Preparation of MgGa Layered Double Hydroxides and Possible Compositional Variation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MgGa–LDHs Intercalated with Iodide
2.3. Anion Exchange from Iodide to Carbonate in MgGa–LDHs
2.4. Reconstruction of MgGa–LDHs in Aqueous Solution of Sodium Dodecyl Sulfate
2.5. Preparation of Suspension
2.6. Characterization
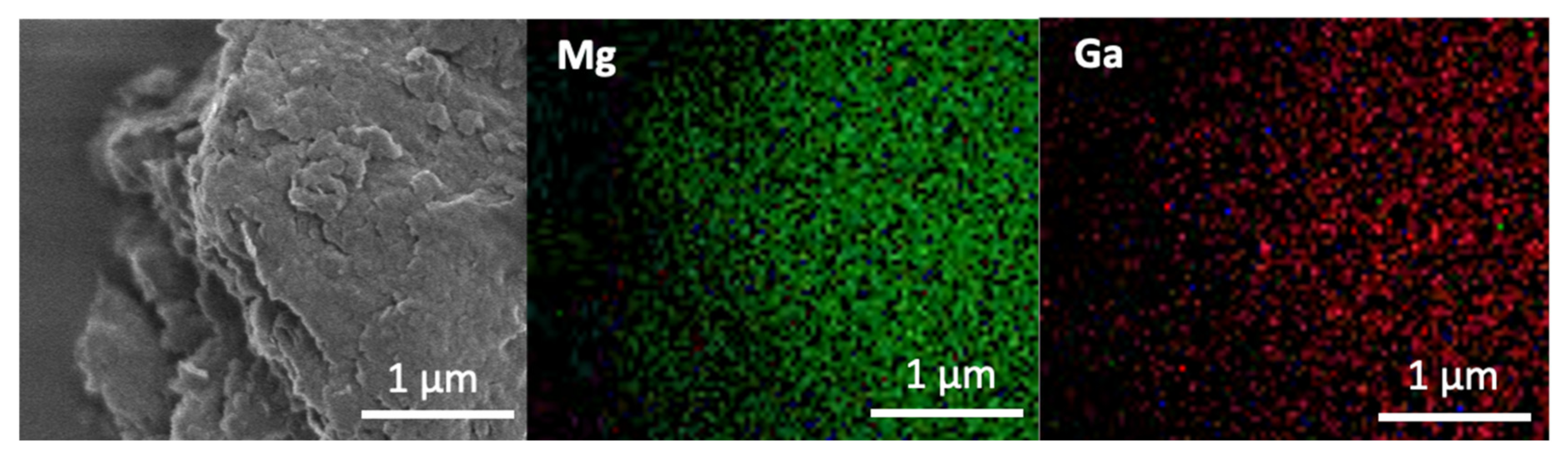
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Gao, Y.; Wang, Q.; Lin, W. The synergistic effect of layered double hydroxides with other flame retardant additives for polymer nanocomposites: A critical review. Dalton Trans. 2018, 47, 14827–14840. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Zhang, C.; Zeng, G.; Tan, X.; Yu, Z.; Zhong, Y.; Wang, H.; Cui, F. Utilization of LDH-based materials as potential adsorbents and photocatalysts for the decontamination of dyes wastewater: A review. RSC Adv. 2016, 6, 79415–79436. [Google Scholar] [CrossRef]
- Claverie, M.; Garcia, J.; Prevost, T.; Brendlé, J.; Limousy, L. Inorganic and hybrid (organic–inorganic) lamellar materials for heavy metals and radionuclides capture in energy wastes management—A review. Materials 2019, 12, 1399. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Ogawa, M. Preparation and properties of Mg/Al layered double hydroxide–oleate and–stearate intercalation compounds. Bull. Chem. Soc. Jpn. 2006, 79, 336–342. