Graphene Oxide: Opportunities and Challenges in Biomedicine
Abstract
1. Introduction
2. Graphene and Its Physicochemical Properties
3. Graphene Oxide and Its Biological Properties
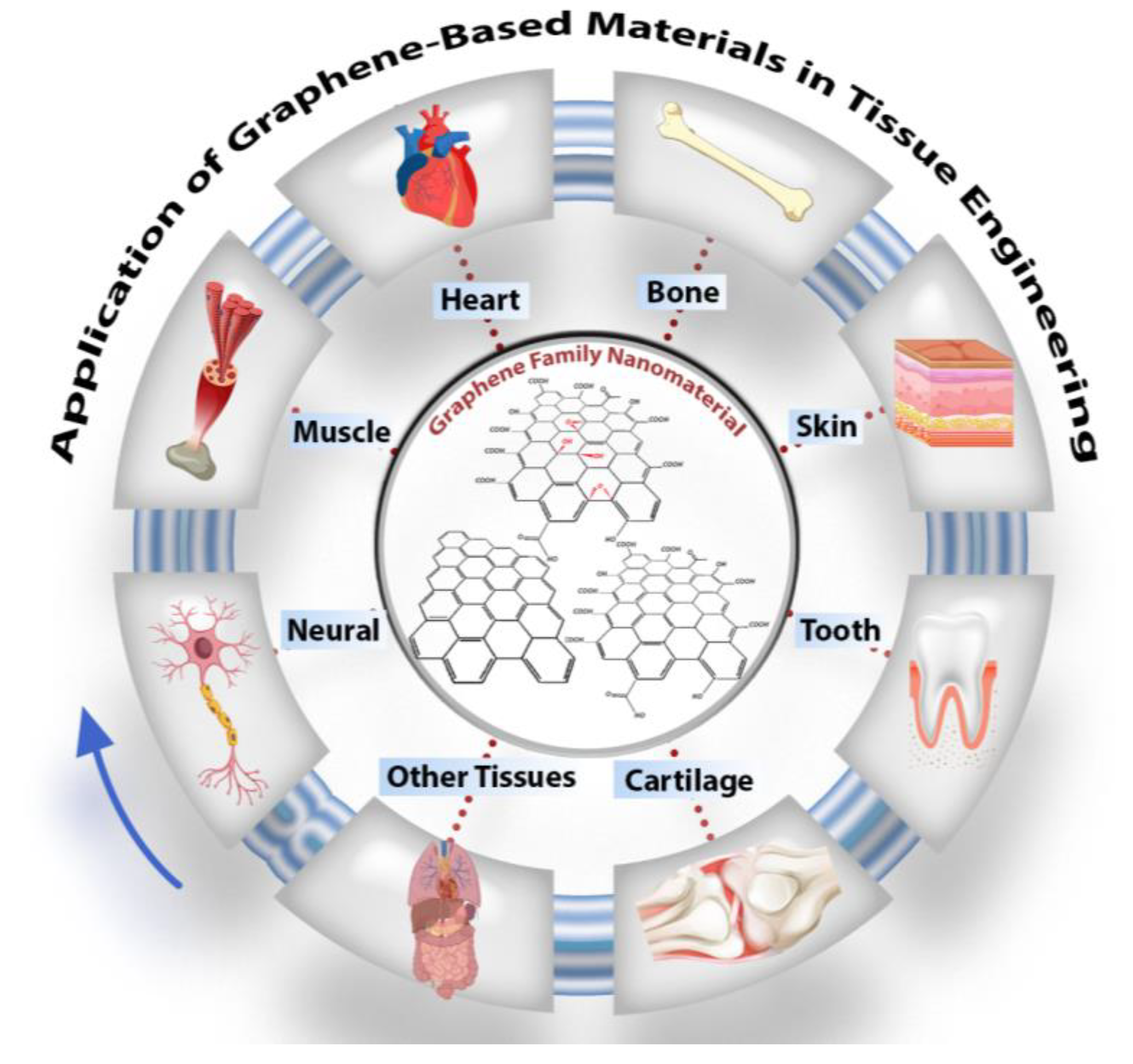
4. Development of Tissues and Organs Using Graphene-Based Materials
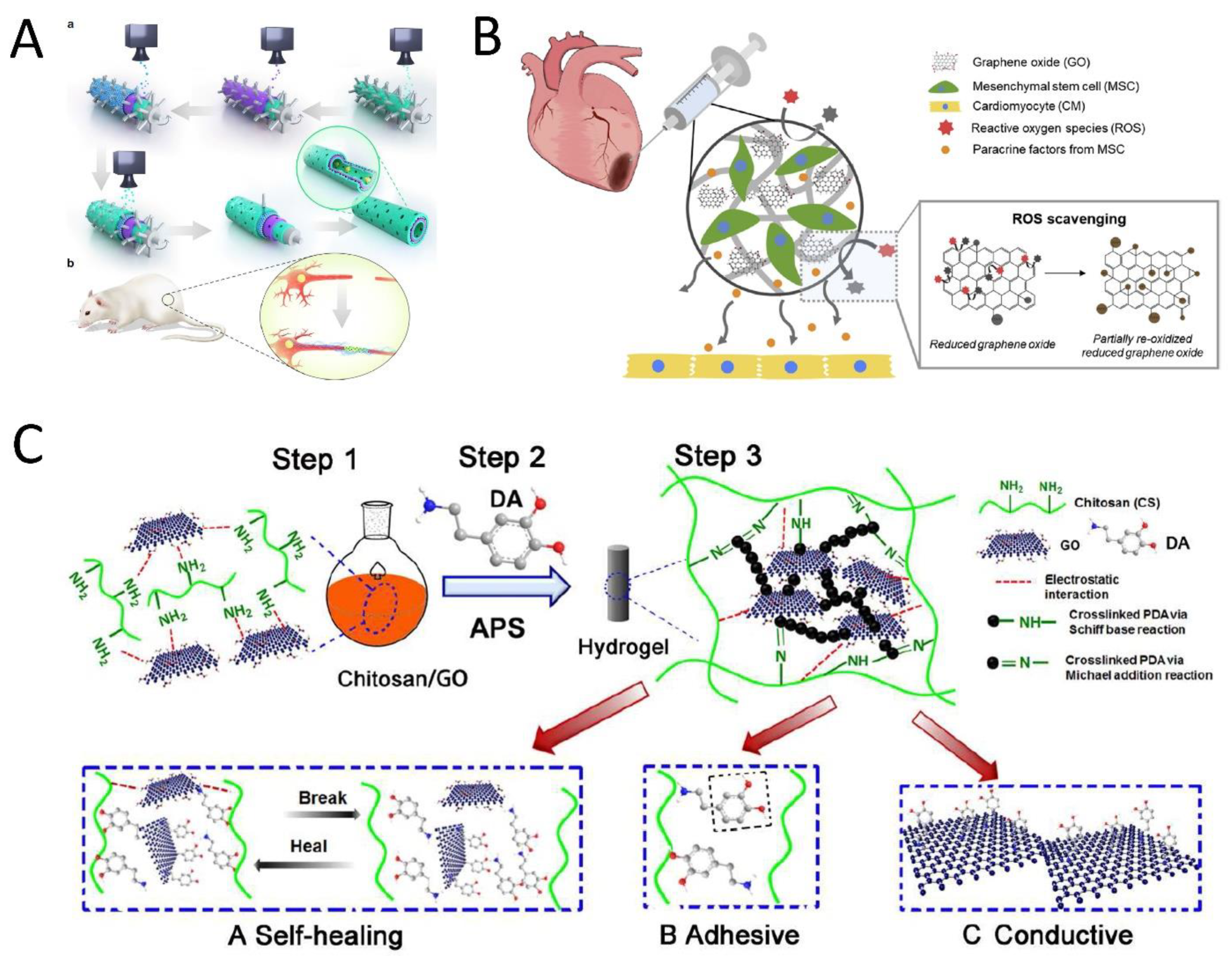
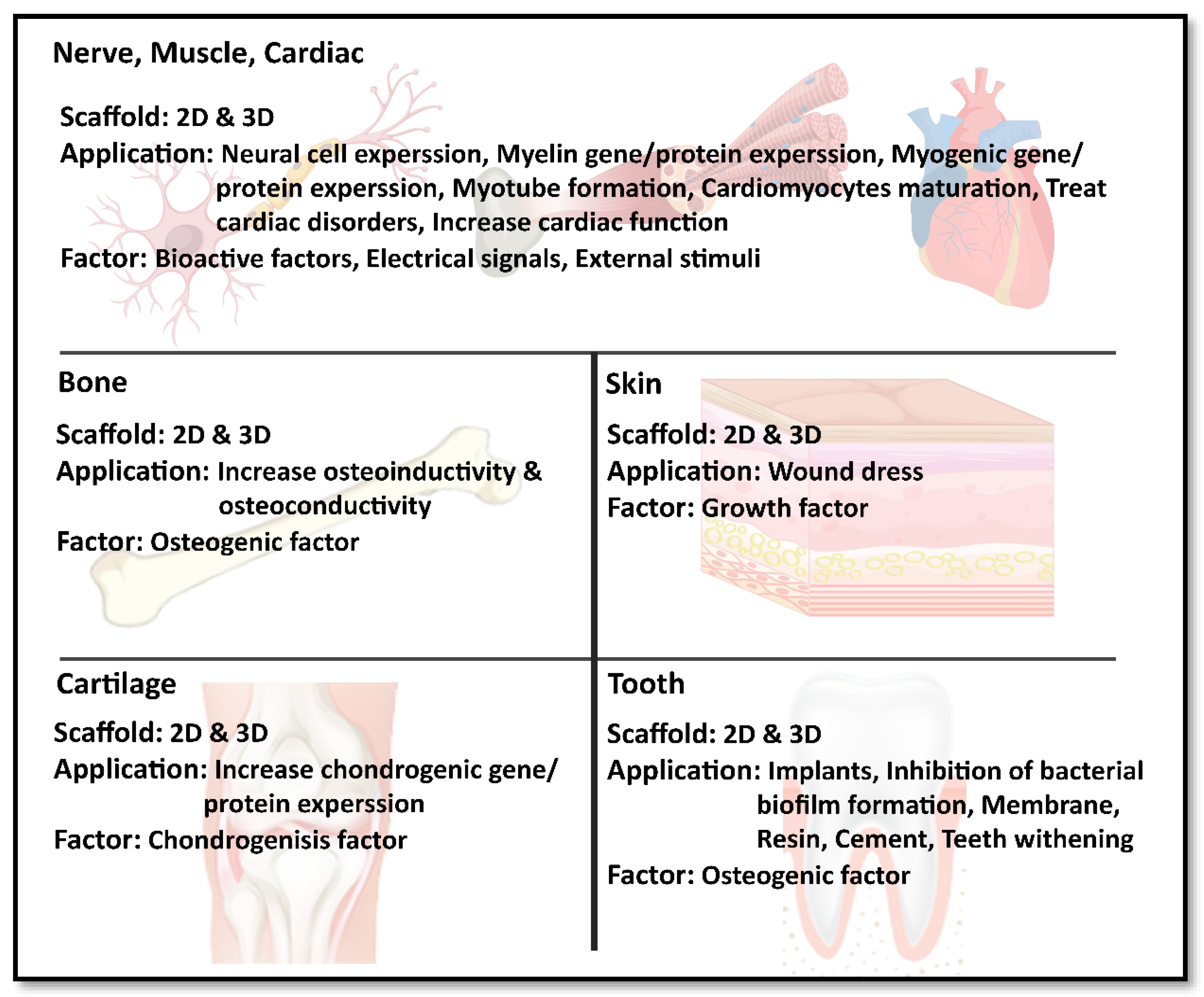
4.1. Nerve Muscle and Cardiac Tissue Engineering
4.2. Bone Tissue Engineering
4.3. Skin Tissue Engineering
4.4. Cartilage Tissue Engineering
4.5. Dental Application
5. Conclusions
6. Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dag Line, P. The Fundamental Challenges in Organ Transplantation. OBM Transpl. 2017, 1, 6. [Google Scholar] [CrossRef]
- Aleemardani, M.; Bagher, Z.; Farhadi, M.; Chahsetareh, H.; Najafi, R.; Eftekhari, B.; Seifalian, A.M. Can tissue engineering bring hope in the development of human tympanic membrane? Tissue Eng. Part B Rev. 2020, 1–62. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef] [PubMed]
- Aydin, T.; Gurcan, C.; Taheri, H.; Yilmazer, A. Graphene based materials in neural tissue regeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1107, pp. 129–142. [Google Scholar]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of nanocellulose/nanocarbon composites: Focus on biotechnology and medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, G.; Huang, Y.; Chen, Z.; Yuan, H.; Du, F. Assembly and Application Advancement of Organic-Functionalized Graphene-Based Materials: A Review. J. Sep. Sci. 2020, 43, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Maulik, N. Graphene-based drug delivery systems in tissue engineering and nanomedicine. Can. J. Physiol. Pharmacol. 2018, 96, 869–878. [Google Scholar] [CrossRef]
- Coleman, B.R.; Knight, T.; Gies, V.; Jakubek, Z.J.; Zou, S. Manipulation and Quantification of Graphene Oxide Flake Size: Photoluminescence and Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 28911–28921. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D graphene-containing structures for tissue engineering. Mater. Today Chem. 2019, 14, 100199. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Shang, L.; Qi, Y.; Lu, H.; Pei, H.; Li, Y.; Qu, L.; Wu, Z.; Zhang, W. Graphene and Graphene Oxide for Tissue Engineering and Regeneration. In Theranostic Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–185. [Google Scholar]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Sayyar, S.; Officer, D.L.; Wallace, G.G. Fabrication of 3D structures from graphene-based biocomposites. J. Mater. Chem. B 2017, 5, 3462–3482. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef]
