Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of B,N-CNTs
2.3. Characterization and Measurement
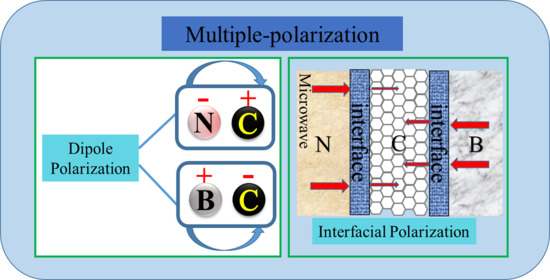
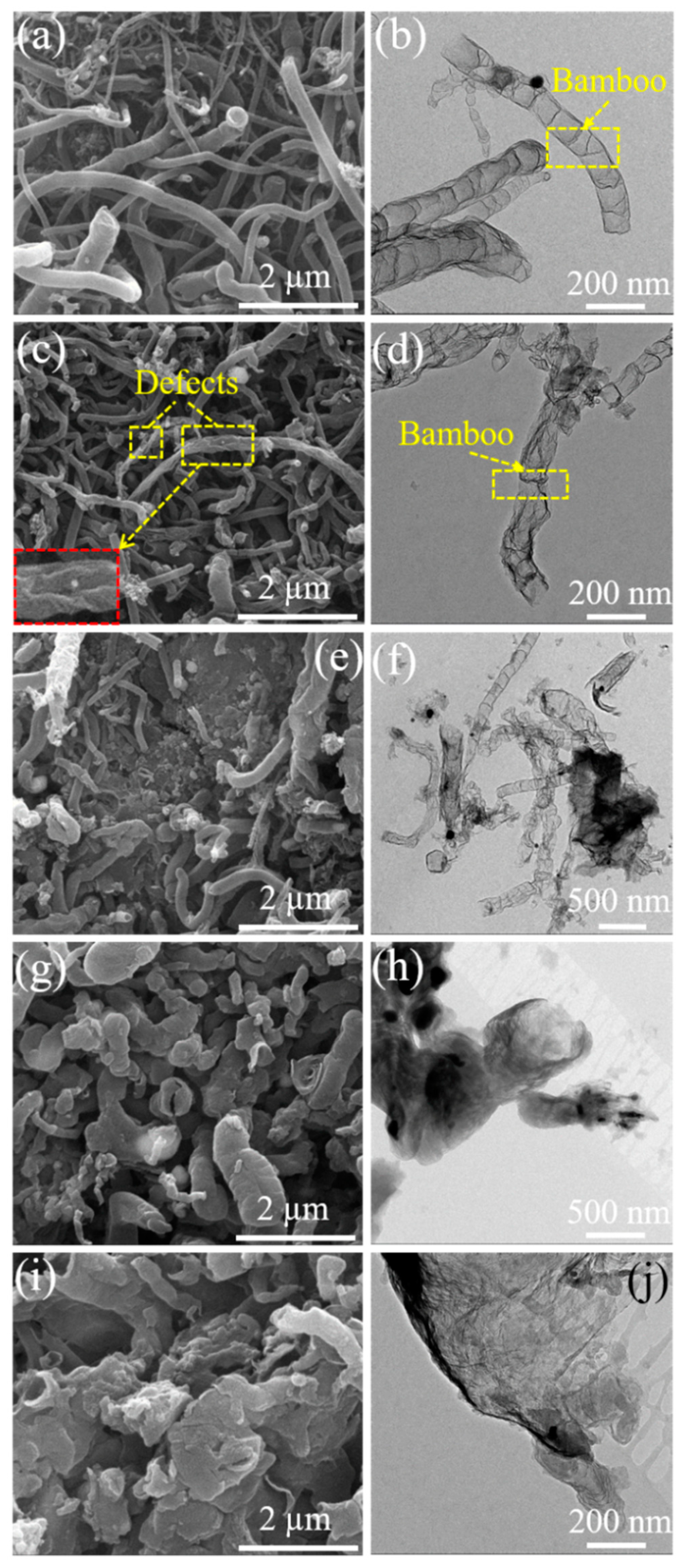
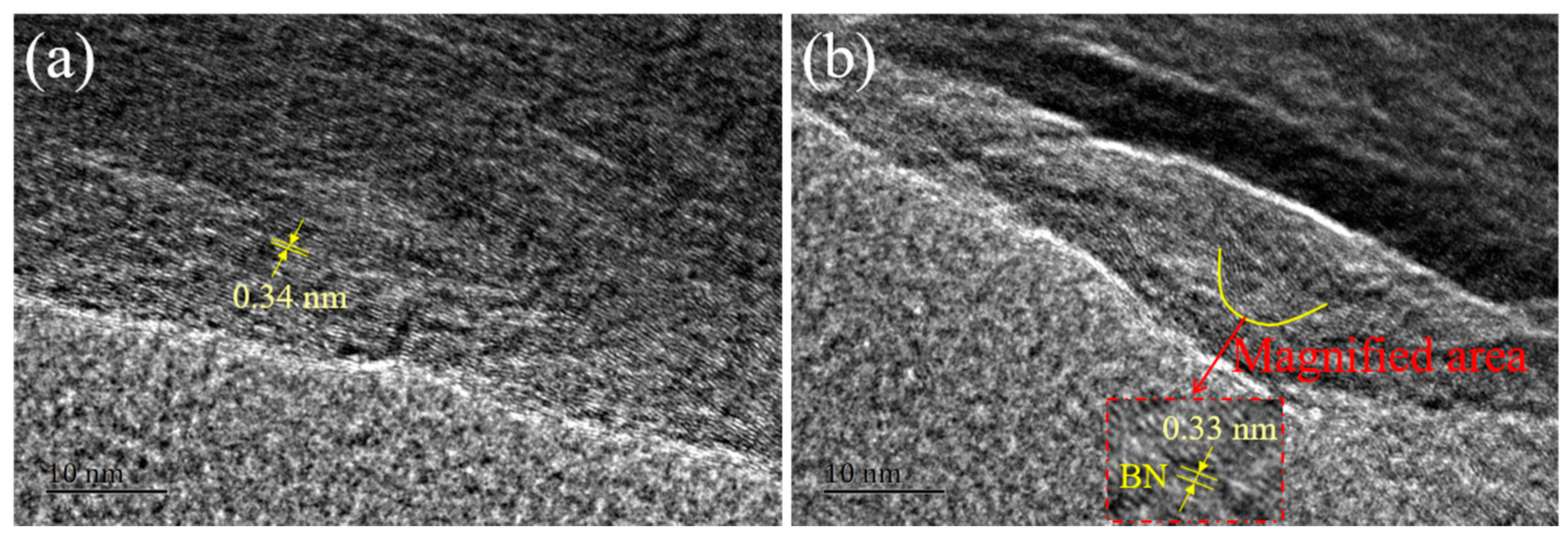
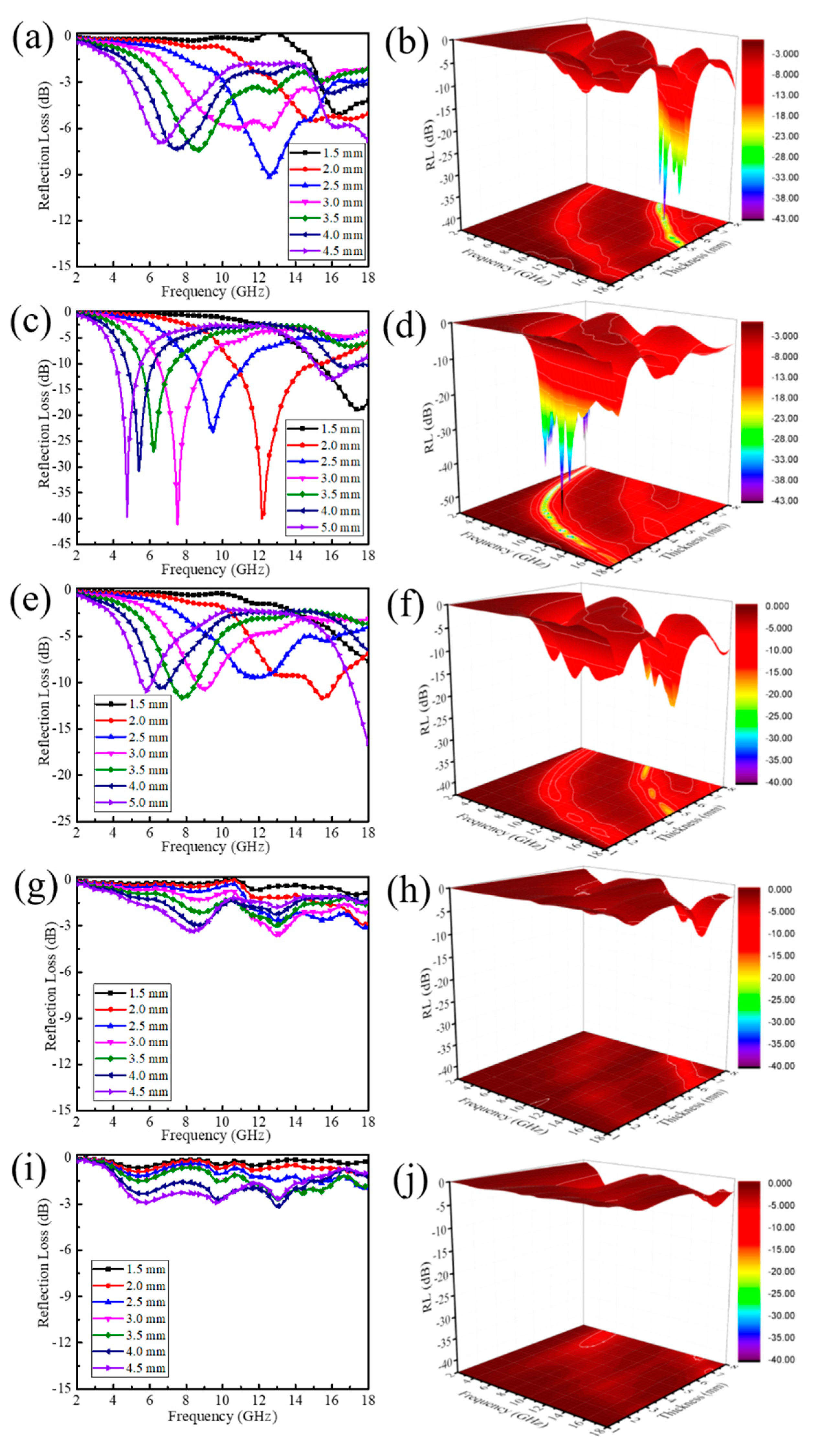
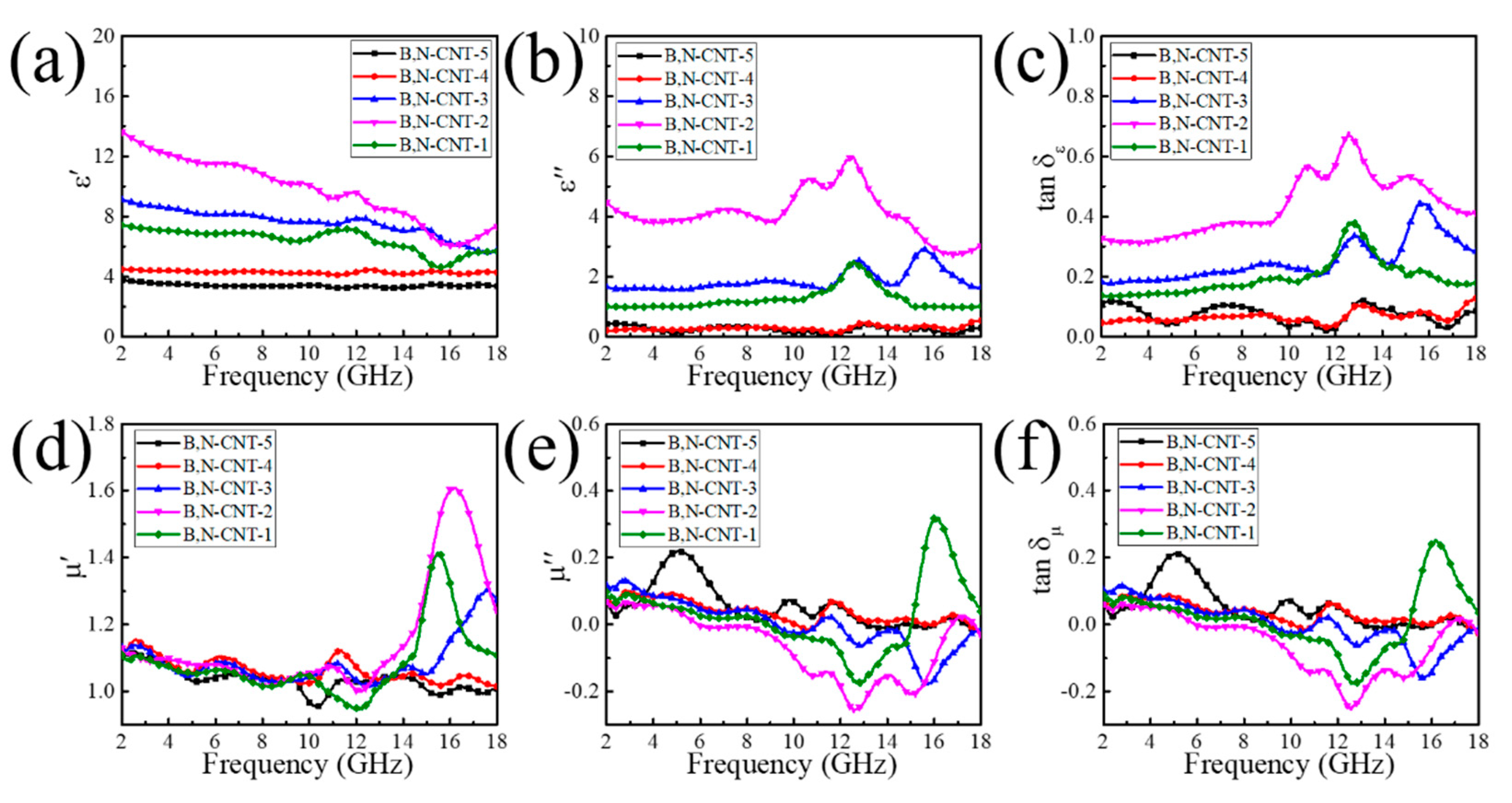
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, W.; Wang, J.; Fan, L.; Li, Y.; Wang, C.; Cao, M. Interfacial engineering of carbon nanofiber-graphene-carbon nanofiber heterojunctions in flexible lightweight electromagnetic shielding networks. ACS Appl. Mater. Inter. 2014, 6, 10516–10523. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Pu, F.; Li, Z.; Li, X.; Hu, X.; Bai, J. Interfacial interactions and synergistic effect of CoNi nanocrystals and nitrogen-doped graphene in a composite microwave absorber. Carbon 2016, 104, 214–225. [Google Scholar] [CrossRef]
- Adebayo, L.; Soleimani, H.; Yahya, N.; Abbas, Z.; Ridwan, A.; Wahaab, F. Investigation of the broadband microwave absorption of citric acid coated Fe3O4/PVDF composite using finite element method. Appl. Sci. 2019, 9, 3877. [Google Scholar] [CrossRef]
- Chaudhary, A.; Kumar, S.; Kumar, R.; Teotia, S.; Singh, B.; Singh, A.; Dhawan, S.; Dhakate, S. Lightweight and easily foldable mcmb-mwcnts composite paper with exceptional electromagnetic interference shielding. ACS Appl. Mater. Inter. 2016, 8, 10600–10608. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, F.; Xiang, Z.; Zeng, Q.; Che, R.; Lu, W. Magnetic vortex core-shell Fe3O4@C nanorings with enhanced microwave absorption performance. Carbon 2020, 157, 130–139. [Google Scholar] [CrossRef]