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring hybrid layered double hydroxides for the development of innovative applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Wijitwongwan, R.P.; Intasa-ard, S.G.; Ogawa, M. Preparation of layered double hydroxides toward precisely designed hierarchical organization. Chem. Eng. 2019, 3, 68. [Google Scholar] [CrossRef]
- Intasa-Ard, G.S.; Imwiset, J.K.; Bureekaew, S.; Ogawa, M. Mechanochemical methods for the preparation of intercalation compounds, from intercalation to the formation of layered double hydroxides. Dalton Trans. 2018, 47, 2896–2916. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers Inc.: New York, NY, USA, 2001. [Google Scholar]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. Struct. Bond. 2006, 119, 1–87. [Google Scholar]
- Mascolo, G.; Marino, O. A new synthesis and characterization of magnesium-aluminium hydroxides. Miner. Mag. 1980, 43, 619–621. [Google Scholar] [CrossRef]
- Lee, J.Y.; Gwak, G.H.; Kim, H.M.; Kim, T.I.; Lee, G.J.; Oh, J.M. Synthesis of hydrotalcite type layered double hydroxide with various Mg/Al ratio and surface charge under controlled reaction condition. Appl. Clay Sci. 2016, 134, 44–49. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. A reexamination of hydrotalcite crystal chemistry. J. Phys. Chem. 1996, 100, 8527–8534. [Google Scholar] [CrossRef]
