 2019. [CrossRef]
- Yigezu, S.; Mahapatra, B.M.; Jha, P. Influence of reinforcement type on microstructure, hardness, and tensile properties of an aluminum alloy metal matrix composite. J. Min. Mater. Charact. Eng. 2014, 1. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Aluko, A.O. Fracture toughness (K1C) and tensile properties of as-cast and age-hardened aluminium (6063)–silicon carbide particulate composites. Sci. Iran. 2012, 19, 992–996. [Google Scholar] [CrossRef]
- Rajmohan, T.; Palanikumar, K.; Ranganathan, S. Evaluation of mechanical and wear properties of hybrid aluminium matrix composites. Trans. Nonferrous Met. Soc. China 2013, 23, 2509–2517. [Google Scholar] [CrossRef]
- Ravindran, P.; Manisekar, K.; Rathika, P.; Narayanasamy, P. Tribological properties of powder metallurgy—Processed aluminium self lubricating hybrid composites with SiC additions. Mater. Des. 2013, 45, 561–570. [Google Scholar] [CrossRef]
- Sharifi, E.M.; Karimzadeh, F. Wear behavior of aluminum matrix hybrid nanocomposites fabricated by powder metallurgy. Wear 2011, 271, 1072–1079. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Sanusi, K.O. Microstructural characteristics, mechanical and wear behaviour of aluminium matrix hybrid composites reinforced with alumina, rice husk ash and graphite. Eng. Sci. Technol. Int. J. 2015, 18, 416–422. [Google Scholar] [CrossRef]
- Jacob Gajakosh, A.K.; Jagadeesha, T. Mechanical, tribological and theological studies on hybrid aluminium matrix composite reinforced with silicon carbide and reduced graphene oxide. Mater. Today Proc. 2020, 24, 343–349. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R Rep. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of Graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Yu, Z.; Asif, M.; Lin, H.; Pan, R. Investigation on microstructural, mechanical and electrochemical properties of aluminum composites reinforced with graphene nanoplatelets. Nat. Sci. Mater. Int. 2015, 25, 460–470. [Google Scholar]
- Irshad, H.M.; Hakeem, A.S.; Ahmed, B.A.; Ali, S.; Ehsan, M.A.; Laoui, T. Effect of Ni content and Al2O3 particle size on the thermal and mechanical properties of Al2O3/Ni composites prepared by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2018, 76, 25–32. [Google Scholar] [CrossRef]
- Dash, K.; Chaira, D.; Ray, B.C. Synthesis and characterization of aluminium–alumina micro- and nano-composites by spark plasma sintering. Mater. Res. Bull. 2013, 48, 2535–2542. [Google Scholar] [CrossRef]
- Elshina, L.A.; Muradymov, R.V.; Kvashnichev, A.G.; Vichuzhanin, D.I.; Molchanova, N.G.; Pankratov, A.A. Synthesis of new metal-matrix Al–Al2O3–graphene composite materials. Russ. Met. 2017, 8, 631–641. [Google Scholar] [CrossRef]
- Sadeghi, B.; Shamanian, M.; Ashrafizadeh, F.; Cavaliere, P.; Sanayei, M.; Szpunar, J.A. Microstructural behaviour of spark plasma sintered composites containing bimodal micro- and nano-sized Al2O3 particles. Powder Metall. 2018, 61, 50–63. [Google Scholar] [CrossRef]
