Gas Porosimetry by Gas Adsorption as an Efficient Tool for the Assessment of the Shaping Effect in Commercial Zeolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Gas Porosimetry
2.2.2. X-ray Diffraction Analysis
2.2.3. Infrared Spectroscopy
2.2.4. ICP-AES Analysis
3. Results and Discussion
3.1. Gas Porosimetry
3.1.1. Argon Adsorption-Desorption at 87 K
3.1.2. CO2 Adsorption–Desorption at 273 K
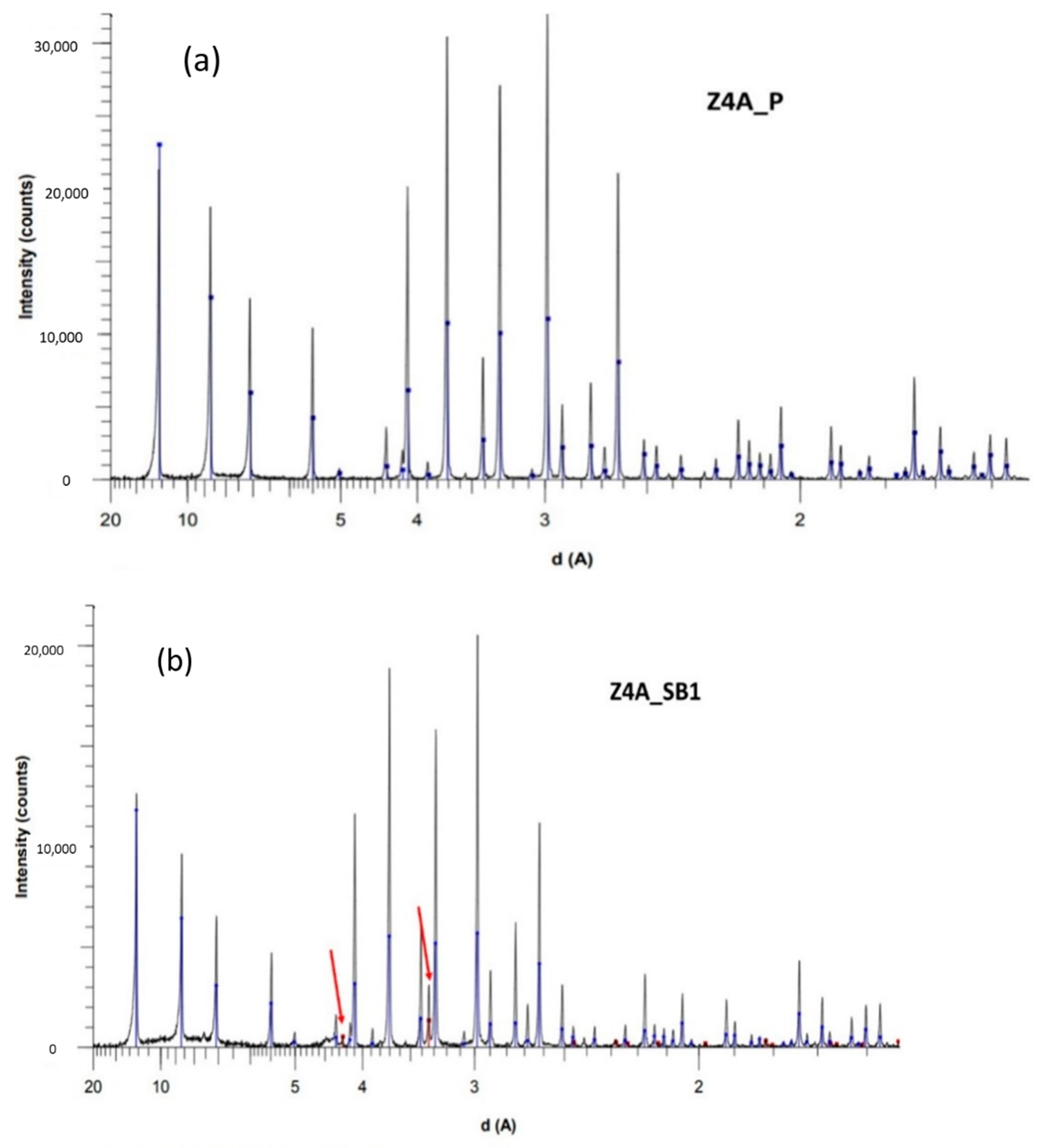
3.2. XRD Analysis
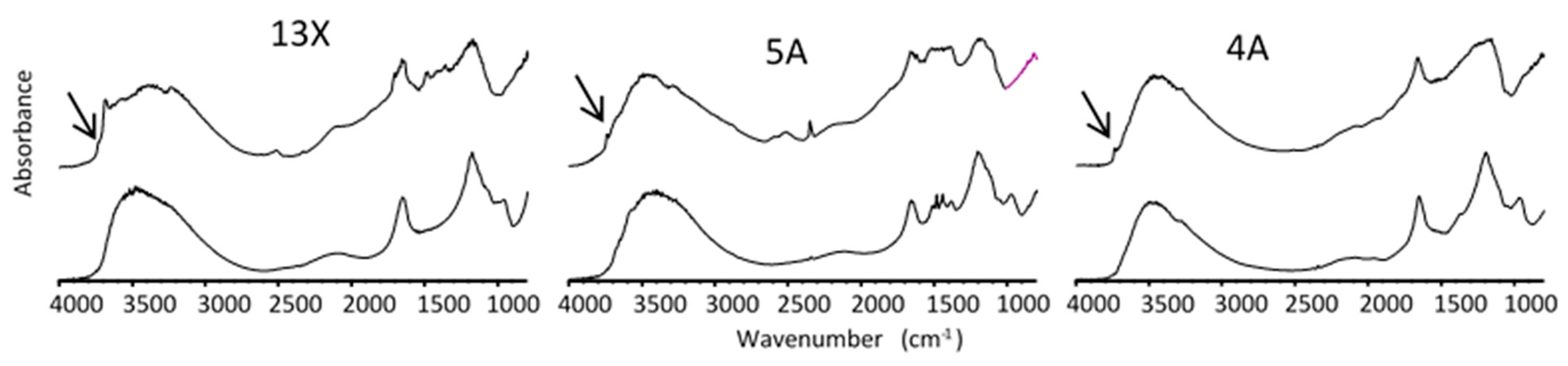
3.3. Infrared Spectroscopy
3.4. ICP-AES Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleichmann, K.; Unger, B.; Brandt, A. Industrial Zeolite Molecular Sieves. In Zeolites; Belviso, C., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Bingre, R.; Louis, B.; Nguyen, P. An overview on zeolite shaping technology and solutions to overcome diffusion limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Pfenninger, A. Manufacture and use of zeolites for adsorption processes. In Structures and Structure