- Pandit, S.; Gaska, K.; Kádár, R.; Mijakovic, I. Graphene-Based Antimicrobial Biomedical Surfaces. ChemPhysChem 2020, 22, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Han, J.; Kim, Y.S.; Lim, M.-Y.; Kim, H.Y.; Kong, S.; Kang, M.; Choo, Y.W.; Jun, J.H.; Ryu, S.; Jeong, H.-Y.; et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano 2018, 12, 1959–1977. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Manga, Y.B.; Ostrikov, K.K.; Chiang, W.H.; Pandey, A.; Lekha, R.; Nyambat, B.; Chuang, E.Y.; Chen, C.H. Microplasma Cross-Linked Graphene Oxide-Gelatin Hydrogel for Cartilage Reconstructive Surgery. ACS Appl. Mater. Interfaces 2020, 12, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019, 97, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Kim, S.-W.; Park, J.; Park, J.; Kim, S.; Kim, Y.S.; Ahn, Y.; Jung, D.-W.; Williams, D.R.; Lee, J.Y. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: Mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials 2019, 225, 119513. [Google Scholar] [CrossRef]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly (ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.Y.; Napiwocki, B.N.; Peng, X.F.; Turng, L.S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef]
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class II furcation defects in dog. Int. J. Nanomed. 2018, 13, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, A.G.; Dolado, E.L.; Gutierrez, M.C.; Collazos-Castro, J.E.; Luisa Ferrer, M.; Del Monte, F.; Serrano, M.C. Favorable biological responses of neural cells and tissue interacting with graphene oxide microfibers. ACS Omega 2017, 2, 8253–8263. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Kumar Shrestha, L.; Ariga, K.; Hsu, S.H. A graphene-polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Burnstine-Townley, A.; Eshel, Y.; Amdursky, N. Conductive Scaffolds for Cardiac and Neuronal Tissue Engineering: Governing Factors and Mechanisms. Adv. Funct. Mater. 2019, 30, 1901369. [Google Scholar] [CrossRef]
- Zhang, Z.; Klausen, L.H.; Chen, M.; Dong, M. Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials. Small 2018, 14, 1801983. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Yan, K.; Shi, C.; Xu, X.; Wang, T.; Li, R.; Dong, W.; Zheng, J. Neurogenic differentiation of adipose derived stem cells on graphene-based mat. Mater. Sci. Eng. C 2018, 90, 685–692. [Google Scholar] [CrossRef]
- Ginestra, P. Journal of the Mechanical Behavior of Biomedical Materials Manufacturing of polycaprolactone—Graphene fi bers for nerve tissue engineering. J. Mech. Behav. Biomed. Mater. 2019, 100, 103387. [Google Scholar] [CrossRef]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, R.; Wang, Z.; Dong, M.; Cui, B.; Chen, M. Visible-Light Neural Stimulation on Graphitic-Carbon Nitride/Graphene Photocatalytic Fibers. ACS Appl. Mater. Interfaces 2017, 9, 34736–34743. [Google Scholar] [CrossRef]