- Miyata, S. Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Brindley, G.W.; Kikkawa, S. A crystal-chemical study of Mg, Al and Ni, N hydroxy-perchlorates and hydroxycarbonates. Am. Mineral. 1979, 64, 836–843. [Google Scholar]
- Kooli, F.; Chisem, I.C.; Vucelic, M.; Jones, W. Synthesis and properties of terephthalate and benzoate intercalates of mg− Al layered double hydroxides possessing varying layer charge. Chem. Mater. 1996, 8, 1969–1977. [Google Scholar] [CrossRef]
- Ogawa, M.; Asai, S. Hydrothermal synthesis of layered double hydroxide− deoxycholate intercalation compounds. Chem. Mater. 2000, 12, 3253–3255. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Vieira, R.S.; Ferreira, S.O.; Reis, C. Preparation and evaluation of the Ca-Al layered double hydroxide for removal of copper (II), nickel (II), zinc (II), chromium (VI) and phosphate from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 5469–5480. [Google Scholar] [CrossRef]
- Sipiczki, M.; Kuzmann, E.; Homonnay, Z.; Megyeri, J.; Pálinkó, I.; Sipos, P. The structure and stability of CaFe layered double hydroxides with various Ca:Fe ratios studied by Mössbauer spectroscopy, X-ray diffractometry and microscopic analysis. J. Mol. Struct. 2013, 1044, 116–120. [Google Scholar] [CrossRef]