- Firestein, K.L.; Corthay, S.; Steinman, A.E.; Matveev, A.T.; Kovalskii, A.M.; Sukhorukova, I.V.; Golberg, D.; Shtansky, D.V. High-strength aluminum-based composites reinforced with BN, AlB2 and AlN particles fabricated via reactive spark plasma sintering of Al-BN powder mixtures. Mater. Sci. Eng. A 2017, 681, 1–9. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Aqeeli, N.A.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]

| Material | Content | wt.% |
|---|---|---|
| Aluminum (Al) Powder | Al | >99.5 |
| Si | <0.25 | |
| Fe | <0.15 | |
| Ti | <0.25 | |
| Alumina Powder | Al2O3 | 99.88 |
| SiO2 | 0.034 | |
| P2O5 | 0.0085 | |
| S | 0.026 | |
| K2O | 0.027 | |
| TiO2 | 0.0022 | |
| V2O5 | 0.0041 | |
| MnO | 0.0016 | |
| Graphene Oxide (GO) | C | 77 |
| O2 | 22 | |
| Other | 1 |
| Material | Speed (RPM) | BPR | Mixing Time (Hours) | PCA | Atmosphere |
|---|---|---|---|---|---|
| Al\X% Al2O3 nanocomposite powder | 200 | 10:1 | 24 h | Ethanol | Argon |
| Al\X% Al2O3\Y% GO hybrid nanocomposite powder | 200 | 10:1 | 48 h | Ethanol | Argon |
| Material | Temp (°C) | Pressure (MPa) | Holding Time (min) | Heating Rate (°C/min) |
|---|---|---|---|---|
| Al\X% Al2O3 nanocomposite powder | 550 | 50 | 10 | 200 |
| Al\X% Al2O3\Y% GO hybrid nanocomposite powder | 550 | 50 | 10 | 200 |
| Material | Synthesis Method | Density (%) | Hardness (HV) | Tensile Strength (TS)/Compressive Strength (CS MPa) | Thermal Expansion (ppm °C−1) | Reference |
|---|---|---|---|---|---|---|
| Al–0.2 wt.% GO | Uniaxial compaction + tube furnace sintering | − | 36 | − | − | [29] |
| Al–4 wt.% Cu - 1 wt.% GO | SPS | 99.26 | 125 | 320 (TS) | − | [30] |
| Al–7 wt.% Al2O3 | SPS | 93.6 | 38.77 | - | − | [43] |
| Al–10% Al2O3-0.2% Graphene | In situ melt casting | − | 198 | 79.91 (TS) | − | [44] |
| Al–10 wt.% Al2O3 | SPS | 95.58 | 85 | 65 (TS) | − | [45] |
| Al-7 wt.% BN | SPS | − | 134 | 170 (TS) | − | [46] |
| Al-10 wt.% Al2O3 | Uniaxial compaction + tube furnace sintering | 98.1 | 81 | - | − | [15] |
| Al-20 wt.% TiB2 | SPS | 96 | 180 | 540 (TS) | − | [47] |
| Al-10%Al2O3-0.25%GO | SPS | 96.8 | 63.2 | 184(CS) | 14.82 | Present research |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.S.; Alahmari, T.S.; Laoui, T.; Hakeem, A.S.; Patel, F. Mechanical and Thermal Evaluation of Aluminum Hybrid Nanocomposite Reinforced with Alumina and Graphene Oxide. Nanomaterials 2021, 11, 1225. https://doi.org/10.3390/nano11051225
Mohammed AS, Alahmari TS, Laoui T, Hakeem AS, Patel F. Mechanical and Thermal Evaluation of Aluminum Hybrid Nanocomposite Reinforced with Alumina and Graphene Oxide. Nanomaterials. 2021; 11(5):1225. https://doi.org/10.3390/nano11051225
Chicago/Turabian StyleMohammed, Abdul Samad, Tawfeeq Saad Alahmari, Tahar Laoui, Abbas Saeed Hakeem, and Faheemuddin Patel. 2021. "Mechanical and Thermal Evaluation of Aluminum Hybrid Nanocomposite Reinforced with Alumina and Graphene Oxide" Nanomaterials 11, no. 5: 1225. https://doi.org/10.3390/nano11051225
APA StyleMohammed, A. S., Alahmari, T. S., Laoui, T., Hakeem, A. S., & Patel, F. (2021). Mechanical and Thermal Evaluation of Aluminum Hybrid Nanocomposite Reinforced with Alumina and Graphene Oxide. Nanomaterials, 11(5), 1225. https://doi.org/10.3390/nano11051225