Determination; Baerlocher, C., Bennett, J.M., Depmeier, W., Fitch, A.N., Jobic, H., van Koningsveld, H., Meier, W.M., Pfenninger, A., Terasaki, O., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 163–198. ISBN 978-3-540-69749-7. [Google Scholar]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Hefti, M.; Marx, D.; Joss, L.; Mazzotti, M. Adsorption equilibrium of binary mixtures of carbon dioxide and nitrogen on zeolites ZSM-5 and 13X. Microporous Mesoporous Mater. 2015, 215, 215–228. [Google Scholar] [CrossRef]
- Rioland, G.; Daou, T.J.; Faye, D.; Patarin, J. A new generation of MFI-type zeolite pellets with very high mechanical performance for space decontamination. Microporous Mesoporous Mater. 2015, 221, 167–174. [Google Scholar] [CrossRef]
- Lakiss, L.; Gilson, J.P.; Valtchev, V.; Mintova, S.; Vicente, A.; Vimont, A.; Bedard, R.; Abdo, S.; Bricker, J. Zeolites in a good shape: Catalyst forming by extrusion modifies their performances. Microporous Mesoporous Mater. 2020, 299, 110114. [Google Scholar] [CrossRef]
- Charkhi, A.; Kazemeini, M.; Ahmadi, S.J.; Ammari Allahyari, S. Effect of bentonite binder on adsorption and cation exchange properties of granulated nano nay zeolite. Adv. Mater. Res. 2011, 335–336, 423–428. [Google Scholar] [CrossRef]
- Jasra, R.V.; Tyagi, B.; Badheka, Y.M.; Choudary, V.N.; Bhat, T.S.G. Effect of clay binder on sorption and catalytic properties of zeolite pellets. Ind. Eng. Chem. Res. 2003, 42, 3263–3272. [Google Scholar] [CrossRef]
- Sun, H.; Shen, B.; Liu, J. N-Paraffins adsorption with 5A zeolites: The effect of binder on adsorption equilibria. Sep. Purif. Technol. 2008, 64, 135–139. [Google Scholar] [CrossRef]
- Chen, N.-Y.; Liu, M.-C.; Yang, S.-C.; Sheu, H.-S.; Chang, J.-R. Impacts of binder-zeolite interactions on the structure and surface properties of NaY–SiO2 extrudates. Ind. Eng. Chem. Res. 2015, 54, 8456–8468. [Google Scholar] [CrossRef]
- Cao, D.V.; Sircar, S. Heats of adsorption of pure SF6 and CO2 on silicalite pellets with alumina binder. Ind. Eng. Chem. Res. 2001, 40, 156–162. [Google Scholar] [CrossRef]