- Ma, R.; Liang, J.; Takada, K.; Sasaki, T. Topochemical synthesis of Co−Fe layered double hydroxides at varied Fe/Co ratios: Unique intercalation of triiodide and its profound effect. J. Am. Chem. Soc. 2011, 133, 613–620. [Google Scholar] [CrossRef]
- Ma, R.; Liang, J.; Liu, X.; Sasaki, T. General insights into structural evolution of layered double hydroxide: Underlying aspects in topochemical transformation from brucite to layered double hydroxide. J. Am. Chem. Soc. 2012, 134, 19915–19921. [Google Scholar] [CrossRef] [PubMed]
- Caravaggio, G.A.; Detellier, C.; Wronski, Z. Synthesis, stability and electrochemical properties of NiAl and NiV layered double hydroxides. J. Mater. Chem. 2001, 11, 912–921. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Salanov, A.N.; Serkova, A.N.; Likholobov, V.A. SEM study of the surface morphology and chemical composition of the MgAl-and MgGa-layered hydroxides in different steps of platinum catalysts Pt/Mg(Al, Ga)Ox synthesis. Appl. Clay Sci. 2018, 157, 267–273. [Google Scholar] [CrossRef]
- Johnsen, R.E.; Wu, Q.; Sjåstad, A.O.; Vistad, Ø.B.; Krumeich, F.; Norby, P. Nanostructured materials produced by mixing and restacking of delaminated layered double hydroxides. J. Phys. Chem. 2008, 112, 16733–16739. [Google Scholar] [CrossRef]
