Abstract
Mg2MnO4 nanoparticles with cubic spinel structure were synthesized by the sol-gel method using polyvinyl alcohol (PVA) as a chelating agent. X-ray powder diffraction, infrared spectrum (IR), scanning electron microscope (SEM), and transmission electron microscope (TEM) were used to characterize the crystalline phase and particle size of as-synthesized nanoparticles. The electronic structure of Mg2MnO4 spinel was studied by X-ray photoelectron spectroscopy (XPS). The results showed that pure cubic Mg2MnO4 spinel nanoparticles were obtained when the annealing temperature was 500–700 °C. The samples had a porous-spongy structure assembled by nanoparticles. XPS studies indicated that Mg2MnO4 nanoparticles were mixed spinel structures and the degree of cation inversion decreased with increasing annealing temperature. Furthermore, the performance of Mg2MnO4 as lithium anode material was studied. The results showed that Mg2MnO4 samples had good cycle stability except for the slight decay in the capacity at 50 cycles. The coulombic efficiency (ratio of discharge and charge capacity) in most cycles was near 100%. The sample annealed at 600 °C exhibited good electrochemical properties, the first discharge capacity was 771.5 mAh/g, and the capacity remained 340 mAh/g after 100 cycles. The effect of calcination temperature on the charge–discharge performance of the samples was studied and discussed.
1. Introduction
Spinel oxides with the general formula of AB2O4 (A and B are the metal cations) are important semiconductor materials and have been widely used in the fields of electronics, ceramics, communication, optics, magnetism, and catalysis because of the advantages of corrosion resistance, high hardness, good thermal stability, high melting point [1,2,3,4,5]. In the structure of AB2O4 spinel, each cell contains eight AB2O4 molecules, that is, thirty-two O2− ions, sixteen B3+ ions, and eight A2+ ions. The spinel structure can be considered as oxygen ions arranged in a cubic close-packed structure, forming tetrahedral and octahedral vacancies. The A2+ and B3+ cations are distributed in these two sites [6]. Considering the cation distribution, the spinel oxide can also be represented by the general formula (A1-xBx) [AxB2-x] O4, where the parentheses ( ) and brackets [ ] represent the tetrahedral and octahedral sites, respectively, and x denotes the inversion parameter. Usually, there are three types of spinel: (1) normal spinel (x = 0), where A ions occupy the tetrahedral sites and B ions occupy the octahedral sites; (2) inverse spinel (x = 1), where the A and one-half of the B cations are in the octahedral sites and the rest of B ions enter the tetrahedral sites; (3) mixed spinel (0 < x < 1), where both the A and B ions are randomly situated at both the sites. The distribution of cations in the spinel is affected by many factors, such as the type and nature of cations, synthesis temperature of materials, particle size, and impurity content [7,8]. The cation distribution of spinel materials has great effects on their properties, such as magnetism, catalytic activity, electrochemical activity. The relationship between the structure and properties can be better understood by studying the distribution of metal cations in the spinel [8,9,10,11].
Among many spinel materials, the spinels containing manganese have attracted much attention and are widely used in the field of secondary batteries and photoelectric catalysis because manganese has a plurality of valence states and is low in price, non-toxic, and environment-friendly. Studies showed that spinels containing a higher content of tetravalent manganese were beneficial to ion migration and could be used as excellent materials for electrochemical cells or rechargeable batteries [3,12]. The Mg2MnO4 spinel has a cubic structure, and it has proven to be a good oxygen evolution photoanode [13,14]. Mg2MnO4 is considered an inverse spinel structure; half of the magnesium ions are located in the tetrahedral site, and half of the magnesium ions and manganese ions are located in the octahedral site together [15]. The structure diagram is shown in Figure 1. Actually, the distribution of magnesium and manganese ions in Mg2MnO4 is influenced by many factors, such as heat treatment temperature and time, and ion doping.
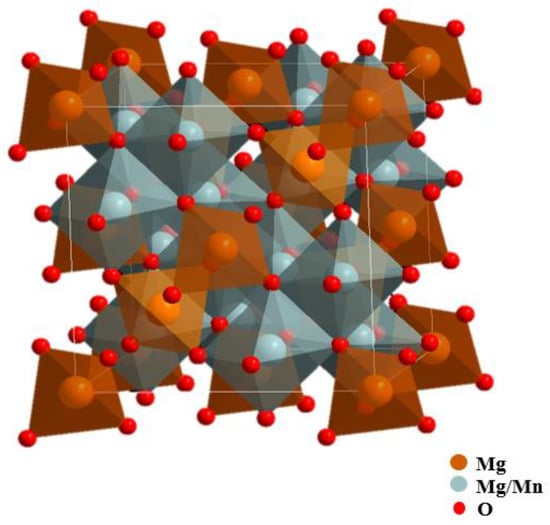
Figure 1.
Theoretical structure of the cubic-phase Mg2MnO4 spinel.