- Wu, F.; Sun, M.; Chen, C.; Zhou, T.; Xia, Y.; Xie, A.; Shang, Y. Controllable Coating of polypyrrole on silicon carbide nanowires as a core-shell nanostructure: A facile method to enhance attenuation characteristics against electromagnetic radiation. ACS Sustain. Chem. Eng. 2019, 7, 2100–2106. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, L.; Zhang, T.; Shi, B.; Huang, L.; Zhong, B.; Zhang, X.; Wang, H.; Zhang, J.; Wen, G. Fe3O4@LAS/RGO composites with a multiple transmission-absorption mechanism and enhanced electromagnetic wave absorption performance. Chem. Eng. J. 2018, 352, 510–518. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.; Ji, G.; Xu, Z. Biomass-derived porous carbon-based nanostructures for microwave absorption. Nano Micro. Lett. 2019, 11, 24. [Google Scholar] [CrossRef]
- Jiao, Y.; Cheng, S.; Wu, F.; Pan, X.; Xie, A.; Zhu, X.; Dong, W. MOF-guest complex derived Cu/C nanocomposites with multiple heterogeneous interfaces for excellent electromagnetic waves absorption. Compos. Part B Eng. 2021, 211, 108643. [Google Scholar] [CrossRef]
- Wu, L.; Wu, F.; Sun, Q.; Shi, J.; Xie, A.; Zhu, X.; Dong, W. TTF-TCNQ complex: An organic charge-transfer system with extraordinary electromagnetic response behaviors. J. Mater. Chem. C 2021, 9, 3316–3323. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, L.; Zhu, H.; Guan, Y.; Zhang, Q. Lightweight and efficient microwave absorbing materials based on walnut shell-derived nanoporous carbon. Nanoscale 2017, 9, 7408–7418. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Yan, J.; Huang, Y.; Xia, L.; Guang, Z. Synthesis of lightweight N-doped graphene foams with open reticular structure for high-efficiency electromagnetic wave absorption. Chem. Eng. J. 2019, 368, 285–298. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Fu, Y.; Wu, X.; Wang, Q.; Zhang, W.; Luo, C. Enhanced microwave absorption performances of polyaniline/graphene aerogel by covalent bonding. Compos. Part B Eng. 2019, 169, 221–228. [Google Scholar] [CrossRef]
