In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroxyapatite Nanoparticles
2.2. Physicochemical Characterization of the nHA Nanoparticles
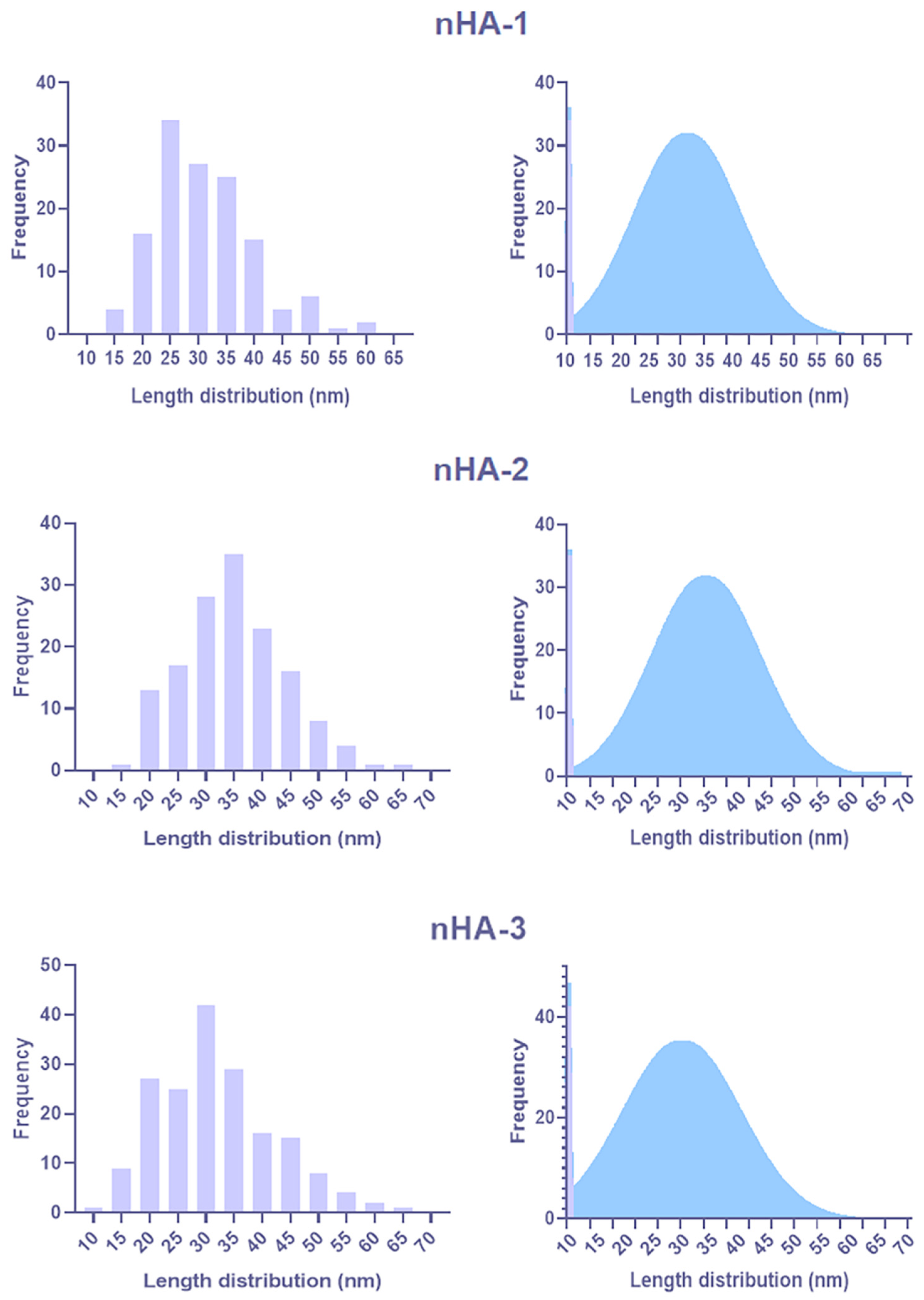
2.2.1. Transmission Electron Microscopy
2.2.2. X-ray Diffraction (XRD)
2.2.3. Fourier Transformed Infrared Spectroscopy (FTIR)
2.2.4. Potassium and Chloride Quantification
2.2.5. Dynamic Light Scattering (DLS)
2.3. Cell Culture Maintenance
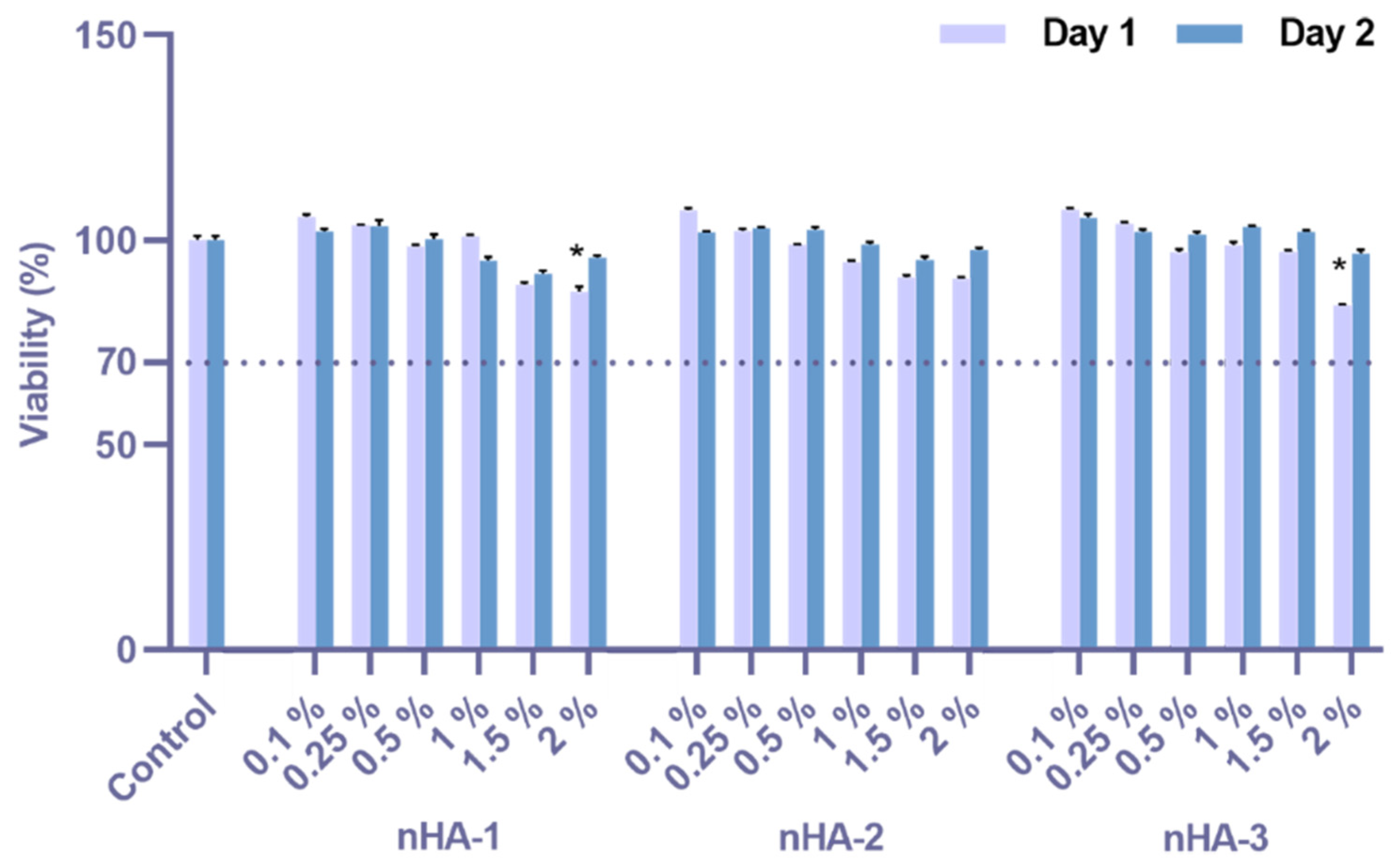
2.4. Cell Cytotoxicity Assessment by Cell Viability Evaluation
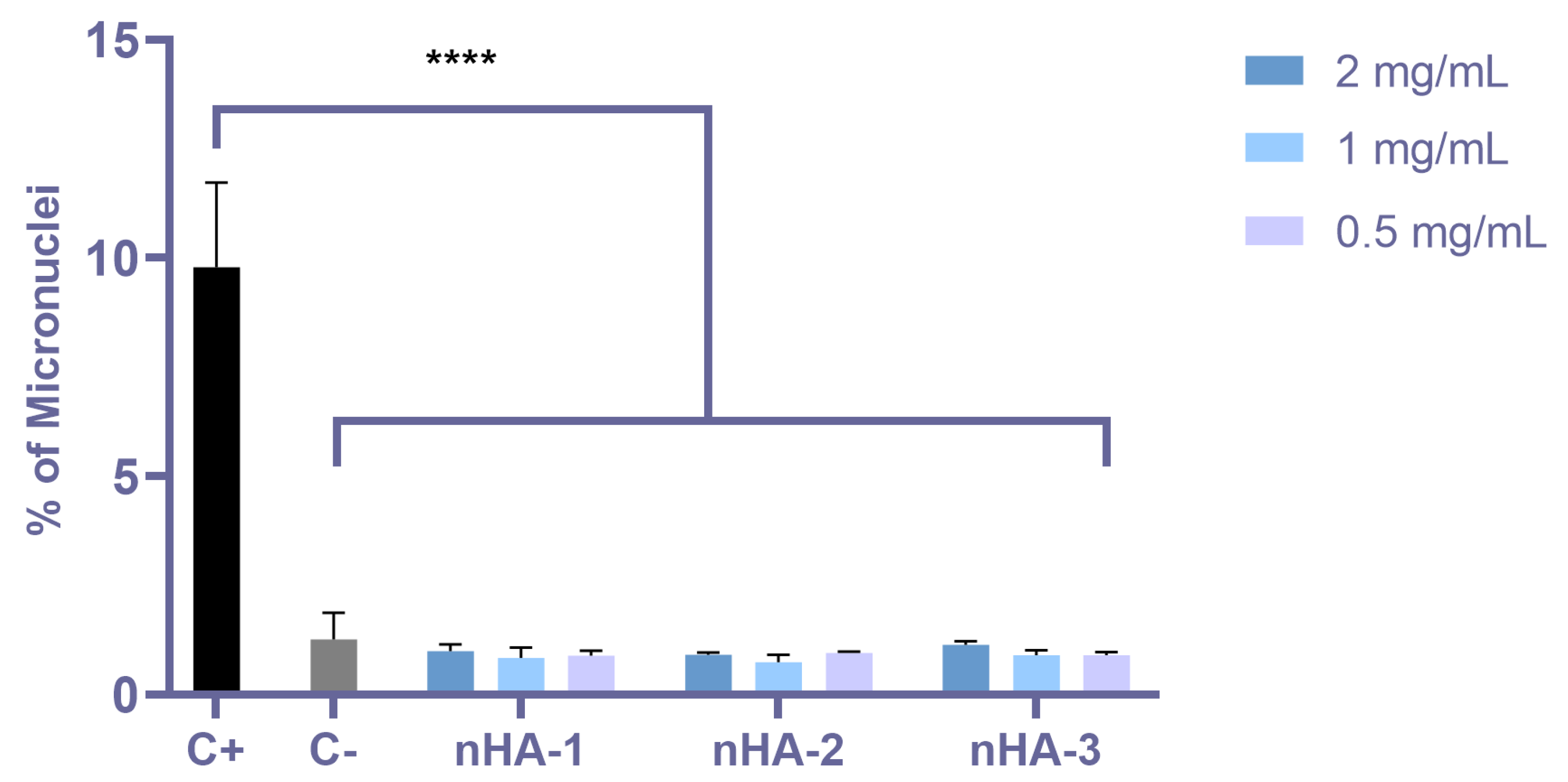
2.5. Genotoxicity Assessment According to OECD 487 (Micronucleus Assay)
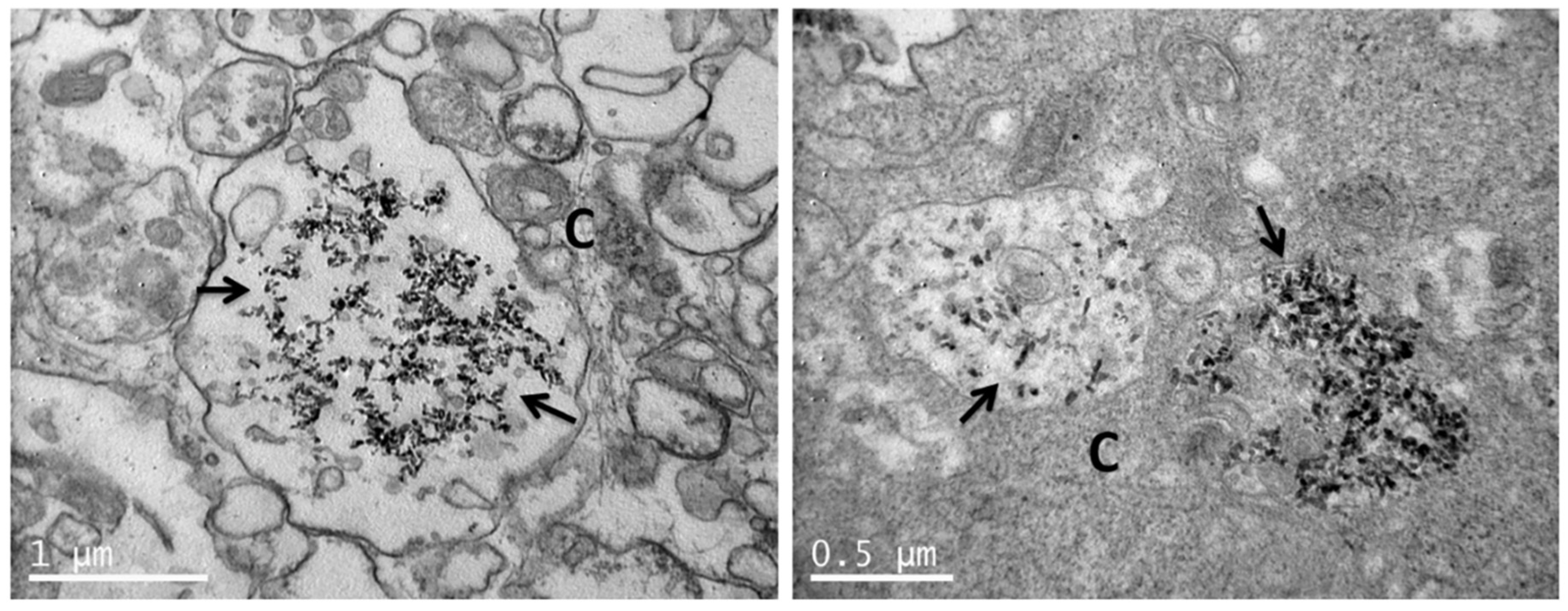
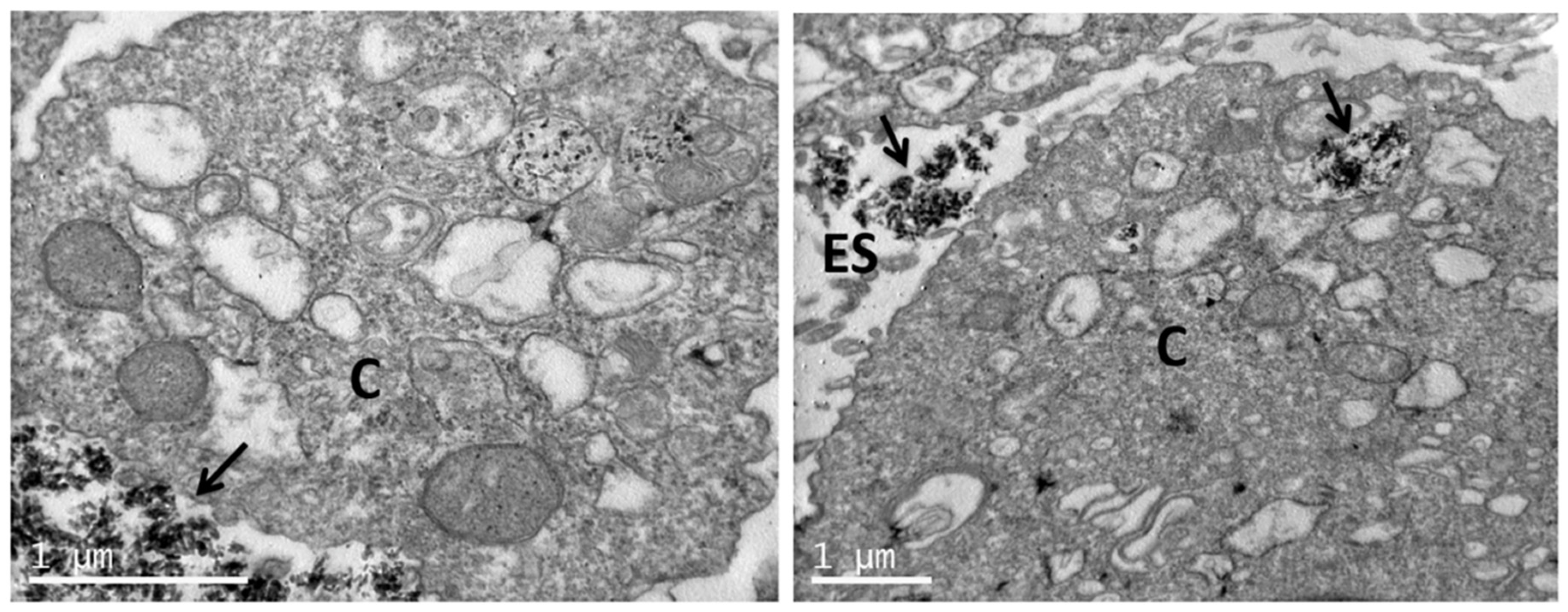
2.6. Visualization of Nanoparticle Uptake with TEM
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.1.1. TEM Analysis
3.1.2. XRD
3.1.3. FTIR
3.1.4. AAS and IC
3.1.5. DLS
3.2. Cytotoxicity Assessment
3.3. Genotoxicity Assessment by Means of the Micronuclei Formation
3.4. Uptake of nHA Samples by Cells Investigated by TEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Vagropoulou, G.; Trentsiou, M.; Georgopoulou, A.; Papachristou, E.; Prymak, O.; Kritis, A.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A.; Koidis, P. Hybrid Chitosan/Gelatin/Nanohydroxyapatite Scaffolds Promote Odontogenic Differentiation of Dental Pulp Stem Cells and In Vitro Biomineralization. Dent. Mater. 2021, 37, E23–E36. [Google Scholar] [CrossRef]