- Askari, N.; Askari, M.B.; Shafieipour, A. Investigation the molecular structure of novel graphene hybrid scaffold in nerve regeneration. J. Mol. Struct. 2019, 1186, 393–403. [Google Scholar] [CrossRef]
- Uehara, T.M.; Paino, I.M.M.; Santos, F.A.; Scagion, V.P.; Correa, D.S.; Zucolotto, V. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym. Adv. Technol. 2020, 31, 1437–1443. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kang, S.H.; Lee, J.H.; Kim, B.; Hong, S.W.; Han, D.W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J. Biomater. Sci. Polym. Ed. 2018, 29, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos. Part B Eng. 2019, 168, 421–431. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pourmokhtari, M.; Saeb, M.R.; Bakhshi, B.; Zomorrod, M.S.; Baheiraei, N. Electroactive cardiac patch containing reduced graphene oxide with potential antibacterial properties. Mater. Sci. Eng. C 2019, 104, 109921. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Tan, B.; Liang, S.; Zhang, N.; Wang, W.; Liu, W. A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials 2017, 122, 63–71. [Google Scholar] [CrossRef]
- Zhao, G.; Qing, H.; Huang, G.; Genin, G.M.; Lu, T.J.; Luo, Z.; Xu, F.; Zhang, X. Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues. NPG Asia Mater. 2018, 10, 982–994. [Google Scholar] [CrossRef]
- Huo, D.; Liu, G.; Li, Y.; Wang, Y.; Guan, G.; Yang, M.; Wei, K.; Yang, J.; Zeng, L.; Li, G. Construction of antithrombotic tissue-engineered blood vessel via reduced graphene oxide based dual-enzyme biomimetic cascade. ACS Nano 2017, 11, 10964–10973. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; et al. A new nanocomposite copolymer Based on functionalised Graphene oxide for Development of Heart Valves. Sci. Rep. 2020, 10, 5271. [Google Scholar] [CrossRef] [PubMed]
- Nazari, H.; Azadi, S.; Hatamie, S.; Zomorrod, M.S.; Ashtari, K.; Soleimani, M.; Hosseinzadeh, S. Fabrication of graphene-silver/polyurethane nanofibrous scaffolds for cardiac tissue engineering. Polym. Adv. Technol. 2019, 30, 2086–2099. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Yoo, H.; Yi, H.; Ahn, E.H.; Lee, J.H.; Shao, G.; Nagornyak, E.; Laflamme, M.A.; Murry, C.E.; Kim, D.-H. Micro-and nano-patterned conductive graphene–PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. 2017, 53, 7412–7415. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Baheiraei, N.; Mohseni, M.; Razavi, M.; Ghaderi, A.; Azizi, B.; Rabiee, N.; Karimi, M. Three-dimensional graphene foam as a conductive scaffold for cardiac tissue engineering. J. Biomater. Appl. 2019, 34, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing cell proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts by BMP-2 delivery in graphene oxide-incorporated PLGA/HA biodegradable microcarriers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Peng, M.; Yao, F.; Yang, Z.; Wan, Y. Effect of highly dispersed graphene and graphene oxide in 3D nanofibrous bacterial cellulose scaffold on cell responses: A comparative study. Mater. Chem. Phys. 2019, 235. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Chiang, W.-H.; Chen, I.-W.P.; Liu, W.-Y.; Chen, Y.-W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C 2017, 73, 726–735. [Google Scholar] [CrossRef]
- Wang, P.; Yu, T.; Lv, Q.; Li, S.; Ma, X.; Yang, G.; Xu, D.; Liu, X.; Wang, G.; Chen, Z.; et al. Fabrication of hydroxyapatite/hydrophilic graphene composites and their modulation to cell behavior toward bone reconstruction engineering. Colloids Surf. B Biointerfaces 2019, 173, 512–520. [Google Scholar] [CrossRef]
- Cabral, C.S.D.; Miguel, S.P.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. In situ green reduced graphene oxide functionalized 3D printed scaffolds for bone tissue regeneration. Carbon 2019, 146, 513–523. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 10, e2001414. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Q.; Pei, X. Graphene family materials in bone tissue regeneration: Perspectives and challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [CrossRef]
- Elkhenany, H.; Bourdo, S.; Hecht, S.; Donnell, R.; Gerard, D.; Abdelwahed, R.; Lafont, A.; Alghazali, K.; Watanabe, F.; Biris, A.S.; et al. Graphene nanoparticles as osteoinductive and osteoconductive platform for stem cell and bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Pathmanapan, S.; Periyathambi, P.; Anandasadagopan, S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In vitro and in vivo study. Nanomed. Nanotechnol. Biol. Med. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Shin, Y.C.; Lee, J.-J.; Bae, E.-B.; Jeon, Y.-C.; Jeong, C.-M.; Yun, M.-J.; Lee, S.-H.; Han, D.-W.; Huh, J.-B. The effect of reduced graphene oxide-coated biphasic calcium phosphate bone graft material on osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725. [Google Scholar] [CrossRef] [PubMed]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Jaidev, L.R.; Kumar, S.; Chatterjee, K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf. B Biointerfaces 2017, 159, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zheng, A.; Liu, Y.; Zhang, X.; Wang, X.; Wu, J.; She, W.; Lv, K.; Cao, L.; Jiang, X. Bidirectional differentiation of BMSCs induced by a biomimetic procallus based on a gelatin-reduced graphene oxide reinforced hydrogel for rapid bone regeneration. Bioact. Mater. 