Mg2MnO4 has been previously prepared by solid-state reactions, coprecipitation method, and microemulsion method [16,17]. The pure phase of Mg2MnO4 cannot be obtained by the former two methods. Although the material prepared by the microemulsion method is relatively pure Mg2MnO4, the synthesis process is complicated and difficult to control. In this study, pure Mg2MnO4 spinel nanoparticles with a cubic structure were synthesized by a simple and easy to operate sol-gel method using polyvinyl alcohol (PVA) as the chelating agent and characterized by XRD, SEM, TEM, and IR techniques. The electronic structure and cation distribution of the Mn and Mg in the Mg2MnO4 spinel were studied by X-ray photoelectron spectroscopy. The as-synthesized Mg2MnO4 nanoparticles were used for the first time as anode material for Li-ion batteries, and the electrochemical properties were determined.
2. Materials and Methods
2.1. Synthesis
Mg2MnO4 nanoparticles with the structure of a cubic spinel were synthesized by the sol-gel method using polyvinyl alcohol (PVA, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) as the chelating agent. The detailed experimental process was as follows: magnesium nitrate (≥99%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and manganese acetate (≥99%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were firstly dissolved in deionized water to form a transparent solution, polyvinyl alcohol was added to the solution under stirring. The molar ratio of PVA to metal ions was 2:1. After mixing and thoroughly stirring, the solution was heated on the magnetic heating agitator until viscous gels were formed. The gels were then dried at 110 °C in an oven and calcined at different temperatures (500–800 °C) for 5 h to obtain the target products.
2.2. Characterization
The X-ray diffraction (XRD) data of the Mg2MnO4 nanoparticles were collected by a D8-Advance type multi-functional powder diffractometer manufactured by Bruker, Germany. Cu target K radiation and carbon monochromator were used in the measurement. The micromorphology of the samples was observed by a ZEISS scanning electron microscope made in Germany. The micrographs of the powders were observed by a high-resolution transmission electron microscope using a JEM-2100F type field produced by Japan. The infrared absorption spectra of the samples were obtained by the NEXUS 670-type Fourier transform Infrared-Raman spectrometer manufactured by Thermo Nicolet. N2 adsorption and desorption tests were mainly used to characterize the specific surface area of the sample. In this experiment, a JW-BK112T automatic gas adsorption analyzer (Beijing Jingwei Gaobo Science and Technology Co., Ltd., Beijing, China) was used for testing.
X-ray photoelectron spectra (XPS) were measured by a Thermofisher ESCALAB 250 (Waltham, MA, USA) X-ray photoelectron spectrometer with the monochromatic Al Kα X-ray source in an ultrahigh vacuum (≤10−7 Pa). The spot size was 500 μm. The neutralizing gun was opened during the test to neutralize the excess positive charge on the sample surface, and the binding energies of all the spectra were calibrated by using the C 1 s peak (284.6 eV) of carbon impurities as a reference. The spectra were deconvoluted after background subtraction, using a mixed Gaussian–Lorentzian function. Fractional atomic concentrations of the elements were calculated using empirically derived atomic sensitivity factors.
2.3. Electrochemical Characterization
Electrochemical measurements of the cubic phase Mg2MnO4 nanoparticles were performed by making coin cells. The working electrode was made by using as-synthesized Mg2MnO4 nanoparticles as the active material. Firstly, Mg2MnO4, acetylene black, and polyvinylidene difluoride were mixed at a mass ratio of 8:1:1 and then dissolved in n-methyl pyrrolidone (NMP). The above slurry was evenly coated on the hydrophilic carbon cloth and dried in a vacuum drying oven at 120 °C for 12 h. The lithium plate was used as the opposite electrode; Celgard 2300 and lithium hexafluorophosphate solutions were used as the separator and electrolyte, respectively. All the above operations were carried out in a glove box filled with argon gas.
The performance of the cells was evaluated in the voltage range of 0–3 V on a LAND battery test system (CT2001A, Wuhan Lambo Test Equipment U Co., Ltd., Wuhan, China), and the charge–discharge current was kept at 0.5 mA. An electrochemical workstation (CHI660E, Shanghai Chenhua Instrument Co., Ltd., Shanghai, China) was used to test the cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The CV test voltage range was 0.1–3 V, and the scanning speed was 0.3 mV/s. The EIS was recorded in the frequency range from 1 MHz to 10 mHz with an amplitude of 10 mV.
3. Results and Discussion
3.1. Preparation of Mg2MnO4 Nanoparticles
X-ray powder diffraction was used to identify the phase of the as-synthesized samples. Figure 2 shows the XRD patterns of all the powder samples. As shown in Figure 2, several obvious diffraction peaks appeared in the XRD pattern of the sample annealed at 500 °C, indicating the formation of nanocrystals. The peak intensity increased gradually with the increase in annealing temperature, indicating that the crystallization degree of the sample increased. All the peaks indexed as (111), (220), (311), (400), (511), (440), and (531) crystal planes were assigned to the cubic Mg2MnO4 phase (ICDD PDF#19-0773). When the annealing temperature was up to 800 °C, a new weak impurity peak of Mg6MnO8 (No.19-0766) occurred. The XRD result showed that the pure cubic Mg2MnO4 spinel could be prepared by the sol-gel method when the annealing temperature was 500–700 °C. In addition, based on the XRD data, the cell parameters of the samples were estimated according to the formula: , where h, k, and l are the Miller indices, d is the interplanar spacing, which is obtained by the Bragg equation (). The parameters were 8.285 (±0.001) Å, 8.298 (±0.001) Å, 8.330 (±0.001) Å, and 8.334 (±0.001) Å for the samples annealed at 500–800 °C.
