Characteristics of P-Type and N-Type Photoelectrochemical Biosensors: A Case Study for Esophageal Cancer Detection
Abstract
1. Introduction
2. Materials and Methods
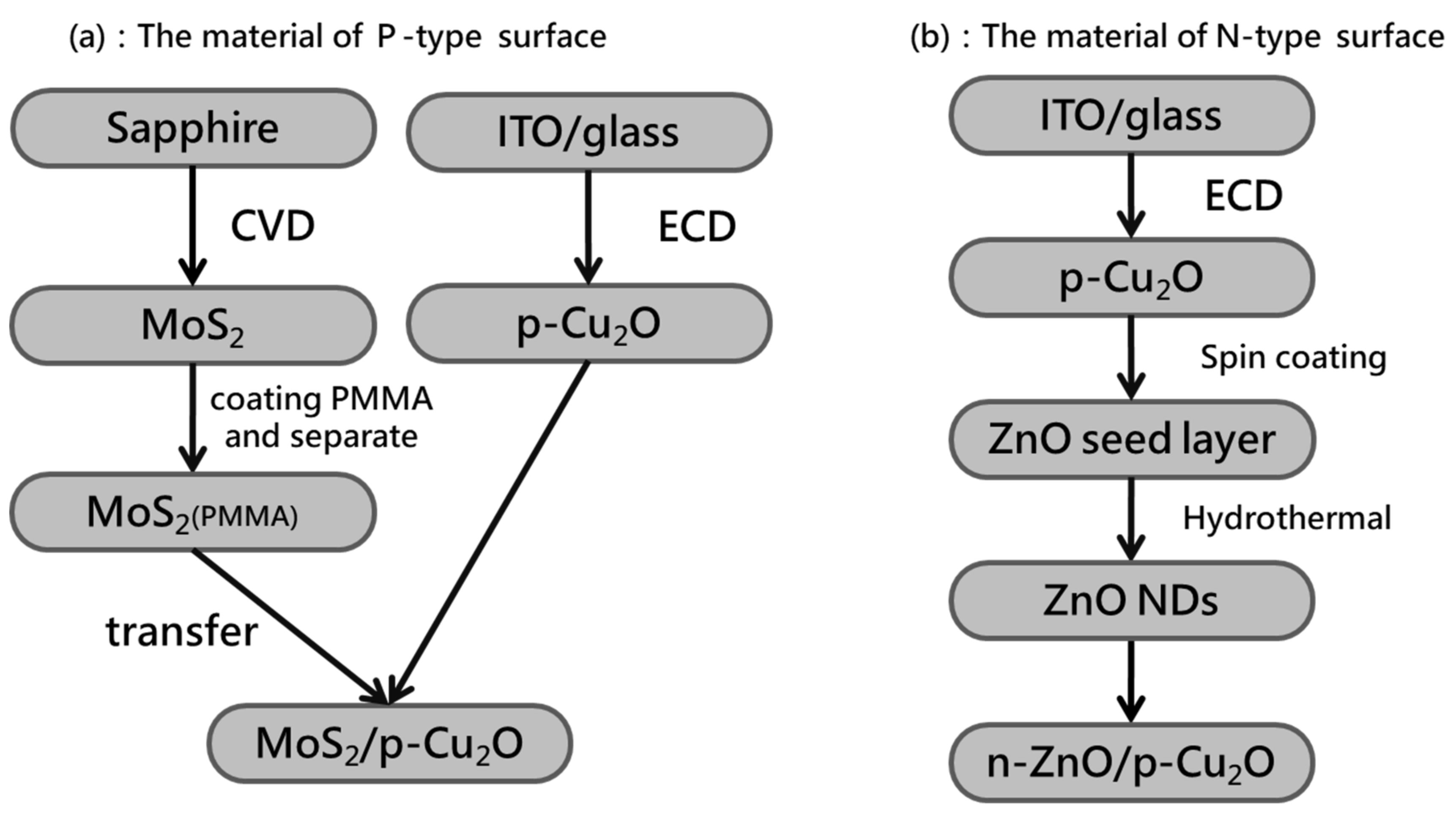
2.1. Preparation of P-Type Biosensor
2.2. Preparation of N-Type Biosensor
2.3. Cell Culture