2021, 6, 2011–2028. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 40572. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-inspired electroactive and antioxidative scaffolds with incorporation of polydopamine-reduced graphene oxide for enhancing skin wound healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- An, J.; Le, T.-S.D.; Huang, Y.; Zhan, Z.; Li, Y.; Zheng, L.; Huang, W.; Sun, G.; Kim, Y.-J. All-graphene-based highly flexible noncontact electronic skin. ACS Appl. Mater. Interfaces 2017, 9, 44593–44601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, Q.; Zhao, Y.; Chen, F.; Wang, Z.; Zhang, Y.; Ni, H.; Deng, H.; Li, Y.; Chen, Y. Graphene oxide-modified electrospun polyvinyl alcohol nanofibrous scaffolds with potential as skin wound dressings. RSC Adv. 2017, 7, 28826–28836. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Electrospun poly (vinyl alcohol)/reduced graphene oxide nanofibrous scaffolds for skin tissue engineering. Colloids Surf. B Biointerfaces 2020, 191, 110994. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Choi, S.M.; Han, S.S. Biofabrication of Lysinibacillus sphaericus-reduced graphene oxide in three-dimensional polyacrylamide/carbon nanocomposite hydrogels for skin tissue engineering. Colloids Surfaces B Biointerfaces 2019, 181, 539–548. [Google Scholar] [CrossRef]
- Nyambat, B.; Chen, C.-H.; Wong, P.-C.; Chiang, C.-W.; Satapathy, M.K.; Chuang, E.-Y. Genipin-crosslinked adipose stem cell derived extracellular matrix-nano graphene oxide composite sponge for skin tissue engineering. J. Mater. Chem. B 2018, 6, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekar, S.; Palanisamy, P.; Jha, P.K.; Murugaraj, J.; Kandasamy, M.; Mohamed Hussain, A.M.K.; Sivaperumal, S. Natural Honeycomb Flavone Chrysin (5, 7-dihydroxyflavone)-Reduced Graphene Oxide Nanosheets Fabrication for Improved Bactericidal and Skin Regeneration. ACS Sustain. Chem. Eng. 2018, 6, 349–363. [Google Scholar] [CrossRef]
- Di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F.; Voli, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60. [Google Scholar] [CrossRef]
- Ur Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef]
- Frontiñán-Rubio, J.; Gómez, M.V.; Martín, C.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E. Differential effects of graphene materials on the metabolism and function of human skin cells. Nanoscale 2018, 10, 11604–11615. [Google Scholar] [CrossRef]
- Bagher, Z.; Asgari, N.; Bozorgmehr, P.; Kamrava, S.K.; Alizadeh, R.; Seifalian, A. Will Tissue-Engineering Strategies Bring New Hope for the Reconstruction of Nasal Septal Cartilage? Curr. Stem Cell Res. Ther. 2019, 15, 144–154. [Google Scholar] [CrossRef]