- Petersen, L.B.; Lipton, A.S.; Zorin, V.; Nielsen, U.G. Local environment and composition of magnesium gallium layered double hydroxides determined from solid-state 1H and 71Ga NMR spectroscopy. J. Solid State Chem. 2014, 219, 242–246. [Google Scholar] [CrossRef]
- Sasaki, T.; Kooli, F.; Iida, M.; Michiue, Y.; Takenouchi, S.; Yajima, Y.; Izumi, F.; Chakoumakos, B.C.; Watanabe, M. A mixed alkali metal titanate with the lepidocrocite-like layered structure. Preparation, crystal structure, protonic form, and acid− base intercalation properties. Chem. Mater. 1998, 10, 4123–4128. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clay Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Carteret, C.; Grégoire, B.; Ruby, C. Tunable composition of NiII–AlIII and NiII–FeIII layered hydroxides within a wide range of layer charge. Solid State Sci. 2011, 13, 146–150. [Google Scholar] [CrossRef]
- López-Salinas, E.; García-Sánchez, M.; Ramon-Garcia, M.L.; Schifter, I. New Gallium-substituted hydrotalcites: [Mg1-xGax (OH)2](CO3)x/2· mH2O. J. Porous Mater. 1996, 3, 169–174. [Google Scholar] [CrossRef]
- López-Salinas, E.; Garcia-Sanchez, M.; Montoya, J.A.; Acosta, D.R.; Abasolo, J.A.; Schifter, I. Structural characterization of synthetic hydrotalcite-like [Mg1-x Gax(OH)2](CO3)x/2· mH2O. Langmuir 1997, 13, 4748–4753. [Google Scholar] [CrossRef]