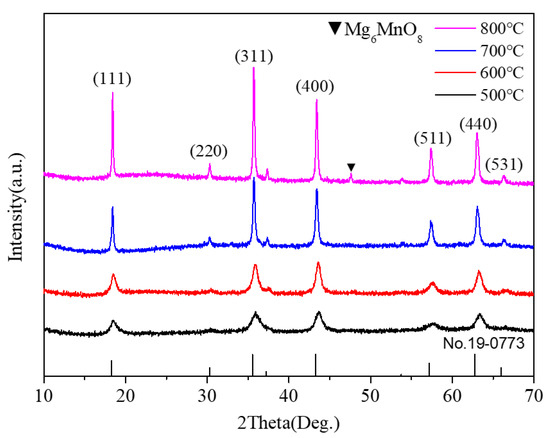
Figure 2.
XRD pattern of Mg2MnO4 samples annealed at different temperatures.
The morphology and particle size of the Mg2MnO4 samples were observed by SEM and TEM, shown in Figure 3 and Figure 4, respectively. From the inset of Figure 3a, it can be seen that the sample annealed at 500 °C had a porous-spongy structure, the samples annealed at 600–800 °C had a similar porous-spongey structure (Supplementary Material, Figure S1). When the magnification was increased, it was found that the structure was assembled by nanoparticles (Figure 3a), and all the annealed samples showed a similar microstructure (Figure 3b–d). It can be seen from Figure 4a,b that the particle size of the samples annealed at 500–600 °C was in the range of 30–50 nm. When the annealing temperature was increased to 700–800 °C, the particles of the sample grew rapidly, and the size was up to 100–200 nm. The high-resolution (HR) TEM image was taken for the particles shown in Figure 4e. The lattice spacing of approximately 0.48 nm corresponded to the d spacing of the (111) crystal plane of the cubic Mg2MnO4. Figure 5 shows the N2 adsorption/desorption isotherms at different annealing temperatures. The specific surface areas of the samples were calculated by the BET method to be 61.04 cm3/g, 34.95 cm3/g, 11.33 cm3/g, and 8.59 cm3/g for the temperatures 500–800 °C, indicating that with the increase in the annealing temperature, the specific surface area decreased because of the increasing particle size, which was confirmed by the TEM results.
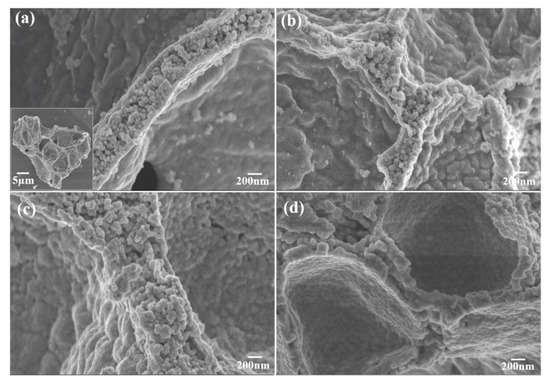
Figure 3.
SEM images of Mg2MnO4 nanoparticles annealed at different temperatures: (a) 500 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C.
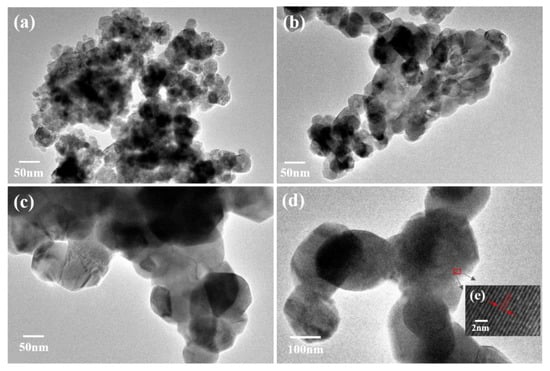
Figure 4.
TEM images of Mg2MnO4 nanoparticles annealed at different temperatures: (a) 500 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C, (e) HR-TEM image marked in (d).
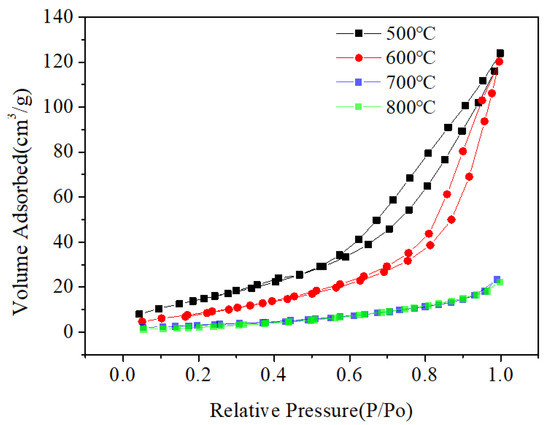
Figure 5.
The N2 adsorption/desorption isotherms of Mg2MnO4 at different annealing temperatures.