- Meng, Y.; Ye, L.; Coates, P.; Twigg, P. In Situ Cross-Linking of Poly(vinyl alcohol)/Graphene Oxide-Polyethylene Glycol Nanocomposite Hydrogels as Artificial Cartilage Replacement: Intercalation Structure, Unconfined Compressive Behavior, and Biotribological Behaviors. J. Phys. Chem. C 2018, 122, 3157–3167. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nowicki, M.; Cui, H.; Zhu, W.; Fang, X.; Miao, S.; Lee, S.J.; Keidar, M.; Zhang, L.G. 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow mesenchymal stem cells. Carbon N. Y. 2017, 116, 615–624. [Google Scholar] [CrossRef]
- Nayyer, L.; Jell, G.; Esmaeili, A.; Birchall, M.; Seifalian, A.M. A Biodesigned Nanocomposite Biomaterial for Auricular Cartilage Reconstruction. Adv. Healthc. Mater. 2016, 5, 1203–1212. [Google Scholar] [CrossRef]
- Seifalian, A.M.; Hancock, S. Composite Material and Its Method of Production. 2017. [Google Scholar]
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Neto, A.H.C.; Rosa, V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2017, 33, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Iaculli, F.; Di Filippo, E.S.; Piattelli, A.; Mancinelli, R.; Fulle, S. Dental pulp stem cells grown on dental implant titanium surfaces: An in vitro evaluation of differentiation and microRNAs expression. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 953–965. [Google Scholar] [CrossRef]
- De Marco, P.; Zara, S.; De Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; Di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 47, 6940–6946. [Google Scholar] [CrossRef]
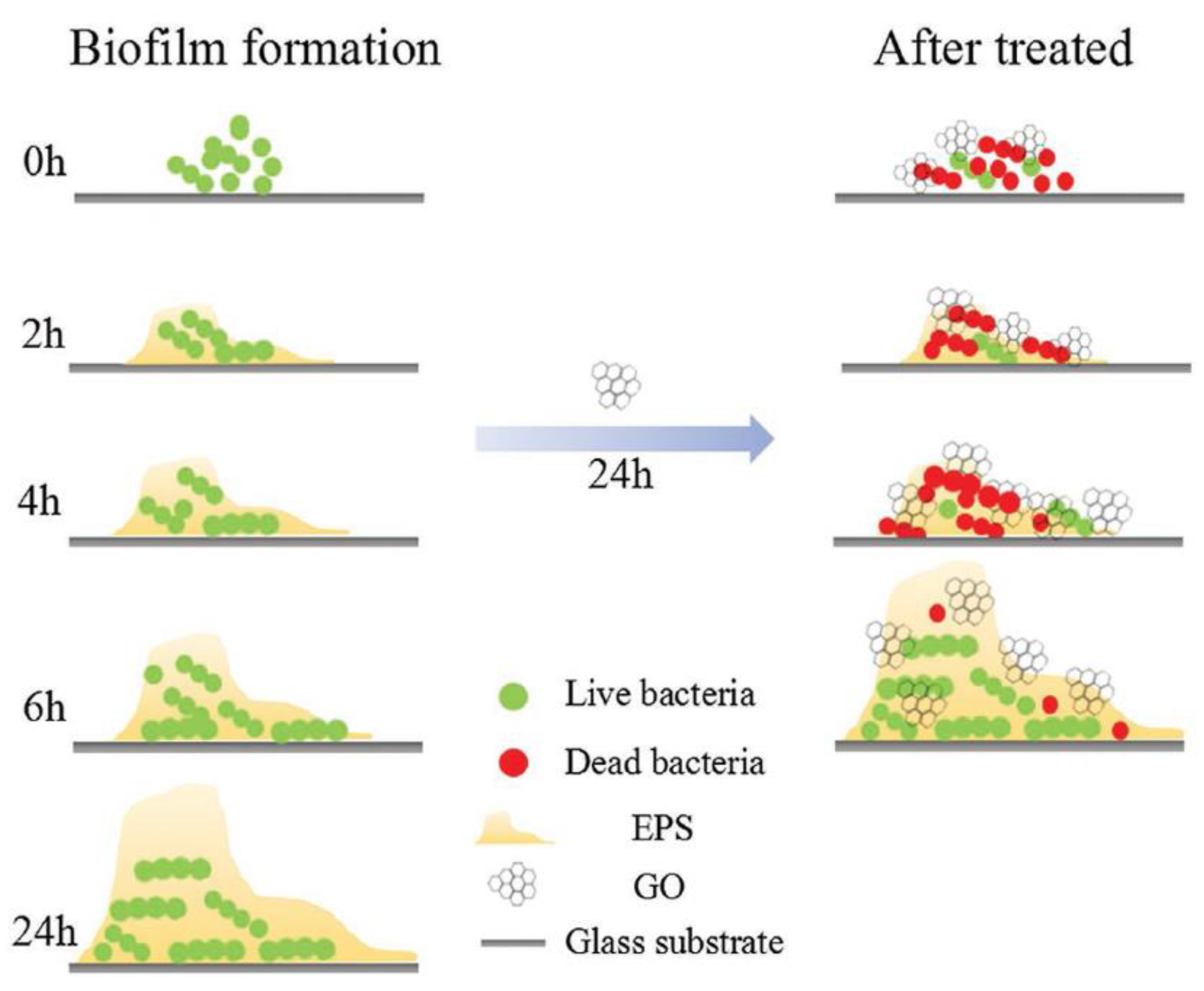
- He, J.; Zhu, X.; Qi, Z.; Wang, L.; Aldalbahi, A.; Shi, J.; Song, S.; Fan, C.; Lv, M.; Tang, Z. The Inhibition Effect of Graphene Oxide Nanosheets on the Development of Streptococcus mutans Biofilms. Part. Part. Syst. Charact. 2017, 34, 1–8. [Google Scholar] [CrossRef]



| Organs | Materials | Animal | Cells/Stem Cells | Experiment | Outcome | Year [Ref] Country |
|---|---|---|---|---|---|---|
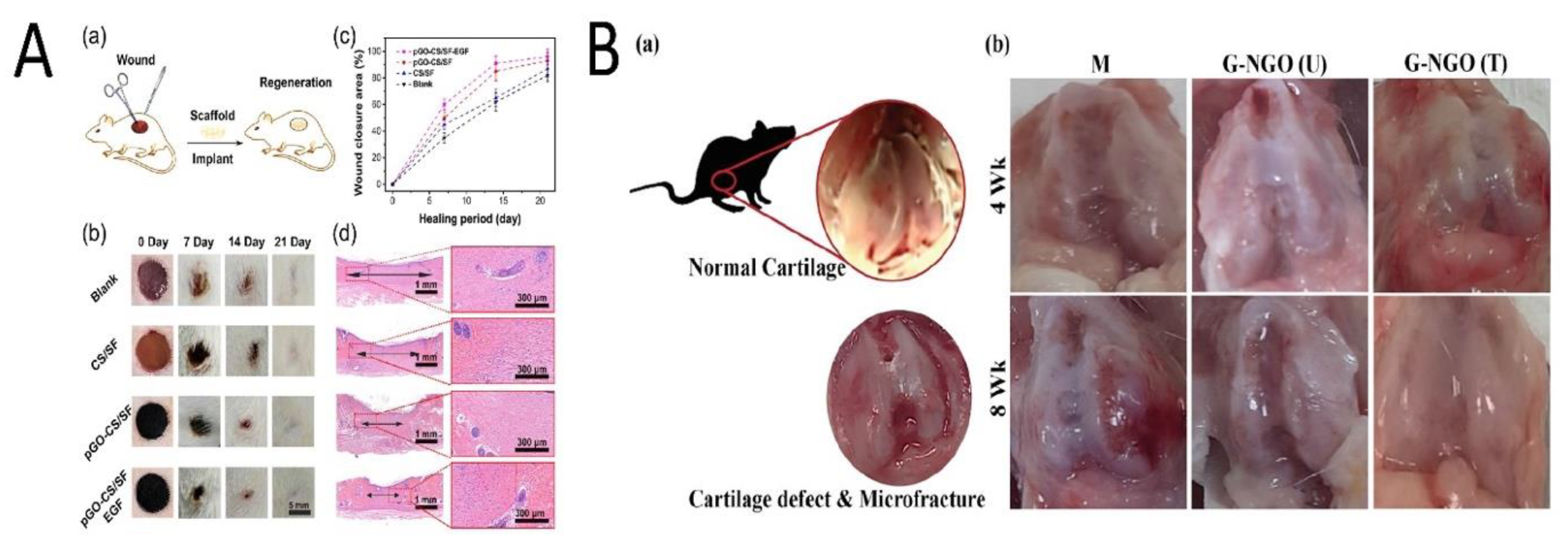
| Cartilage | Gelatin (G) and GO | Rats | Human chondrosarcoma cell, rat BMSCs, and rat chondrocyte cells | Preparing nano-GO (NGO) solution => hydrogel crosslinking => three groups: non-crosslinked hydrogel (NGO (U)), crosslinked hydrogel (NGO(T)), control group (G) | NGO(T) vs. NGO(U): ↑ mechanical properties. No significant cytotoxicity After implantation (8 weeks): fibrous tissue repair for U and Complete repair for T | 2020 [24] Taiwan |