- Miyata, S. The syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties—I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clay Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, H.C. Abrupt structural transformation in hydrotalcite-like compounds Mg1-xAlx(OH)2(NO3)x∙nH2O as a continuous function of nitrate anions. J. Phys. Chem. B 2001, 105, 1743–1749. [Google Scholar] [CrossRef]
- Palin, L.; Milanesio, M.; Van Beek, W.; Conterosito, E. Understanding the ion exchange process in LDH nanomaterials by fast in situ XRPD and PCA-assisted kinetic analysis. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Iyi, N.; Fujii, K.; Okamoto, K.; Sasaki, T. Factors influencing the hydration of layered double hydroxides (LDHs) and the appearance of an intermediate second staging phase. Appl. Clay Sci. 2007, 35, 218–227. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhu, Y.Q.; Xu, S.M.; Liu, H.M.; Yin, P.; Feng, Y.L.; Yan, H. Anion exchange behavior of MIIAl layered double hydroxides: A molecular dynamics and DFT study. Phys. Chem. Chem. Phys. 2020, 22, 19758–19768. [Google Scholar] [CrossRef]
- Roobottom, H.K.; Jenkins, H.D.B.; Passmore, J.; Glasser, L. Thermochemical radii of complex ions. J. Chem. Educ. 1999, 76, 1570. [Google Scholar] [CrossRef]
- Toson, V.; Conterosito, E.; Palin, L.; Boccaleri, E.; Milanesio, M.; Gianotti, V. Facile intercalation of organic molecules into hydrotalcites by liquid-assisted grinding: Yield optimization by a chemometric approach. Crys. Growth Des. 2015, 15, 5368–5374. [Google Scholar] [CrossRef]