- Yousefi, A.M.; Oudadesse, H.; Akbarzadeh, R.; Wers, E.; Lucas-Girot, A. Physical and Biological Characteristics of Nanohydroxyapatite and Bioactive Glasses Used for Bone Tissue Engineering. Nanotechnol. Rev. 2014, 3, 527–552. [Google Scholar] [CrossRef]
- Ohta, K.; Kawamata, H.; Ishizaki, T.; Hayman, R. Occlusion of Dentinal Tubules by Nano-Hydroxyapatite. J. Dent. Res. 2007, 86, 21–24. [Google Scholar]
- Kikuchi, M. Hydroxyapatite/Collagen Bone-Like Nanocomposite. Biol. Pharm. Bull. 2013, 36, 1666–1669. [Google Scholar] [CrossRef]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of Hydrothermal Synthesis Parameters on the Properties of Hydroxyapatite Nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef]
- Barros, J.; Ferraz, M.P.; Azeredo, J.; Fernandes, M.H.; Gomes, P.S.; Monteiro, F.J. Alginate-Nanohydroxyapatite Hydrogel system: Optimizing the Formulation for Enhanced Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 109985. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Fernandes, M.H.; Beppu, M.M.; Monteiro, F.J.; Ferraz, M.P. Silk Fibroin/Nanohydroxyapatite Hydrogels for Promoted Bioactivity and Osteoblastic Proliferation and Differentiation of Human Bone Marrow Stromal Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 336–345. [Google Scholar] [CrossRef]