| Nervous system | GO, antheraea pernyi silk fibroin (ApF) and PLCL | Rats | Schwann and PC12 cells | Coating GO on ApF/PLCL nanofibers => GO reduction => applying ES => preparing the AP/RGO nerve guidance conduit | ↑ CPAM and ↑ MPs. GO: ↑ focal adhesion kinase expression of PC12 cells. ↑ Repair in animal model’s sciatic nerve | 2019 [25] China |
| Muscle | GO, rGO, polyacry- lamide (PAAm) | Mice | C2C12 myoblasts | Incorporating GO into PAAm (GO-PAAm) => micropatterning of GO-PAAm with femtosecond laser ablation (FLA) => production of micropatterned conductive r(GO/PAAm) | Micropatterned: ↑ differentiation and myoblast alignment r(GO/PAAm) vs. GO/PAAm: ↓ impedance values PD50/r(GO/PAAm) (optimum): ↑ tissue compatibility and ES => ↑ myogenesis. | 2019 [26] Korea |
| Heart | GO, rGO and alginate | Rats with MI | Human mesenchymal stem cells | GO/Ag blend => hMSCs encapsulation => electrospraying and then crosslinking => GO/Ag microgels => reductive treatment => r(GO/Ag) | rGO vs. GO: ↑ CPAM, ↑ antioxidant activity, and ↓ Oxidative stress hMSCs-CMs vs. CMs: ↑ cell viability and cardiac maturation => expressing cardiac markers | 2019 [27] Korea |
| Bone | GO and poly(ɛ-caprolactone) | Rat | MC3T3 preosteoblastic cells | Synthesizing GO => PCL/GO pellets => melt blending => 3D printing | ↑ Protein absorbent and ↑ CPAM ↓ Immunogenicity. Treating a rat calvaria critical size defect => well-organized tissue deposition and bone remodeling. | 2019 [28] UK |
| Skin | Polydopamine (P), rGO (pGO), chitosan (CS), and silk fibroin (SF) (pGO-CS/SF) | Rats with a full-thickness skin defect | RAW 2467 cells and C2C12 myoblast cells | Dispersing pGO into CS/SF mixture=> dual-crosslinking by poly(ethylene glycol) diglycidyl ether (PEGDE) and glutaraldehyde (GA) => freeze-drying => pGO-CS/SF scaffold | ↑ CPAM and ↑ mechanical properties ↑ Antioxidant activity => reduce cellular oxidation. Well-connected electric pathway ↑ Wound healing and ↓ oxidative stress and inflammatory responses | 2019 [29] China |
| Nervous system | Single (SG) and multilayered (GM) graphene PCL, RGD, polydopamine | Rats | Schwann cells | Fabricating nanoscaffolds => seeding Schwann cells => implanting the 3D scaffold in sciatic nerve defect models. | ↑ CPAM and ↑ neural cell expressionPDA/RGD-SG/PCL and PDA/RGD-MG/PCL nerve conduits: ↑ neural regeneration | 2018 [30] China |
| Heart | GO, gold nanoparticles (AuNPs) (GO-AuNPs), chitosan (CS) | Rats with MI | Rat smooth muscle cells, mouse fibroblasts, and human iPSC-CMs | GO => embedding with AuNPs by thermal-reduction => GO-AuNPs => CS solution addition and freeze-drying => CS-GO-Au scaffolds | ↑ Electrical conductivity (at 0.5% w/v GO- AuNPs). ↑ CPAM, no immune response ↑ QRS interval (by ↑ conduction velocity and ↑ contractility). ↑ Connexin43 (Cx43) ↑ Electrical conduction and ventricular function | 2018 [31] Canada |