- Shams, K.; Mirmohammadi, S.J. Preparation of 5A zeolite monolith granular extrudates using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Microporous Mesoporous Mater. 2007, 106, 268–277. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle, T., Ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Wang, Y.; LeVan, M.D. Adsorption equilibrium of carbon dioxide and water vapor on zeolites 5A and 13X and silica gel: Pure components. J. Chem. Eng. Data 2009, 54, 2839–2844. [Google Scholar] [CrossRef]
- Giencke, J. Introduction to EVA. Bruker Coop. Billerica 2007, 31. [Google Scholar]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 49–56. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 31 July 2019).

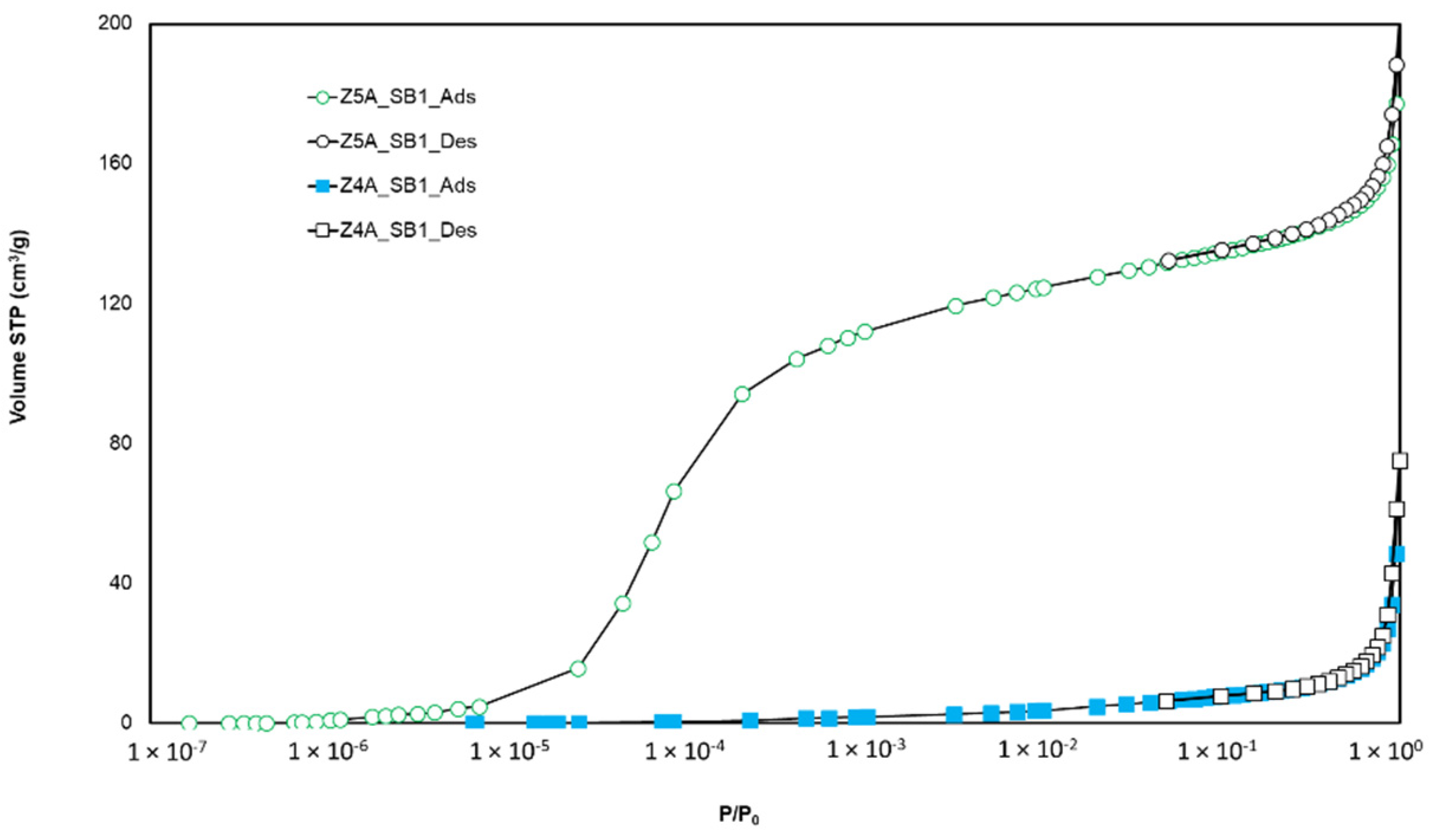
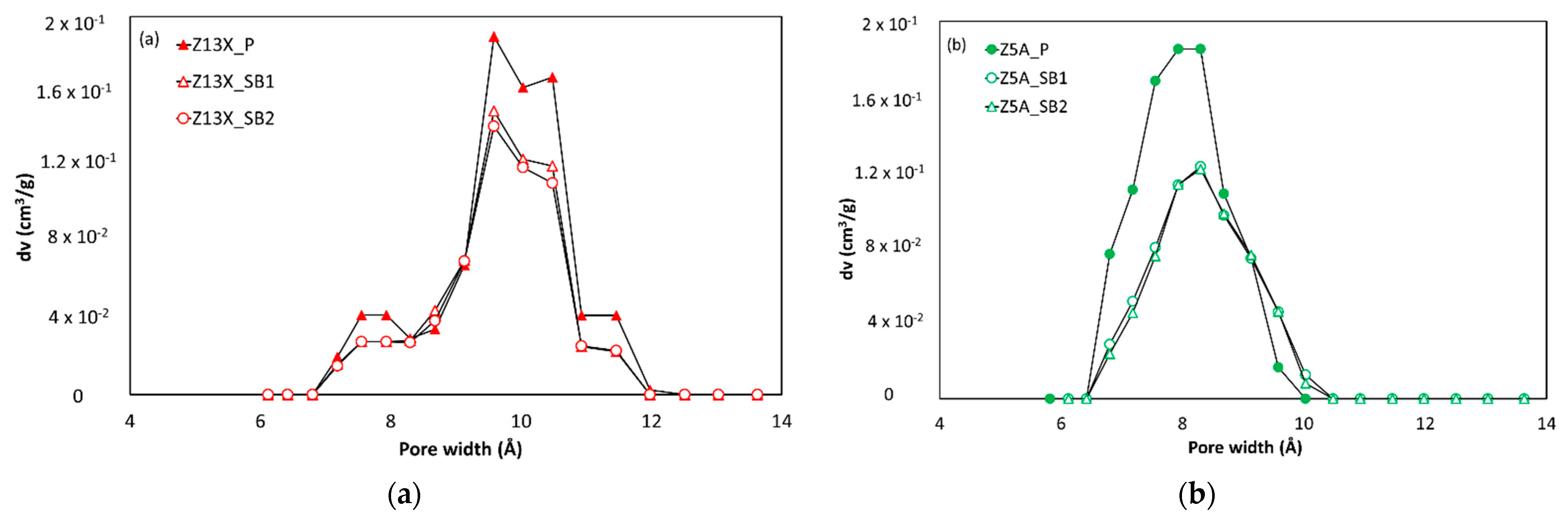

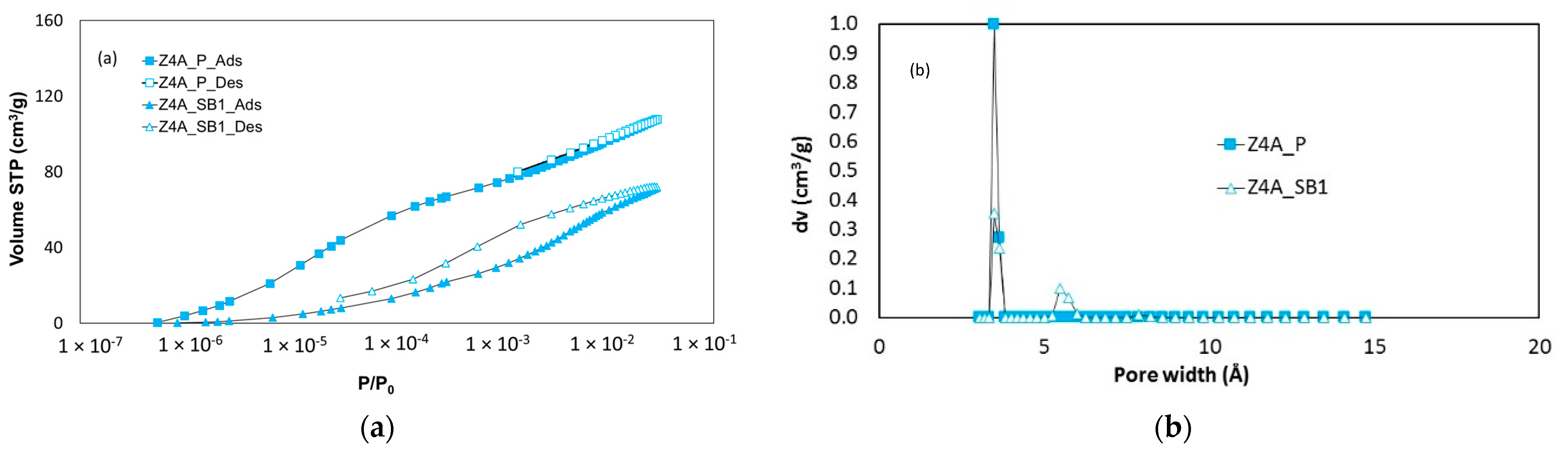
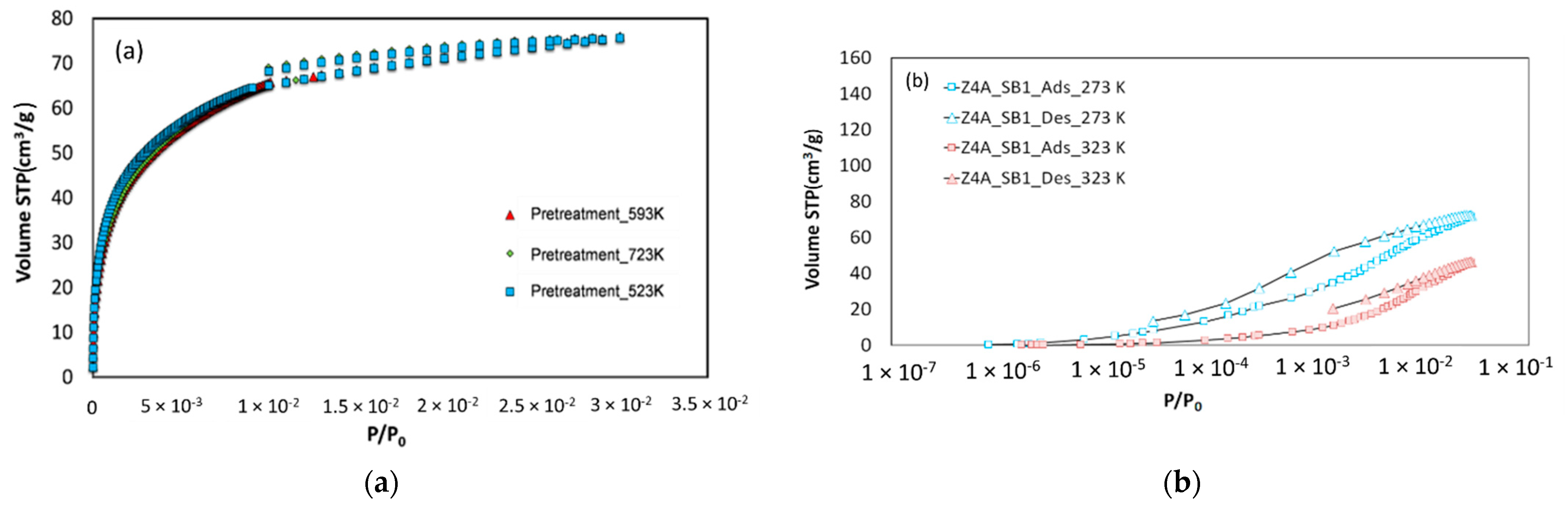

| Zeolite Sample | 13X | 5A | 4A |
|---|---|---|---|
| Powder (P) | Z13X_P | Z5A_P | Z4A_P |