The IR spectra of the sintered samples are presented in Figure 6. From the spectrum of the precursor (Figure 6), we could see that there were several intense absorption peaks: the absorption peak at 1450–1700 cm−1 was caused by the deformation and vibration of water, the wide absorption peak at 3400 cm−1 was due to the stretching vibration of lattice -OH, and the asymmetric stretching of CO2 appeared at 2400 cm−1 [18]. When the sample was annealed at 500 °C, an obvious absorption peak at 695 cm−1 appeared in the IR spectra, and the new peak was the characteristic absorption peak of Mg2MnO4. When the heat treatment temperature of the sample was increased to 600 °C, the peak at 695 cm−1 was enhanced, indicating the crystallization was strengthened. The result was consistent with the XRD analysis.
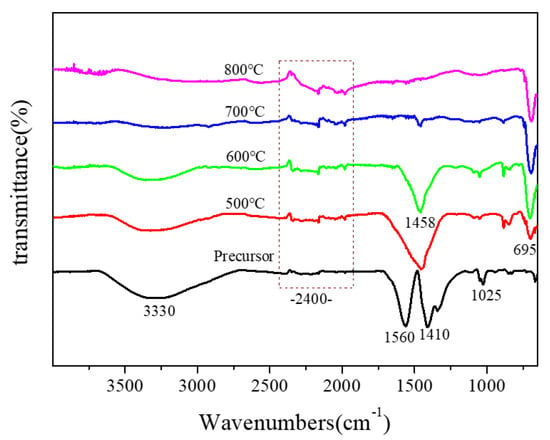
Figure 6.
IR spectra of Mg2MnO4 samples annealed at different temperatures.
3.2. XPS Studies
The survey XPS spectra of Mg2MnO4 samples annealed under different temperatures were determined and shown in Figure 7a. The results showed that no other elements were detected except for the original components and contaminated carbon. The C 1 s peak of the carbon contamination at 284.6 eV was used as reference (Figure 7b). The O 1 s spectra (Figure 7c) could be deconvoluted into two peaks. The peak located at the high-binding energy side (530.6 eV) was mainly from the adsorbed oxygen, while the peak with the low-binding energy (529.6) was allocated to the metal-oxygen bond corresponding to the lattice oxygen [19]. In order to analyze the chemical environments and valence states of Mn and Mg ions, Mn 2p3/2 and Mg 1 s high-resolution photoelectron spectra were measured, and the results are shown in Figure 7d,e, respectively.
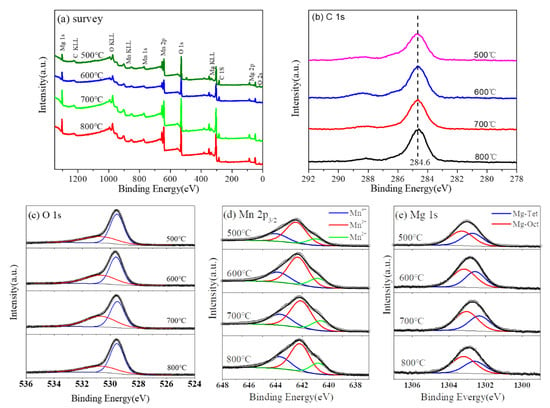
Figure 7.
XPS spectra of Mg2MnO4 samples annealed at different temperatures: (a) survey, (b) C 1 s, (c) O 1s, (d) Mn 2p3/2, and (e) Mg 1 s.
XPS spectra of Mn 2p3/2 were wide and asymmetric, indicating that Mn ions occupied more than one coordination environment or had different valence states in the samples. In order to analyze the distribution of Mn ion in the sample, XPS spectra of Mn 2p3/2 were deconvolved; the analysis results are shown in Figure 7d. We fixed the binding energy difference between the two adjacent peaks of Mn 2p3/2 to 1.5 eV to obtain accurate values. After deconvolution, the Mn 2p3/2 orbital consisted of three peaks. The data of the binding energy, full width at half maximum, and relative content of Mn 2p3/2 are listed in Table 1. It is generally known that Mn has a plurality of valence states. It was reported that the binding energy values of Mn2+, Mn3+, and Mn4+ were in the ranges of 640.6–641.2 eV, 642.0–642.5 eV, and 643.5–644.2 eV, respectively [20,21,22]. Therefore, the three peaks of Mn 2p3/2 orbital at about 640.8 eV, 642.3 eV, and 643.8 eV corresponded to Mn2+, Mn3+, and Mn4+, respectively [23,24]. Similarly, the XPS spectra of Mg 1 s were analyzed. After deconvolution, the analysis results are shown in Figure 7e. Mg 1 s orbital was divided into two peaks, indicating that Mg2+ ions occupy two different sites in Mg2MnO4 spinel. The binding energy data are listed in Table 2. The peaks at 1302.6 eV and 1302.2 eV were attributed to Mg ions at octahedral and tetrahedral positions, respectively [25,26,27].

Table 1.
XPS data for Mn 2p3/2 spectra of Mg2MnO4 nanoparticles annealed at different temperatures.

Table 2.
XPS data for Mg 1 s spectra of Mg2MnO4 nanoparticles annealed at different temperatures.