| Dental | GO, chitosan (CS), hydroxyapatite (HA) and Titanium (Ti) | Rats | Bone marrow stromal cells (BMSCs) | Coating GO/CS/HA on Ti substrates by electrophoretic deposition (EPD) | ↑ CPAM and ↑ osseointegration in vivo | 2018 [32] China |
| Dental | GO and Collagen | Dog | Mouse osteoblastic MC3T3-E1 cells | Coating Ti on the 3D collagen scaffold => evaluation of bone augmentation on the rat cranial bone => assessing the periodontal healing of class II furcation defects | ↓ Cytotoxicity. GO: cellular ingrowth behavior and angiogenesis => ↑ rat bone augmentation ↑ Periodontal attachment | 2018 [33] Japan |
| Nervous system | GO, rGO, and Gelatin | Rats | Embryonic neural progenitor cells | Synthesizing rGO microfibers from GO => assembling rGO microfibers into the 3D gelatin hydrogel for stable implantation | Microfiber coated with adhesive molecules => interconnected culture ↑ Differentiation in the defect site | 2017 [34] Spain |
| Bone | GO and chitosan (CHT) | Mice | Murine preosteoblasts belonging to the 3T3-E1 cell line | CHT/GO blend => freeze-drying | ↑ Alkaline phosphatase activity (ALP), ↑ osteogenesis, and ↑ bone morphogenetic protein expression ↑ Differentiation of osteoprogenitor cells ↑ New bone formation | 2017 [35] Romania |
| Bone | rGO and nanohydroxyapatite (nHA) | Rabbits | Bone mesenchymal stem cells | Self-assembling of GO and nHA => nHA@RGO | ↑ CPAM, ↑ ALP, and ↑ osteogenic gene expression ↑ Healing circular calvarial defects (optimum: 20% nHA@RGO) ↑ Collagen deposition and ↑ mineralization | 2017 [11] China |
| Tissue | Stem cells |
|---|---|
| Bone | hMSCs, hADMSCs, MC3T3-E1, DPSCs, PDLSCs |
| Nerve | NSCs, hMSCs, hADMSCs, ESCs, iPSCs, SCAP |
| Muscle and cardiac | C2C12, MSCs, hMSCs, cardiomyocytes, and EC |
| Cartilage | Human mesenchymal stem cell |
| Skin | MSCs, human dermal fibroblasts (HDFs) |
| Dental | DPSCs, PDLSCs, hMSCs, BMSCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials 2021, 11, 1083. https://doi.org/10.3390/nano11051083
Zare P, Aleemardani M, Seifalian A, Bagher Z, Seifalian AM. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials. 2021; 11(5):1083. https://doi.org/10.3390/nano11051083
Chicago/Turabian StyleZare, Pariya, Mina Aleemardani, Amelia Seifalian, Zohreh Bagher, and Alexander M. Seifalian. 2021. "Graphene Oxide: Opportunities and Challenges in Biomedicine" Nanomaterials 11, no. 5: 1083. https://doi.org/10.3390/nano11051083
APA StyleZare, P., Aleemardani, M., Seifalian, A., Bagher, Z., & Seifalian, A. M. (2021). Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials, 11(5), 1083. https://doi.org/10.3390/nano11051083