- Xu, Z.; Du, Y.; Liu, D.; Wang, Y.; Ma, W.; Wang, Y.; Xu, P.; Han, X. Pea-like Fe/Fe3C nanoparticles embedded in nitrogen-doped carbon nanotubes with tunable dielectric/magnetic loss and efficient electromagnetic absorption. ACS Appl. Mater. Inter. 2019, 11, 4268–4277. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, J.; Xie, A.; Wu, F.; Zhang, K.; Dong, W.; Zhu, X. Confined polymerization strategy to construct polypyrrole/zeolitic imidazolate frameworks (PPy/ZIFs) nanocomposites for tunable electrical conductivity and excellent electromagnetic absorption. Compos. Sci. Technol. 2019, 174, 232–240. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Wang, Z.; Li, Y.; He, X.; Yuan, Y. Microwave absorption enhancement of porous C@CoFe2O4 nanocomposites derived from eggshell membrane. Carbon 2019, 143, 507–516. [Google Scholar] [CrossRef]
- Wang, J.; Jia, X.; Wang, T.; Geng, S.; Zhou, C.; Yang, F.; Tian, X.; Zhang, L.; Yang, H.; Li, Y. Synthesis and microwave absorption property of two-dimensional porous nickel oxide nanoflakes/carbon nanotubes nanocomposites with a threaded structure. J. Alloy Compd. 2016, 689, 366–373. [Google Scholar] [CrossRef]
- Xu, W.; Pan, Y.; Wei, W.; Wang, G. Nanocomposites of oriented nickel chains with tunable magnetic properties for high-performance broadband microwave absorption. ACS Appl. Nano Mater. 2018, 1, 1116–1123. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Jiang, L.; Chen, Y.; Nan, C. High permittivity Li and Al doped NiO ceramics. Appl. Phys. Lett. 2004, 85, 5664. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Kong, L.; Li, M.; Duan, W.; Zhang, L.; Cheng, L. Graphene-wrapped ZnO hollow spheres with enhanced electromagnetic wave absorption properties. J. Mater. Chem. A 2014, 2, 16403–16409. [Google Scholar] [CrossRef]