- Lichtinger, T.K.; Müller, R.T.; Schürmann, N.; Oldenburg, M.; Rumpf, H.M.; Wiemann, M.; Chatzinikolaidou, M.; Jennissen, H.P. Osseointegration of Titanium Implants by Addition of Recombinant Bone Morphogenetic Protein 2 (rhBMP-2). Mater. Werkst. Mater. Sci. Eng. Technol. 2001, 32, 937–941. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing Dentine Hypersensitivity with Nano-Hydroxyapatite Toothpaste: A Double-Blind Randomized Controlled Trial. Clin. Oral Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in Preventive Dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and Dentine Remineralization by Nano-Hydroxyapatite Toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Monteiro, F.J.; Laranjeira, M.S. Duality of Iron (III) Doped Nano Hydroxyapatite in Triple Negative Breast Cancer Monitoring and as a Drug-Free Therapeutic Agent. Ceram. Int. 2020, 46, 16590–16597. [Google Scholar] [CrossRef]
- Jafari, S.; Maleki-Dizaji, N.; Barar, J.; Barzegar-Jalali, M.; Rameshrad, M.; Adibkia, K. Methylprednisolone Acetate-Loaded Hydroxyapatite Nanoparticles as a Potential Drug Delivery System for Treatment of Rheumatoid Arthritis: In Vitro and in Vivo Evaluations. Eur. J. Pharm. Sci. 2016, 91, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Geuli, O.; Metoki, N.; Zada, T.; Reches, M.; Eliaz, N.; Mandler, D. Synthesis, Coating, and Drug-Release of Hydroxyapatite Nanoparticles Loaded with Antibiotics. J. Mater. Chem. B 2017, 5, 7819–7830. [Google Scholar] [CrossRef] [PubMed]