The change of cation distribution in the microstructure of the material led to lattice distortion, which had a macro effect on the properties of the material. We analyzed the cationic space occupation and the reversion degree of the samples at different sintering temperatures. The theoretical structure of the cubic Mg2MnO4 could be described as (Mg2+) [Mn4+1/2Mg2+1/2]2O4. However, due to the influence of the heat treatment temperature, synthesis method, and other experimental conditions, manganese ions were many more than one valence state and chemical environments in manganese-containing spinels [24,28]. According to the report, the absolute Octahedral Site Preference Energy (OSPE) value of Mn3+ (95.4 kJ mol−1) was higher than that of Mn2+(0 kJ mol−1), indicating that Mn3+ and Mn4+ tended to occupy the octahedral site and Mn2+ tended to occupy the tetrahedral site [1,29]. Considering the distribution of Mn ions with different valence states, the general formula of Mg2MnO4 could be described as (Mg2+xMn2+1-x) [Mn3+/4+x/2Mg2+2-x/2] O4. It can be seen from Table 1 that the percent of Mn4+ ions was 28.85% for the 500 °C heated sample and decreased with the increase in heat treatment temperature. The sum of Mn3+ and Mn4+ at the octahedral sites decreased from 87% to 80% when the sintering temperature increased from 500 °C to 800 °C. According to the distribution of manganese ions in the sample, the inversion degree calculated is listed in Table 1. It could be found that with the increase in heat treatment temperature of the sample, the inversion degree dropped from 0.87 to 0.80. Similarly, the inversion degree calculated according to the distribution of magnesium ions in the sample is listed in Table 2. By comparing the inversion parameters calculated at different temperatures, it could be found that the inversion parameters calculated for Mn and Mg ions were consistent, and the value decreased with the increase in the temperature. The result indicated that as the temperature increased, more Mn2+ ions emerged and occupied the tetrahedral sites in the samples. Meanwhile, the fraction of Mg2+ ions in the tetrahedral sites was reduced. As a result, we concluded that the sample was a mixed spinel and the degree of cationic disorder decreased as the temperature increased.
3.3. Electrochemical Properties
The curves of the charge–discharge performance of the Mg2MnO4 samples annealed at different temperatures are shown in Figure 8. All the samples showed good cycle stability, and the coulombic efficiency was near 100%. The first discharge capacities were 537.0, 771.5, 531.7, and 522 mAh/g for the samples annealed at 500–800 °C. The capacity was higher than the commercial graphite anode materials, for which the theoretical specific capacity was 372 mAh/g [30]. It can be seen from Figure 8 that the capacities increased with the temperature increasing from 500 °C to 600 °C, and then decreased when the temperature was higher than 700 °C. This change could be explained in combination with the particle size and microstructure of the samples. From the above analysis, we knew that the samples annealed at 500–600 °C had a small crystallite size (30–50 nm), which was helpful for the electrochemical properties. From the view of microstructure, the sample annealed at 600 °C had a relatively small inversion degree when compared with the 500-heated sample. The previous studies indicated that the spinel materials with less inversion degree showed better electrochemical performance [31,32]. Therefore, the 600-heated sample displayed the higher capacity. However, the crystal particle of the sample grew rapidly when annealed at 700–800 °C, and the size was more than 100 nm; thus, the specific surface area decreased greatly. Although the inversion degree of the samples decreased with increasing annealing temperature, the large crystallite particle was not conducive to the migration of lithium ions in the material, so the discharge capacity of the sample annealed at these two temperatures decreased. After 100 cycles, the sample annealed at 600 °C remained the largest capacity of 340.0 mAh/g, and all the samples showed almost no decay in the capacities; the cycle stability of Mg2MnO4 was better than that of the MgCo2O4 spinel [33]. As a result, the sample annealed at 600 °C as lithium battery anode material had a good electrochemical performance and showed a highest charge–discharge capacity.
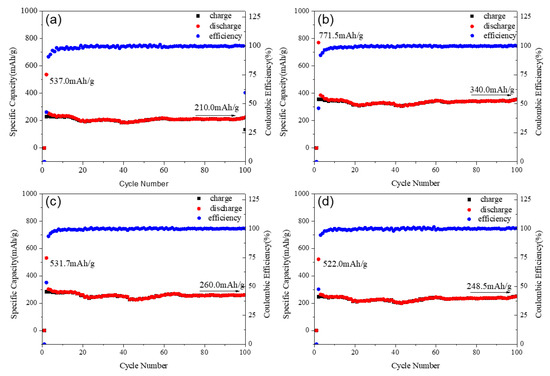
Figure 8.
Specific capacity and cycle performance of Mg2MnO4 samples annealed at different temperatures: (a) 500 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C.
The detailed electrochemical properties of the 600-annealed sample were measured and shown in Figure 9. Figure 9a shows the capacity retention of Mg2MnO4 electrode materials. It could be seen that the discharge capacities were 343, 290, 166, 104, and 80 mAh/g when the current densities were 50, 100, 400, 800, and 1000 mA/g, respectively. When the current density returned to 100 mAh/g, the discharge capacity restored to 290 mAh/g, indicating that Mg2MnO4 had a good capacity recovery. From the cycle volt–ampere curve of the Mg2MnO4 sample shown in Figure 9b, it could be seen that the pairs of redox peaks of Mg2MnO4 appeared in the first three cycles. The position of the oxidation peak in the first cycle was about 0.4 V, and the position increased to 0.7 V for the second and third cycles. The position of the reduction peak in the first three cycles was basically kept the same value at 0.63 V. The results indicated that Mg2MnO4 as a lithium negative electrode had good electrochemical reversibility.