- Meyn, M.; Beneke, K.; Lagaly, G. Anion-exchange reactions of layered double hydroxides. Inorg. Chem. 1990, 29, 5201–5207. [Google Scholar] [CrossRef]
- Naik, V.V.; Vasudevan, S. Effect of alkyl chain arrangement on conformation and dynamics in a surfactant intercalated layered double hydroxide: Spectroscopic measurements and MD simulations. J. Phys. Chem. C 2011, 115, 8221–8232. [Google Scholar] [CrossRef]
- Clearfield, A.; Kieke, M.; Kwan, J.; Colon, J.L.; Wang, R.C. Intercalation of dodecyl sulfate into layered double hydroxides. J. Incl. Phenom. Mol. Recognit. Chem. 1991, 11, 361–378. [Google Scholar] [CrossRef]
- Alansi, A.M.; Alkayali, W.Z.; Al-qunaibit, M.H.; Qahtan, T.F.; Saleh, T.A. Synthesis of exfoliated polystyrene/anionic clay MgAl-layered double hydroxide: Structural and thermal properties. RSC Adv. 2015, 5, 71441–71448. [Google Scholar] [CrossRef]

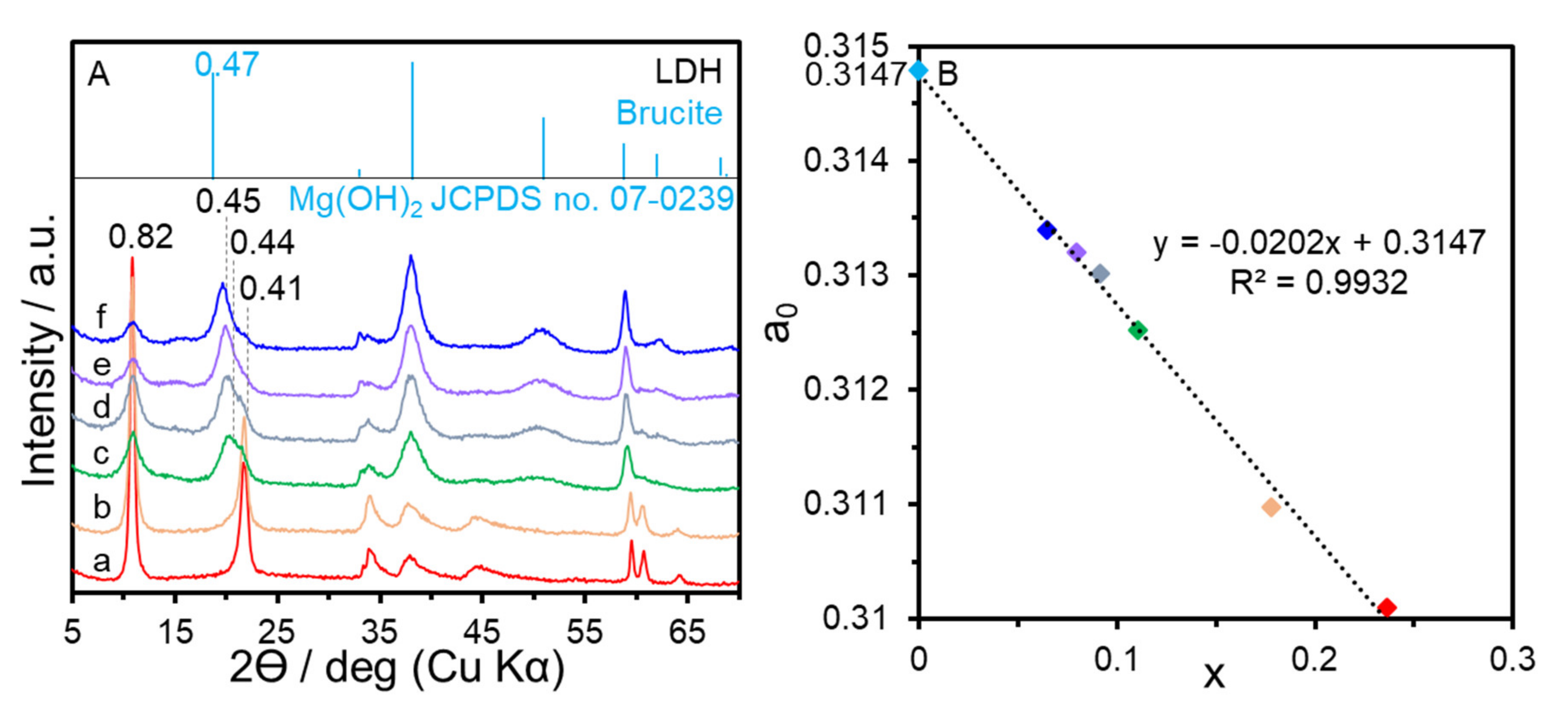
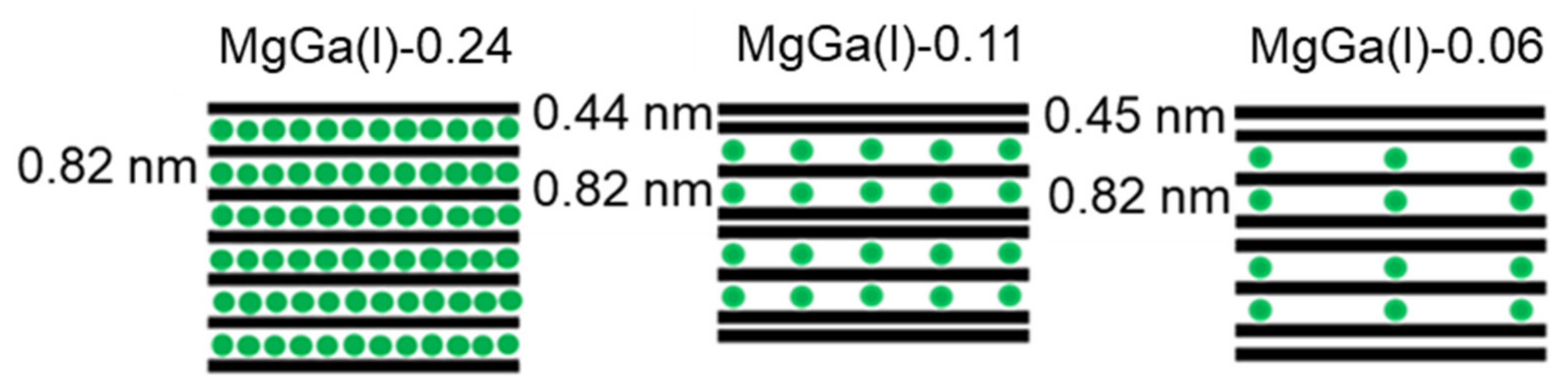
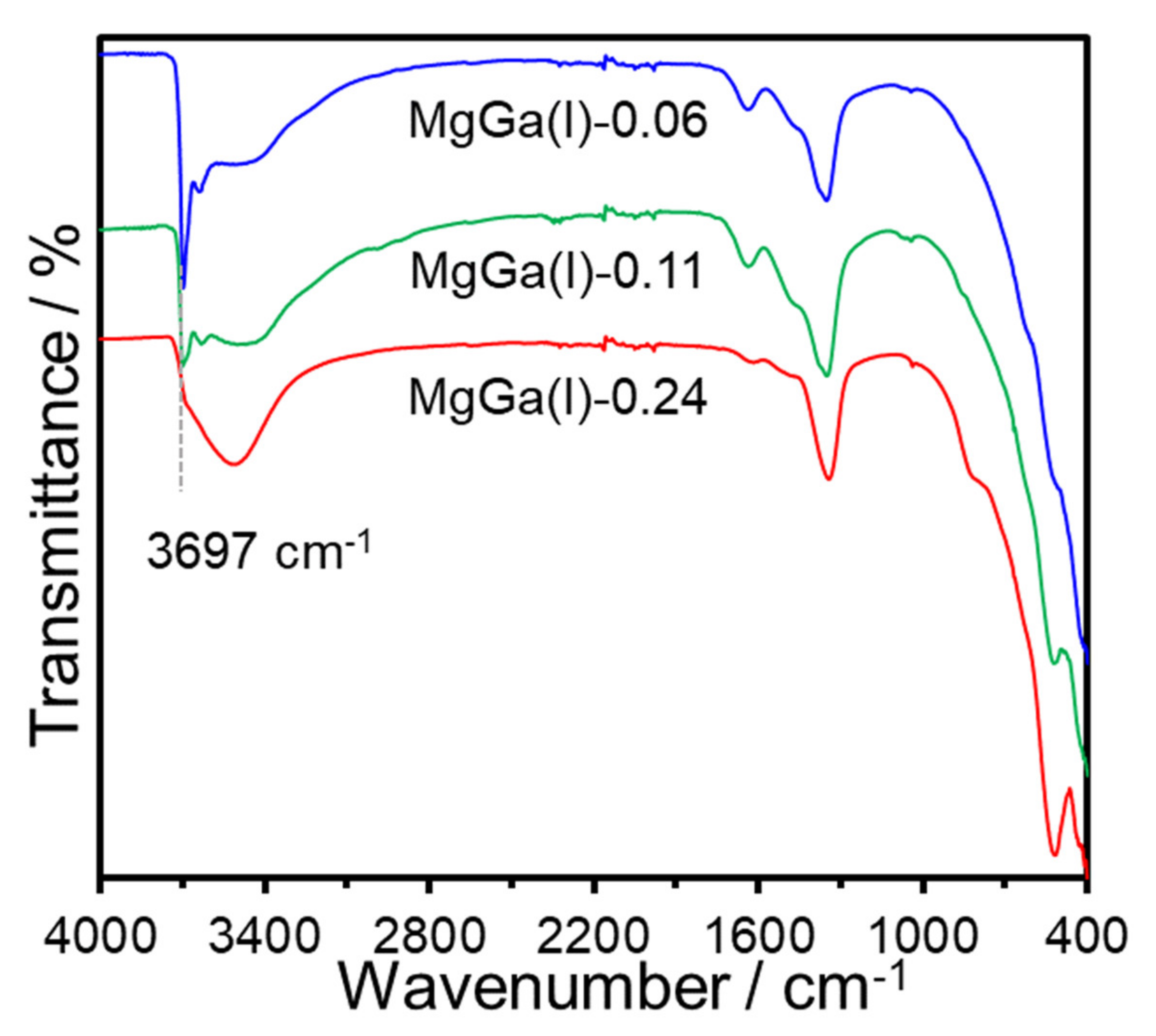

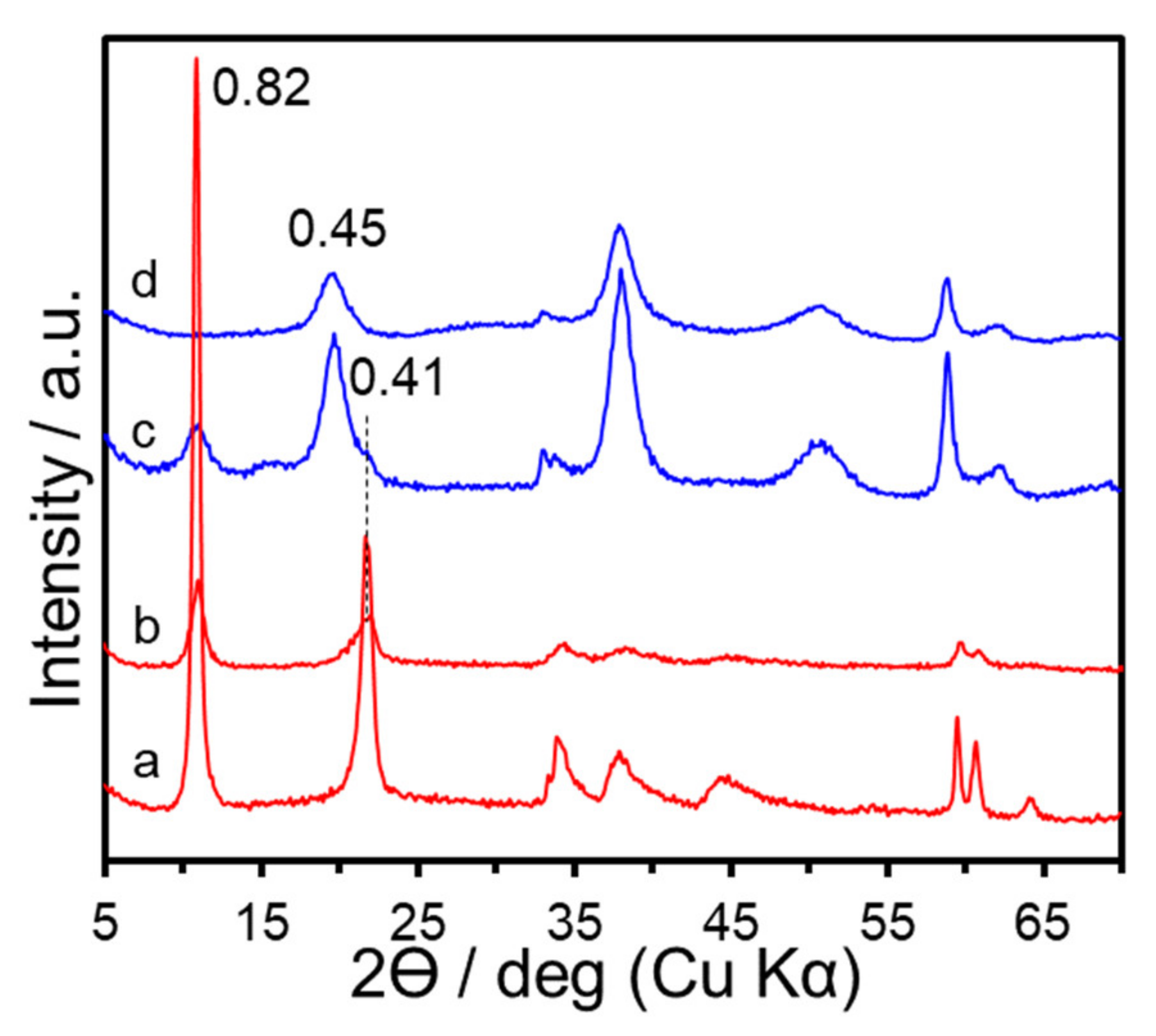
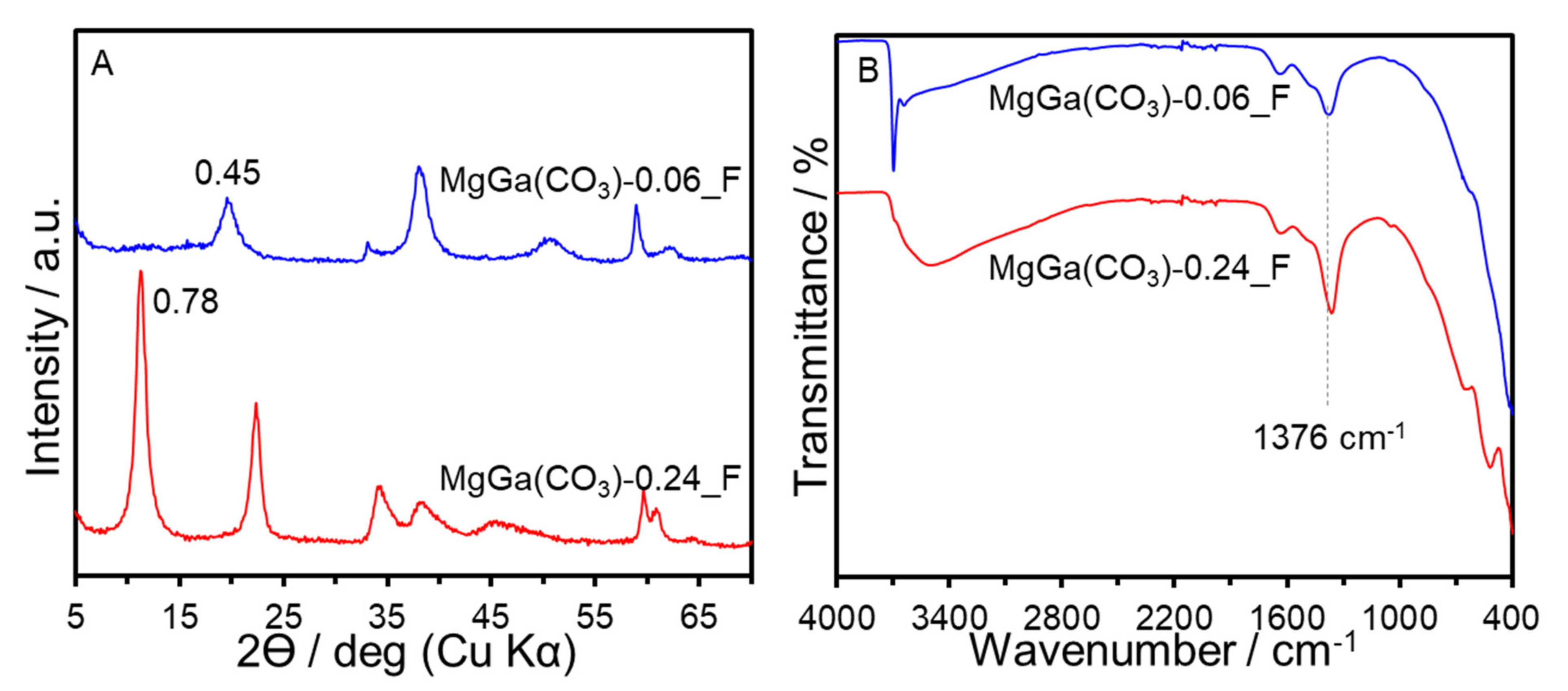
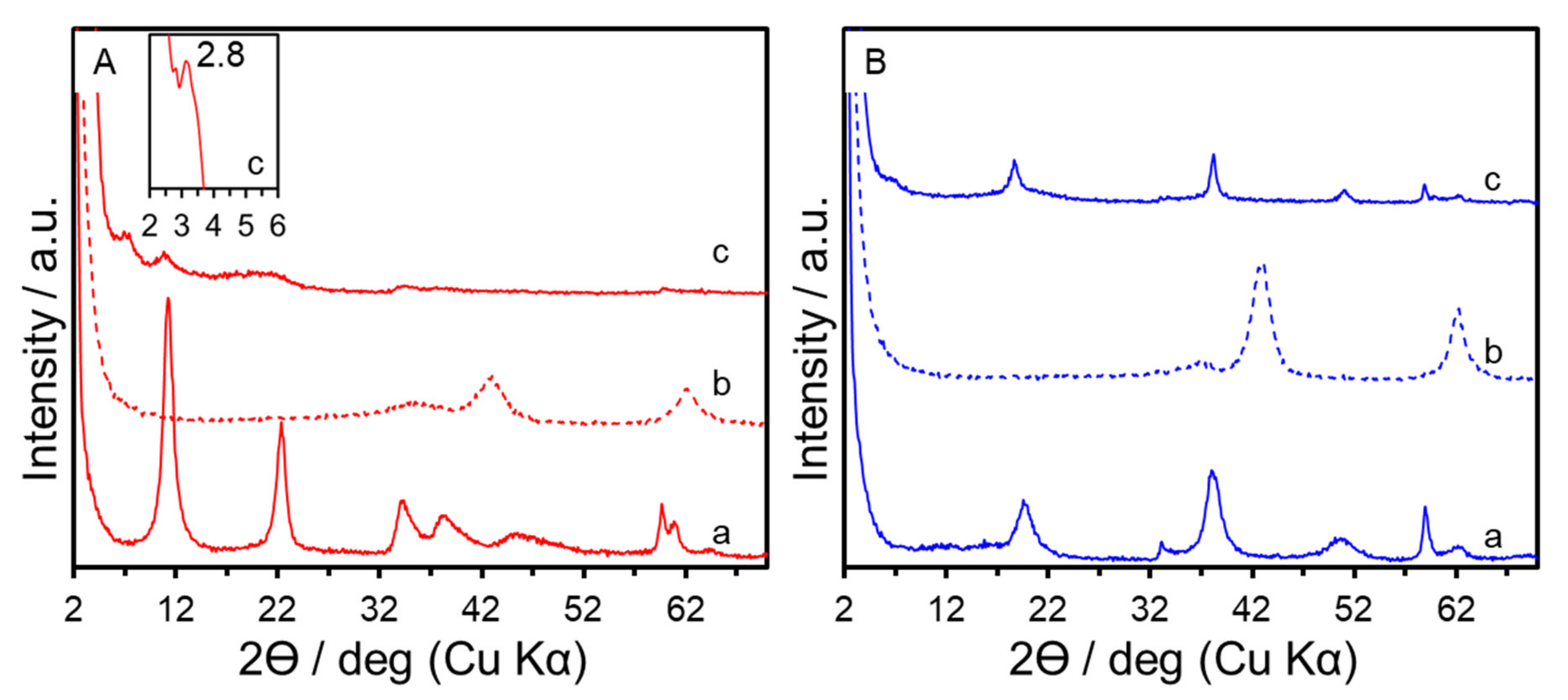
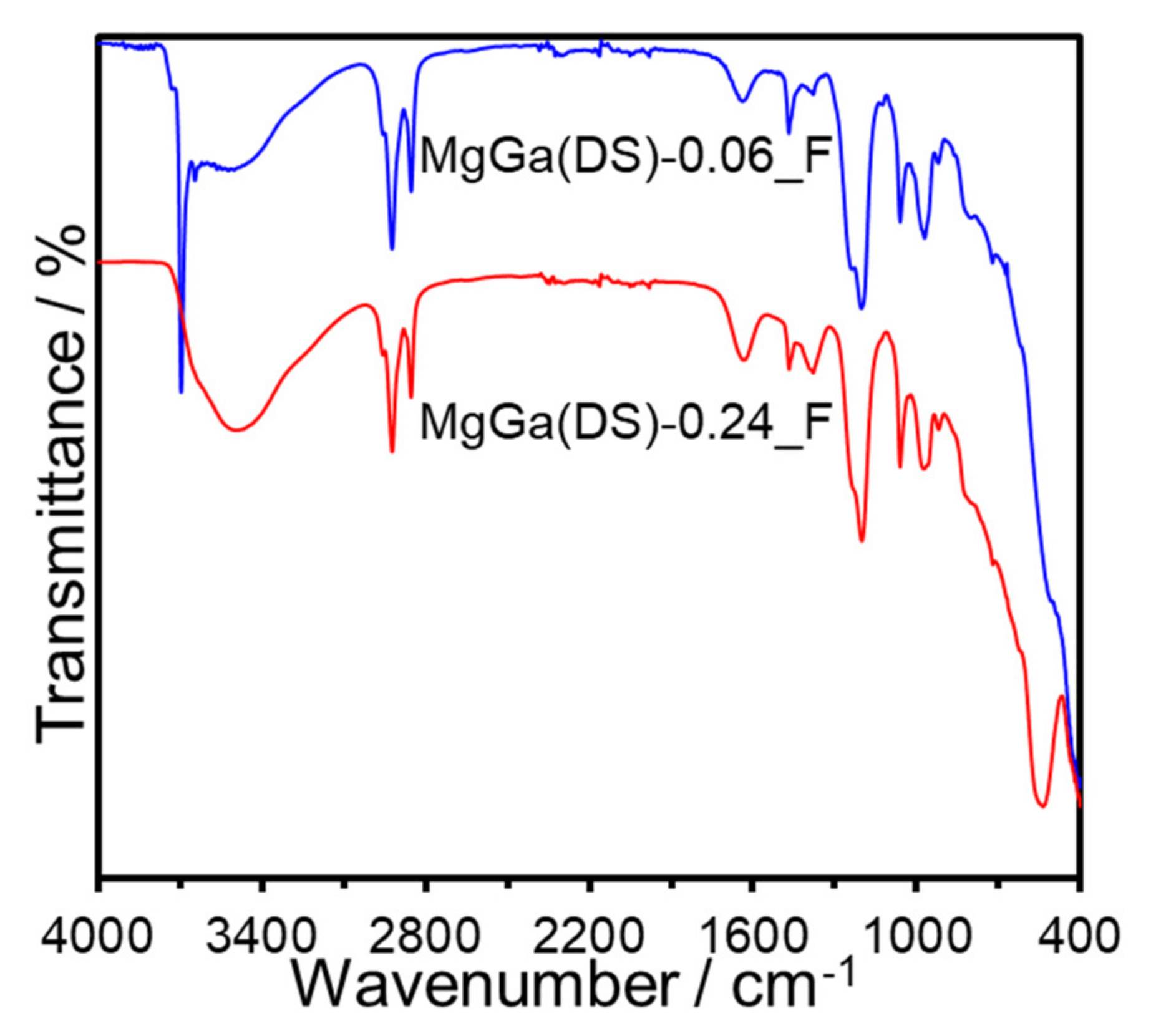
| Sample | x (starting solution) | x (products) | Ga/I (products) | Estimated Area per Charge (nm2/(+)) 1 | Estimated Layer Charge Density ((+)/nm2) 2 | ζ-Potential (mV) |