- SCCS. Opinion on Hydroxyapatite (Nano) 2016. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_191.pdf (accessed on 8 April 2021).
- Ramis, J.M.; Coelho, C.C.; Córdoba, A.; Quadros, P.A.; Monjo, M. Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient According to the EU Cosmetics Regulation. Cosmetics 2018, 5, 53. [Google Scholar] [CrossRef]
- Silva, V.M.T.M.; Quadros, P.A.; Laranjeira, P.E.M.S.C.; Dias, M.M.; Lopes, J.C.B. A Novel Continuous Industrial Process for Producing Hydroxyapatite Nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 542–547. [Google Scholar] [CrossRef]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The Effect of Silver Nanoparticles Size, Produced Using Plant Extract from Arbutus Unedo, on Their Antibacterial Efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.S.; Langari, R. Chapter 3—Measurement Uncertainty. In Measurement and Instrumentation; Morris, A.S., Langari, R., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2012; pp. 39–102. [Google Scholar]
- Loppinet, B.; Sigel, R.; Larsen, A.; Fytas, G.; Vlassopoulos, D.; Liu, G. Structure and Dynamics in Dense Suspensions of Micellar Nanocolloids. Langmuir 2000, 16, 6480–6484. [Google Scholar] [CrossRef]
- Sentjabrskaja, T.; Jacob, A.R.; Egelhaaf, S.U.; Petekidis, G.; Voigtmann, T.; Laurati, M. Binary Colloidal Glasses: Linear Viscoelasticity and Its Link to the Microscopic Structure and Dynamics. Soft Matter 2019, 15, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, C.; Buyakov, A.; Buyakova, S.; Kulkov, S.; Chatzinikolaidou, M. Porous Alumina, Zirconia and Alumina/Zirconia for Bone Repair: Fabrication, Mechanical and In Vitro Biological Response. Biomed. Mater. 2015, 10, 025012. [Google Scholar] [CrossRef]
- Balabanidou, V.; Kampouraki, A.; MacLean, M.; Blomquist, G.J.; Tittiger, C.; Juárez, M.P.; Mijailovsky, S.J.; Chalepakis, G.; Anthousi, A.; Lynd, A. Cytochrome P450 Associated with Insecticide Resistance Catalyzes Cuticular Hydrocarbon Production in Anopheles gambiae. In Proceedings of the National Academy of Sciences of the United States of America, Washongton, DC, USA, 16 August 2016; Volume 113, pp. 9268–9273. [Google Scholar]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-Hydroxyapatite in Oral Care Cosmetics: Characterization and Cytotoxicity Assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef] [PubMed]
- Padrão, T.; Coelho, C.C.; Costa, P.; Alegrete, N.; Monteiro, F.J.; Sousa, S.R. Combining Local Antibiotic Delivery with Heparinized Nanohydroxyapatite/Collagen Bone Substitute: A Novel Strategy for Osteomyelitis Treatment. Mater. Sci. Eng. C 2021, 119, 111329. [Google Scholar] [CrossRef] [PubMed]