| Spherical beads 1 (SB1) | Z13X_SB1 | Z5A_SB1 | Z4A_SB1 |
| Spherical beads 2 (SB2) | Z13X_SB2 | Z5A_SB2 | ---- |
| Zeolite Sample | 13X | 5A | ||||
|---|---|---|---|---|---|---|
| Z13X_P | Z13X_SB1 | Z13X_SB2 | Z5A_P | Z5A_SB1 | Z5A_SB2 | |
| SBET (m2/g) | 799 | 624 | 607 | 641 | 482 | 461 |
| Vp (NLDFT) (cm3/g) | 0.37 | 0.28 | 0.28 | 0.35 | 0.25 | 0.23 |
| Compound | Al (396.152 nm) | Na (396.152 nm) | Si (396.152 nm) | Ca (396.152 nm) | Molar Si/Al Ratio | Molar Na/AlRatio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| % mass | %RSD | % mass | %RSD | % mass | %RSD | % mass | %RSD | |||
| Z4A_P | 9.38 | 0.54 | 6.99 | 0.81 | 15.8 | 1.03 | 0.29 | 0.98 | 1.6 | 0.9 |
| Z4A_SB1 | 5.56 | 3.14 | 11.4 | 0.60 | 17.2 | 0.58 | - | - | 3 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsikowsky-Sanchez, A.; Franke, C.; Sachse, A.; Ferrage, E.; Petit, S.; Brunet, J.; Plantier, F.; Miqueu, C. Gas Porosimetry by Gas Adsorption as an Efficient Tool for the Assessment of the Shaping Effect in Commercial Zeolites. Nanomaterials 2021, 11, 1205. https://doi.org/10.3390/nano11051205
Orsikowsky-Sanchez A, Franke C, Sachse A, Ferrage E, Petit S, Brunet J, Plantier F, Miqueu C. Gas Porosimetry by Gas Adsorption as an Efficient Tool for the Assessment of the Shaping Effect in Commercial Zeolites. Nanomaterials. 2021; 11(5):1205. https://doi.org/10.3390/nano11051205
Chicago/Turabian StyleOrsikowsky-Sanchez, Alejandro, Christine Franke, Alexander Sachse, Eric Ferrage, Sabine Petit, Julien Brunet, Frédéric Plantier, and Christelle Miqueu. 2021. "Gas Porosimetry by Gas Adsorption as an Efficient Tool for the Assessment of the Shaping Effect in Commercial Zeolites" Nanomaterials 11, no. 5: 1205. https://doi.org/10.3390/nano11051205
APA StyleOrsikowsky-Sanchez, A., Franke, C., Sachse, A., Ferrage, E., Petit, S., Brunet, J., Plantier, F., & Miqueu, C. (2021). Gas Porosimetry by Gas Adsorption as an Efficient Tool for the Assessment of the Shaping Effect in Commercial Zeolites. Nanomaterials, 11(5), 1205. https://doi.org/10.3390/nano11051205