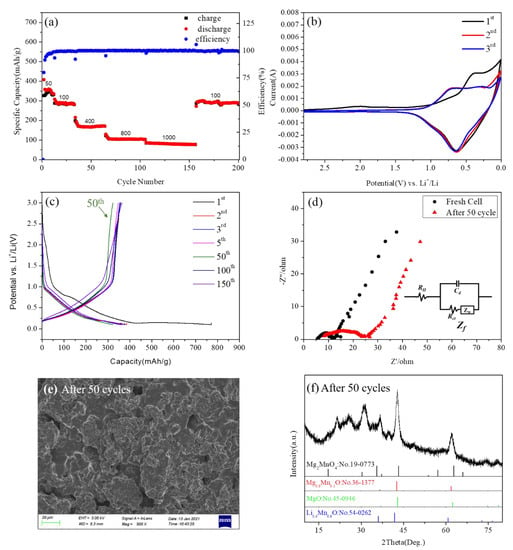
Figure 9.
(a) The charge–discharge capacity of cycles for Mg2MnO4 at different current densities. (b) CV curves of Mg2MnO4 at a scan rate of 0.3mV/s. (c) The 1, 2, 3, 5, 50, 100, and 150 cycles of charge–discharge curves at a rate of 1C. (d) Nyquist plots of the electrode of Mg2MnO4 operating at the fresh cell and after 50 cycles. (e) The SEM image of the anode material after 50 cycles. (f) The XRD pattern of the anode material after 50 cycles.
Figure 9c shows the voltage-capacity distribution curves of the sample at cycles 1, 2, 3, 5, 50, 100, and 150. We could see that in the 2, 3, 5, 100, and 150 cycles, the sample exhibited almost no capacity attenuation and good cycle stability. However, at the 50th cycle, there was a capacity fading, but this phenomenon returned to its previous state in later cycles. By comparing the voltage–capacity distribution curves at 500 °C, 700 °C, and 800 °C (Figure S3), it was found that the samples at different annealing temperatures all experienced a certain degree of capacity attenuation at 50 cycles. In order to explore the reason for this phenomenon, we conducted an electrochemical impedance analysis, morphology, and phase analysis on the samples at 50 cycles. Figure 9d is the EIS diagram of the material in a fresh cell and after 50 cycles. Figure 9d shows the electrochemical impedance spectra of Mg2MnO4 electrode material in a fresh cell and after 50 cycles. Firstly, in the equivalent circuit, RΩ represented the internal resistance of the battery, mainly including electrolyte resistance (Re), surface film resistance (Rsf), etc., Cd represented the double-layer capacitance, and Zf represented the Faraday impedance. The Faraday impedance could be further divided into two parts: the charge transfer resistance (Rct) and the Warburg impedance (ZW) [34,35]. Nyquist plots showed that the Mg2MnO4 electrode presented a semicircular arc and an inclined line distributed from high frequency to low frequency, indicating that, in the high-frequency region, the electrode process was controlled by charge transfer, while in the low-frequency region, the electrode process was dominated by mass transfer [36]. The diameter of the semicircular arc in the high-frequency region of the Nyquist diagram corresponded to the internal resistance of the battery. The impedance for the fresh cell of Mg2MnO4 was estimated to be 12 ohm; this indicated that the material had a high electronic conductivity. After 50 cycles, the impedance of the electrode was changed to 24 ohm, indicating that the conductivity of the material decreased, and the resistance increased. It was speculated that the reason for this phenomenon was the change of the morphology and structure of the material. In order to verify this assumption, the electrode material after 50 cycles was tested by SEM and XRD, and the results are shown in Figure 9e,f. According to the SEM figure (Figure 9e), the morphology of the material collapsed. XRD results showed that the phase of the material changed after 50 cycles, part of the cubic phase Mg2MnO4 changed into Mg0.9Mn0.1O and MgO, some manganese ions combined with lithium ions in the electrolyte to form Li0.4Mn0.6O, the diffraction peaks around 2θ = 22° and 25 ° belonged to the carbon cloth collector. Presumably, this change was reversible because the capacity returned to its previous state in the later cycles. The voltage–capacity curves of the samples at 500 °C, 700 °C, and 800 °C showed similar capacity attenuation at about 50 cycles (Figure S3). By comparing the changes of EIS, SEM, and XRD of the fresh cell and after 50 cycles (Figures S4–S6), all the samples showed a similar process of change.