- Savchyn, P.; Karbovnyk, I.; Vistovskyy, V.; Voloshinovskii, A.; Pankratov, V.; Cestelli Guidi, M.; Mirri, C.; Myahkota, O.; Riabtseva, A.; Mitina, N.; et al. Vibrational Properties of LaPO4 Nanoparticles in Mid-and Far-Infrared Domain. J. Appl. Phys. 2012, 112, 124309. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Fernandes, M.H.; Monteiro, F.J. Innovative Macroporous Granules of Nanostructured-Hydroxyapatite agglomerates: Bioactivity and Osteoblast-Like Cell Behaviour. J. Biomed. Mater. Res. Part A 2010, 95A, 891–900. [Google Scholar] [CrossRef]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical Characterization of Silicon-Substituted Hydroxyapatite. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 44, 422–428. [Google Scholar] [CrossRef]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.G.; Porter, A.E.; Skepper, J.N. Hydroxyapatite Nano and Microparticles: Correlation of Particle Properties with Cytotoxicity and Biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef]
- Liu, Z.S.; Tang, S.L.; Ai, Z.L. Effects of Hydroxyapatite Nanoparticles on Proliferation and Apoptosis of Human Hepatoma BEL-7402 Cells. World J. Gastroenterol. 2003, 9, 1968. [Google Scholar] [CrossRef]