- Ding, Y.; Kopold, P.; Hahn, K.; Aken, P.; Maier, J.; Yu, Y. Facile solid-state growth of 3D well-interconnected nitrogen-rich carbon nanotube-graphene hybrid architectures for lithium-sulfur batteries. Adv. Funct. Mater. 2016, 26, 1112–1119. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; He, W.; Huang, W.; Luo, J. Carbon nanocages with N-doped carbon inner shell and Co/N-doped carbon outer shell as electromagnetic wave absorption materials. Chem. Eng. J. 2020, 381, 122653. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Huang, W.; Ren, J.; Yu, D.; He, W. Hybrid zeolite imidazolate framework derived N-implanted carbon polyhedrons with tunable heterogeneous interfaces for strong wideband microwave attenuation. Carbon 2020, 159, 83–93. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, C.; Gao, S.; Guan, C.; Huang, Y.; He, W. N-doped porous carbon nanoplates embedded with CoS2 vertically anchored on carbon cloths for flexible and ultrahigh microwave absorption. Carbon 2020, 163, 348–359. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Chen, C.; Zhou, F.; Meng, Z.; Huang, Y.; Wang, Y. Organic polymer aerogel derived N-doped carbon aerogel with vacancies for ultrahigh microwave absorption. Carbon 2020, 169, 276–287. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Zhou, F.; Huang, Y.; Huang, W.; Chang, N. Core-shell Ni@C encapsulated by N-doped carbon derived from nickel-organic polymer coordination composites with enhanced microwave absorption. Carbon 2020, 170, 503–516. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Zhou, F.; Huang, Y.; Luo, J. Metal-organic polymer coordination materials derived Co/N-doped porous carbon composites for frequency-selective microwave absorption. Compos. Part B Eng. 2020, 202, 108406. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Wu, Y.; Zhang, J.; Zhang, G. Nitrogen-doped Co-C/MWCNTs nanocomposites derived from bimetallic metal-organic frameworks for electromagnetic wave absorption in the X-band. Chem. Eng. J. 2019, 362, 513–524. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Y.; Chen, J.; Li, B.; Guo, L.; Ouyang, J.; Jia, D.; Zhou, Y. Metal organic framework-derived CoZn alloy/N-doped porous carbon nanocomposites: Tunable surface area and electromagnetic wave absorption properties. J. Mater. Chem. C 2018, 6, 10–18. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Shi, G.; Zhou, W.; Gong, Y.; Lei, S.; Yang, X.; Zhang, J.; Yu, J.; Hackenberg, K.; et al. In-plane heterostructures of graphene and hexagonal boron nitride with controlled domain sizes. Nat. Nanotechnol. 2013, 8, 119–124. [Google Scholar] [CrossRef]
- Nozaki, H.; Itoh, S. Structural stability of BC2N. J. Phys. Chem. Solids 1996, 57, 41–49. [Google Scholar] [CrossRef]
- Kang, Y.; Chu, Z.; Zhang, D.; Li, G.; Jiang, Z.; Cheng, H.; Li, X. Incorporate boron and nitrogen into graphene to make BCN hybrid nanosheets with enhanced microwave absorbing properties. Carbon 2013, 61, 200–208. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; Wang, Y.; Lou, J. Core−shell CoNi@graphitic carbon decorated on B, N-codoped hollow carbon polyhedrons toward lightweight and high-efficiency microwave attenuation. ACS Appl. Mater. Inter. 2019, 11, 25624–25635. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, S.; Yan, Q.; Chen, Z.; Ding, Y.; Li, C.; Liang, H.; Yu, S. Transition metal-assisted carbonization of small organic molecules toward functional carbon materials. Sci. Adv. 2018, 4, eaat0788. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; Chen, X.; Wu, L.; Yu, X. Graphene oxide based recyclable dehydrogenation of ammonia borane within a hybrid nanostructure. J. Am. Chem. Soc. 2012, 134, 5464–5467. [Google Scholar] [CrossRef]