|---|---|---|---|---|---|---|
| MgGa(I)-0.24 | 0.25 | 0.24 | 2.0 | 0.35 | 2.86 | 38.71 |
| MgGa(I)-0.18 | 0.20 | 0.18 | 2.1 | 0.47 | 2.13 | NA.3 |
| MgGa(I)-0.11 | 0.13 | 0.11 | 2.4 | 0.76 | 1.31 | 34.47 |
| MgGa(I)-0.10 | 0.10 | 0.10 | 2.7 | 0.93 | 1.07 | NA.3 |
| MgGa(I)-0.08 | 0.09 | 0.08 | 2.9 | 1.07 | 0.93 | NA.3 |
| MgGa(I)-0.06 | 0.08 | 0.06 | 3.4 | 1.31 | 0.76 | 30.81 |
| Mg(OH)2 | - | - | - | - | - | 16.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijitwongwan, R.; Intasa-ard, S.; Ogawa, M. Preparation of MgGa Layered Double Hydroxides and Possible Compositional Variation. Nanomaterials 2021, 11, 1206. https://doi.org/10.3390/nano11051206
Wijitwongwan R, Intasa-ard S, Ogawa M. Preparation of MgGa Layered Double Hydroxides and Possible Compositional Variation. Nanomaterials. 2021; 11(5):1206. https://doi.org/10.3390/nano11051206
Chicago/Turabian StyleWijitwongwan, Rattanawadee (Ploy), Soontaree (Grace) Intasa-ard, and Makoto Ogawa. 2021. "Preparation of MgGa Layered Double Hydroxides and Possible Compositional Variation" Nanomaterials 11, no. 5: 1206. https://doi.org/10.3390/nano11051206
APA StyleWijitwongwan, R., Intasa-ard, S., & Ogawa, M. (2021). Preparation of MgGa Layered Double Hydroxides and Possible Compositional Variation. Nanomaterials, 11(5), 1206. https://doi.org/10.3390/nano11051206