- Seyedmajidi, S.; Seyedmajidi, M.; Zabihi, E.; Hajian-Tilaki, K. A Comparative Study on Cytotoxicity and Genotoxicity of the Hydroxyapatite-Bioactive Glass and Fluorapatite-Bioactive Glass Nanocomposite Foams as Tissue Scaffold for Bone Repair. J. Biomed. Mater. Res. Part A 2018, 106, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; da Silva, V.H.P.; Ruiz, P.L.M.; Ussui, V.; Lazar, D.R.R.; Renno, A.C.M.; Ribeiro, D.A. Physico-Chemical Characterization and Biocompatibility of Hydroxyapatite Derived from Fish Waste. J. Mech. Behav. Biomed. Mater. 2018, 80, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Cilli, E.M.; Ribeiro, S.J.L.; Scarel-Caminaga, R.M.; de Oliveira Capote, T.S. Silk Fibroin/Hydroxyapatite Composite Membranes: Production, Characterization and Toxicity Evaluation. Toxicol. In Vitro 2020, 62, 104670. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In Vitro Uptake of Hydroxyapatite Nanoparticles and Their Effect on Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef]
- Chen, L.; Mccrate, J.M.; Lee, J.C.M.; Li, H. The Role of Surface Charge on the Uptake and Biocompatibility of Hydroxyapatite Nanoparticles with Osteoblast Cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, C.; Kozlova, D.; Sokolova, V.; Epple, M.; Chatzinikolaidou, M. Calcium Phosphate Nanoparticles Carrying BMP-7 Plasmid DNA Induce an Osteogenic Response in MC3T3-E1 Pre-Osteoblasts. J. Biomed. Mater. Res. Part A 2015, 103, 3834–3842. [Google Scholar] [CrossRef]