- Fan, M.; Wu, J.; Yuan, J.; Deng, L.; Zhong, N.; He, L.; Cui, J.; Wang, Z.; Behera, S.; Zhang, C.; et al. Doping nanoscale graphene domains improves magnetism in hexagonal boron nitride. Adv. Mater. 2019, 31, 1805778. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, T.; Satti, A.; May, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.; Coleman, J. Oxygen radical functionalization of boron nitride nanosheets. J. Am. Chem. Soc. 2012, 134, 18758. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cheng, L.; Zhang, Y.; Yang, Y.; Deng, C.; Yang, Z.; Chen, Q.; Du, X.; Zhao, C.; Zheng, L. Enhanced flexibility and microwave absorption properties of HfC/SiC nanofiber mats. ACS Appl. Mater. Inter. 2018, 10, 29876–29883. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, J.; Ding, Z.; Wu, J.; Wang, P.; Jin, S.; Bi, H. Magnetic and microwave absorption properties of Ni microcrystals with hierarchical branch-like and flowers-like shapes. Mater. Chem. Phys. 2013, 142, 119–123. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.S.; Hou, Z.L.; Song, W.L.; Zhang, L.; Lu, M.M.; Jin, H.B.; Fang, X.Y.; Wang, W.Z.; Yuan, J. Temperature dependent microwave attenuation behavior for carbon-nanotube/silica composites. Carbon 2013, 65, 124–139. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhang, X.; Liu, R.; Shen, S.; Wu, F.; Xie, A. Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion. Nanomaterials 2021, 11, 1164. https://doi.org/10.3390/nano11051164
Sun Q, Zhang X, Liu R, Shen S, Wu F, Xie A. Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion. Nanomaterials. 2021; 11(5):1164. https://doi.org/10.3390/nano11051164
Chicago/Turabian StyleSun, Qingya, Xinfang Zhang, Ruonan Liu, Shaofeng Shen, Fan Wu, and Aming Xie. 2021. "Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion" Nanomaterials 11, no. 5: 1164. https://doi.org/10.3390/nano11051164
APA StyleSun, Q., Zhang, X., Liu, R., Shen, S., Wu, F., & Xie, A. (2021). Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion. Nanomaterials, 11(5), 1164. https://doi.org/10.3390/nano11051164