4. Conclusions
Using PVA as the chelating agent, and Mg2MnO4 with cubic spinel structure was successfully prepared by the sol-gel method. XRD, SEM, TEM, and IR tests were carried out to analyze the effect of sintering temperature on the phase, crystallinity, and particle size of the samples. The results showed that the pure Mg2MnO4 phase could be obtained at temperatures of 500–700 °C. All the samples had a porous-spongy structure; the particle size was 30–50 nm for the samples annealed at 500–600 °C and increased to 100–200 nm when the temperature was up to 700–800 °C. The coordination environments and valence states of metal ions in the samples were analyzed by XPS, and the inversion parameters were calculated. It was found that manganese existed in three valence states in the Mg2MnO4 samples, in which Mn3+ and Mn4+ were located in the octahedral sites, and Mn2+ was located in the tetrahedral sites. With the increase in the heat treatment temperature of the samples, the inversion parameters and degree of cation disorder decreased. The electrochemical properties of Mg2MnO4 powders were also studied. It could be found that Mg2MnO4 as the lithium anode material had good cycling stability. The sample annealed at 600 °C had an initial discharge capacity of 771.5 mAh/g and a capacity of 340 mAh/g after 100 cycles. Mg2MnO4 was a potential anode material for lithium-ion batteries, and it was meaningful to improve the performance by decreasing the particle size and the degree of inversion of the material.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11051122/s1, Figure S1. SEM images of Mg2MnO4 nanoparticles annealed at different temperatures: (a) 500 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C, Figure S2. The 1, 2, 3, 5, 50, 100, and 150 cycles of charge–discharge curves at a rate of 1C. (a) 500 °C; (b) 700 °C; (c) 800 °C, Figure S3. Nyquist plots of the electrode of Mg2MnO4 operating at the fresh cell and after 50 cycles. (a) 500 °C; (b) 700 °C; (c) 800 °C, Figure S4. The SEM image of the anode material after 50 cycles. (a) 500 °C; (b) 700 °C; (c) 800 °C. Figure S5. The XRD pattern of the anode material after 50 cycles. (a) 500 °C; (b) 700 °C; (c) 800 °C.
Author Contributions
Z.W. and X.D. conceived and designed the research and wrote the manuscript. Z.W. carried out all of the experiments. H.Z., B.L., and L.A. helped with the electrochemical test section. J.D., P.Z., Z.L., and H.J. (Hechun Jiang) helped with the XPS test section. H.J. (Huaidong Jiang) and F.Y. helped to revise the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (51672160).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.G.; Depeyrot, J.; Campos, A.F.C.; Aquino, R.; Fiorani, D.; Peddis, D. Structural and Magnetic Properties of Spinel Ferrite Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 4888–4902. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Sarac, M.F. Magnetic, Structural, and Optical Properties of Gadolinium-Substituted Co0.5Ni0.5Fe2O4 Spinel Ferrite Nanostructures. J. Supercond. Nov. Magn. 2019, 33, 397–406. [Google Scholar] [CrossRef]
- Torruella, P.; Ruiz-Caridad, A.; Walls, M.; Roca, A.G.; López-Ortega, A.; Blanco-Portals, J.; López-Conesa, L.; Nogués, J.; Peiró, F.; Estradé, S. Atomic-Scale Determination of Cation Inversion in Spinel-Based Oxide Nanoparticles. Nano Lett. 2018, 18, 5854–5861. [Google Scholar] [CrossRef]
- Sun, Q.; Bijelić, M.; Djurisic, A.B.; Suchomski, C.; Liu, X.; Xie, M.; Ng, A.M.C.; Li, H.K.; Shih, K.; Burazer, S.; et al. Graphene-oxide-wrapped ZnMn2O4 as a high performance lithium-ion battery anode. Nanotechnology 2017, 28, 455401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Du, Y.; Xi, S.; Xu, Z.J. Spinel Manganese Ferrites for Oxygen Electrocatalysis: Effect of Mn Valency and Occupation Site. Electrocatalysis 2017, 9, 287–292. [Google Scholar] [CrossRef]
- Vitor, P.A.M.; Venturini, J.; da Cunha, J.B.M.; Bergmann, C.P. The influence of cation distribution on the magnetic properties of mixed Co1-yNiyFe2O4 nanofer-rites produced by the sol-gel method. J. Alloys Compd. 2021, 851, 156799. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Hölscher, J.; Petrecca, M.; Albino, M.; Garbus, P.G.; Saura-Múzquiz, M.; Sangregorio, C.; Christensen, M. Magnetic Property Enhancement of Spinel Mn–Zn Ferrite through Atomic Structure Control. Inorg. Chem. 2020, 59, 11184–11192. [Google Scholar] [CrossRef]
- Harudin, N.; Osman, Z.; Majid, S.R.; Othman, L.; Hambali, D.; Silva, M.M. Improved electrochemical properties of MgMn2O4 cathode materials by Sr doping for Mg ion cells. Ionics 2020, 26, 3947–3958. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hudak, B.M.; Wallace, D.K.; Kim, D.Y.; Guiton, B.S. Simple synthetic route to manganese-containing nanowires with the spinel crystal structure. J. Solid State Chem. 2016, 240, 23–29. [Google Scholar] [CrossRef]