- Ochieng, J.; Nangami, G.; Sakwe, A.; Rana, T.; Ingram, S.; Goodwin, J.S.; Moye, C.; Lammers, P.; Adunyah, S.E. Extracellular Histones are the Ligands for the Uptake of Exosomes and Hydroxyapatite-Nanoparticles by Tumor Cells via Syndecan-4. FEBS Lett. 2018, 592, 3274–3285. [Google Scholar] [CrossRef] [PubMed]
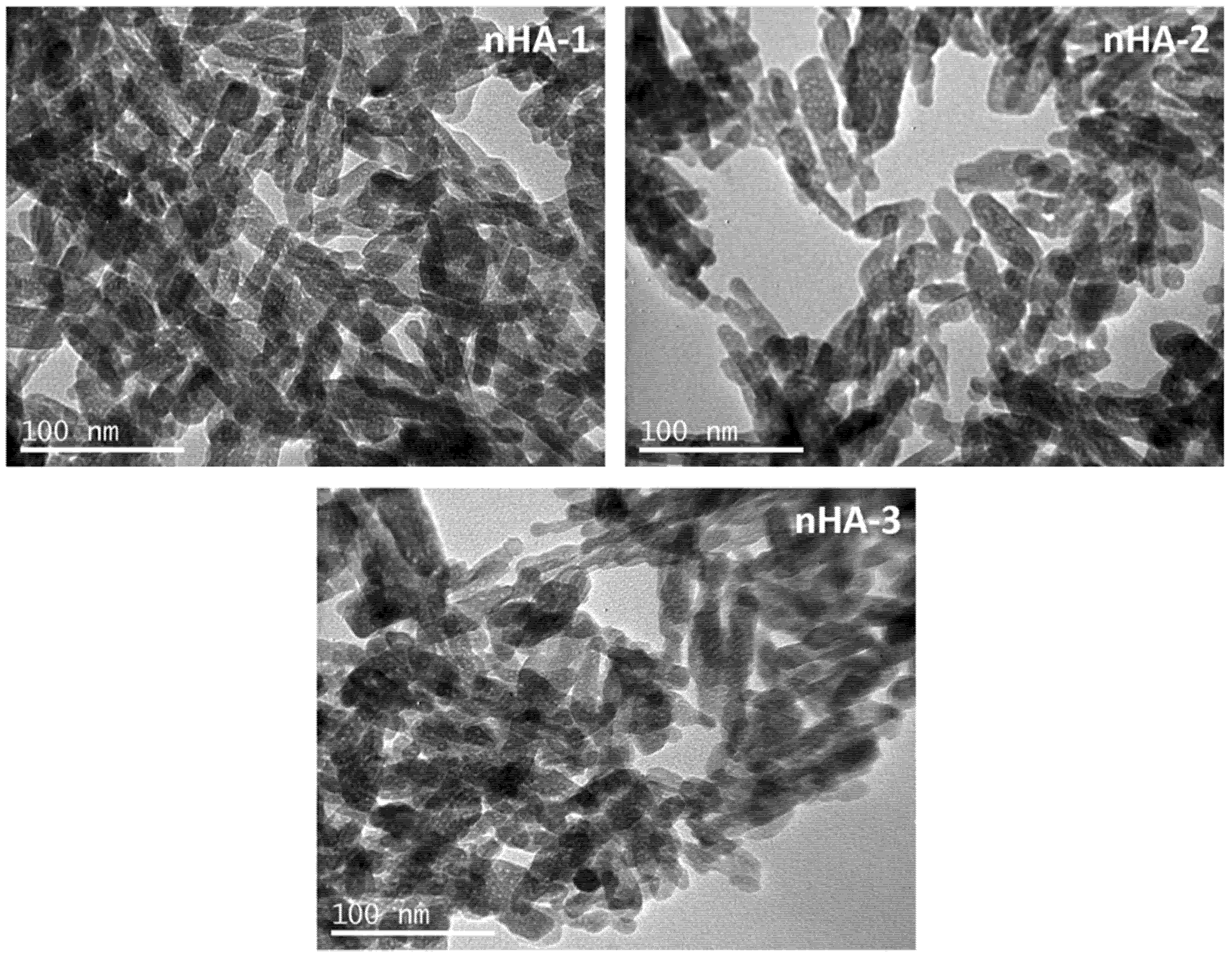





| K+ (%) | Cl− (%) | |
|---|---|---|
| nHA-1 | 2.2 | 2.3 |
| nHA-2 | 2.0 | 2.3 |
| nHA-3 | 2.0 | 2.2 |
| Average | 2.1 | 2.3 |
| SD | 0.1 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavasi, R.-M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials 2021, 11, 1152. https://doi.org/10.3390/nano11051152
Kavasi R-M, Coelho CC, Platania V, Quadros PA, Chatzinikolaidou M. In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials. 2021; 11(5):1152. https://doi.org/10.3390/nano11051152
Chicago/Turabian StyleKavasi, Rafaela-Maria, Catarina C. Coelho, Varvara Platania, Paulo A. Quadros, and Maria Chatzinikolaidou. 2021. "In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite" Nanomaterials 11, no. 5: 1152. https://doi.org/10.3390/nano11051152
APA StyleKavasi, R.-M., Coelho, C. C., Platania, V., Quadros, P. A., & Chatzinikolaidou, M. (2021). In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials, 11(5), 1152. https://doi.org/10.3390/nano11051152