- Noh, J.; Kim, S.; Gu, G.H.; Shinde, A.; Zhou, L.; Gregoire, J.M.; Jung, Y. Unveiling new stable manganese based photoanode materials via theoretical high-throughput screening and experiments. Chem. Commun. 2019, 55, 13418–13421. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Nanostructured dimagnesium manganese oxide (Spinel): Control of size, shape and their magnetic and electro catalytic properties. J. Solid State Chem. 2013, 197, 392–397. [Google Scholar] [CrossRef]
- Ghozza, A.M. Solid-solid interactions in pure and Li2O-doped manganese and magnesium mixed oxides system. J. Therm. Anal. Calorim. 2004, 75, 135–151. [Google Scholar] [CrossRef]
- Izawa, N.; Oi, T. Ion and lithium isotope selectivities of sorbents prepared from Mg2MnO4. J. Mater. Sci. 1997, 32, 675–679. [Google Scholar] [CrossRef]
- Duan, X.; Yuan, D.; Cheng, X.; Wang, L.; Yu, F. Preparation of Co2+-doped MgGa2O4 nanocrystals by citrate sol–gel method. J. Alloy. Compd. 2007, 439, 355–357. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.-T.; Huq, M.M.; Hsu, J.-P.; Gandomi, Y.A.; Li, J. Preparation of MgCo2O4/graphite composites as cathode materials for magnesium-ion batteries. J. Solid State Electrochem. 2019, 23, 1399–1407. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, R.; Lei, M. FeCo1.6Mn0.4O4 microspheres as catalysts for oxygen reduction reaction. In Proceedings of the 2019 3rd International Workshop on Renewable Energy and Development, Guangzhou, China, 8–10 March 2019. [Google Scholar]
- Beyreuther, E.; Grafström, S.; Eng, L.M.; Thiele, C.; Dörr, K. XPS investigation of Mn valence in lanthanum manganite thin films under variation of oxygen content. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Wang, J.; Gu, T. Improvement of activity, selectivity and H2O&SO2-tolerance of micro-mesoporous CrMn2O4 spinel catalyst for low-temperature NH3-SCR of NOx. Appl. Surf. Sci. 2019, 466, 411–424. [Google Scholar] [CrossRef]
- Dudric, R.; Bortnic, R.; Souca, G.; Ciceo-Lucacel, R.; Stiufiuc, R.; Tetean, R. XPS on Nd0.6-xBixSr0.4MnO3 nano powders. Appl. Surf. Sci. 2019, 487, 17–21. [Google Scholar] [CrossRef]
- Ge, T.; Guo, Z.; Wu, M.; Sun, R.; Li, W.; Yang, G. Preparation and characterization of spinel-layered mixed structural 0.2 LiNi0.5Mn1.5O4·0.8 Li[Li0.2Ni0.2Mn0.6]O2 as cathode materials for lithium-ion batteries. J. Alloy. Compd. 2019, 801, 254–261. [Google Scholar] [CrossRef]
- Liu, J.; Duan, X.; Li, N.; Jiang, H. Effects of synthesis method on cation distribution and optical properties of Co/Cr co-doped MgGa2O4 nanoparti-cles. J. Alloys Compd. 2015, 640, 169–174. [Google Scholar] [CrossRef]
- Mittal, V.; Chandramohan, P.; Bera, S.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Cation distribution in NixMg1−xFe2O4 studied by XPS and Mössbauer spectroscopy. Solid State Commun. 2006, 137, 6–10. [Google Scholar] [CrossRef]
- Bennet, J.; Tholkappiyan, R.; Vishista, K.; Jaya, N.V.; Hamed, F. Attestation in self-propagating combustion approach of spinel AFe2O4 (A = Co, Mg and Mn) complexes bearing mixed oxidation states: Magnetostructural properties. Appl. Surf. Sci. 2016, 383, 113–125. [Google Scholar] [CrossRef]
- Kutty, R.K.N.; Kasturi, P.R.; Jaganath, J.; Padmanapan, S.; Lee, Y.S.; Meyrick, D.; Selvan, R.K. Structural and magnetic properties of CoMn2O4 synthesized by auto combustion method. J. Mater. Sci. Mater. Electron. 2018, 30, 975–981. [Google Scholar] [CrossRef]
- Reynolds, J.G. Spinel structure and liquidus temperature relationships in nuclear waste glass. J. Mater. Sci. 2005, 40, 3987–3991. [Google Scholar] [CrossRef]
- Kaskhedikar, N.A.; Maier, J. Lithium Storage ion Carbon Nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Morando, C.; Cofrancesco, P.; Tealdi, C. Zn ion diffusion in spinel-type cathode materials for rechargeable batteries: The role of point defects. Mater. Today Commun. 2020, 25, 101478. [Google Scholar] [CrossRef]
- Sai Gautam, G.; Canepa, P.; Urban, A.; Bo, S.H.; Ceder, G. Influence of Inversion on Mg Mobility and Electrochemistry in Spinels. Chem. Mater. 2017, 29, 7918–7930. [Google Scholar] [CrossRef]
- Darbar, D.; Reddy, M.; Sundarrajan, S.; Pattabiraman, R.; Ramakrishna, S.; Chowdari, B. Anodic electrochemical performances of MgCo2O4 synthesized by oxalate decomposition method and electrospinning technique for Li-ion battery application. Mater. Res. Bull. 2016, 73, 369–376. [Google Scholar] [CrossRef]
- Moya, A. Identification of characteristic time constants in the initial dynamic response of electric double layer capacitors from high-frequency electrochemical impedance. J. Power Sources 2018, 397, 124–133. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.M. Doped Graphene Sheets as Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS NANO 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Zhuang, Q.-C.; Qiu, X.-Y.; Xu, S.-D.; Qiang, Y.-H.; Su, S.-G. Diagnosis of Electrochemical Impedance Spectroscopy in Lithium-Ion Batteries. Lithium Ion Batter. New Dev. 2012, 22, 1044–1057. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).