2.4. Optical and Material Analysis Instruments
3. Results and Discussion
3.1. Material Structure Characteristics
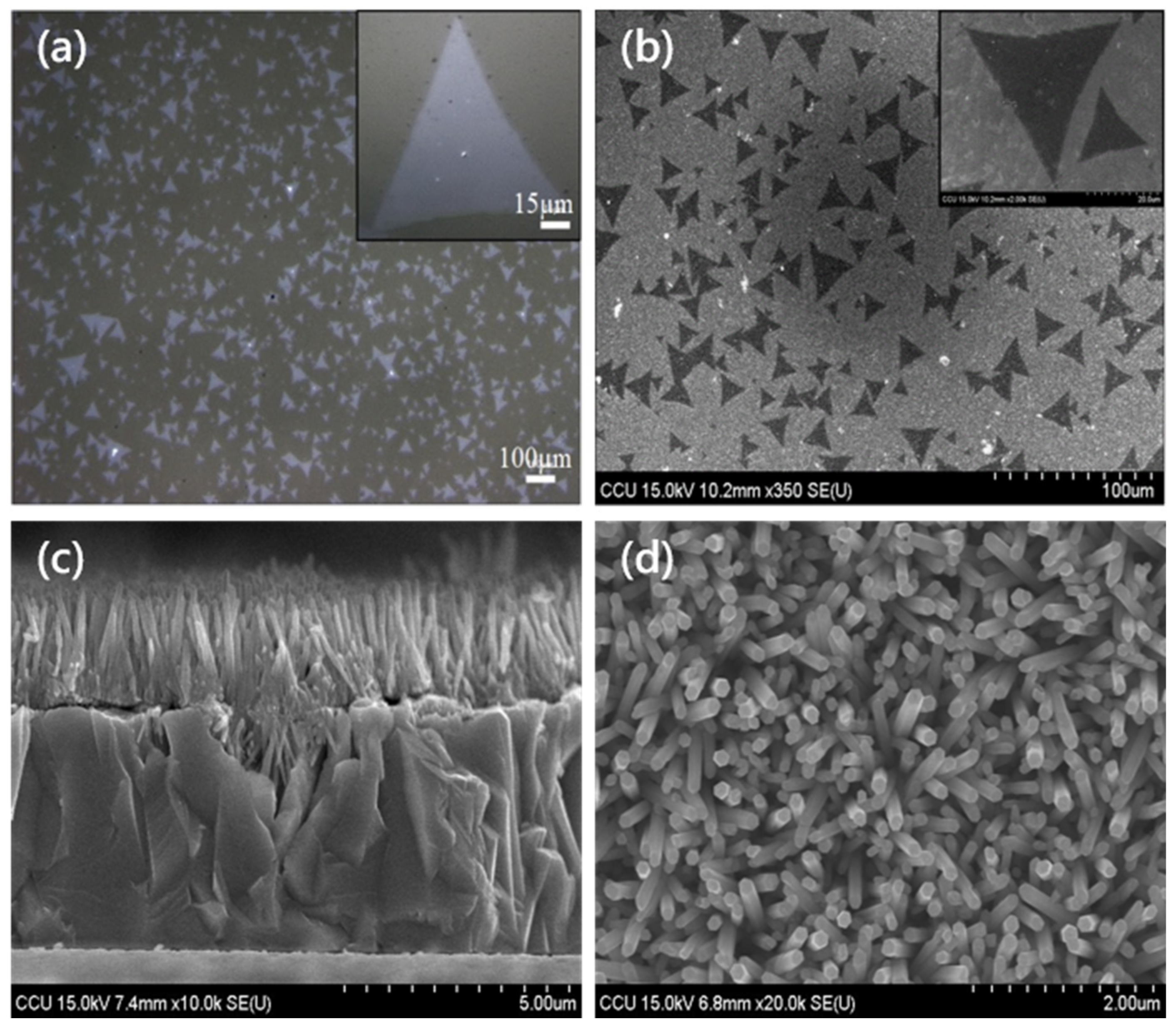
3.1.1. Optical Microscope (OM) and Scanning Electron Microscope (SEM) Images
3.1.2. X-ray Diffraction (XRD) Analysis
3.1.3. Absorption Analysis
3.2. Photoelectrochemical Response Analysis
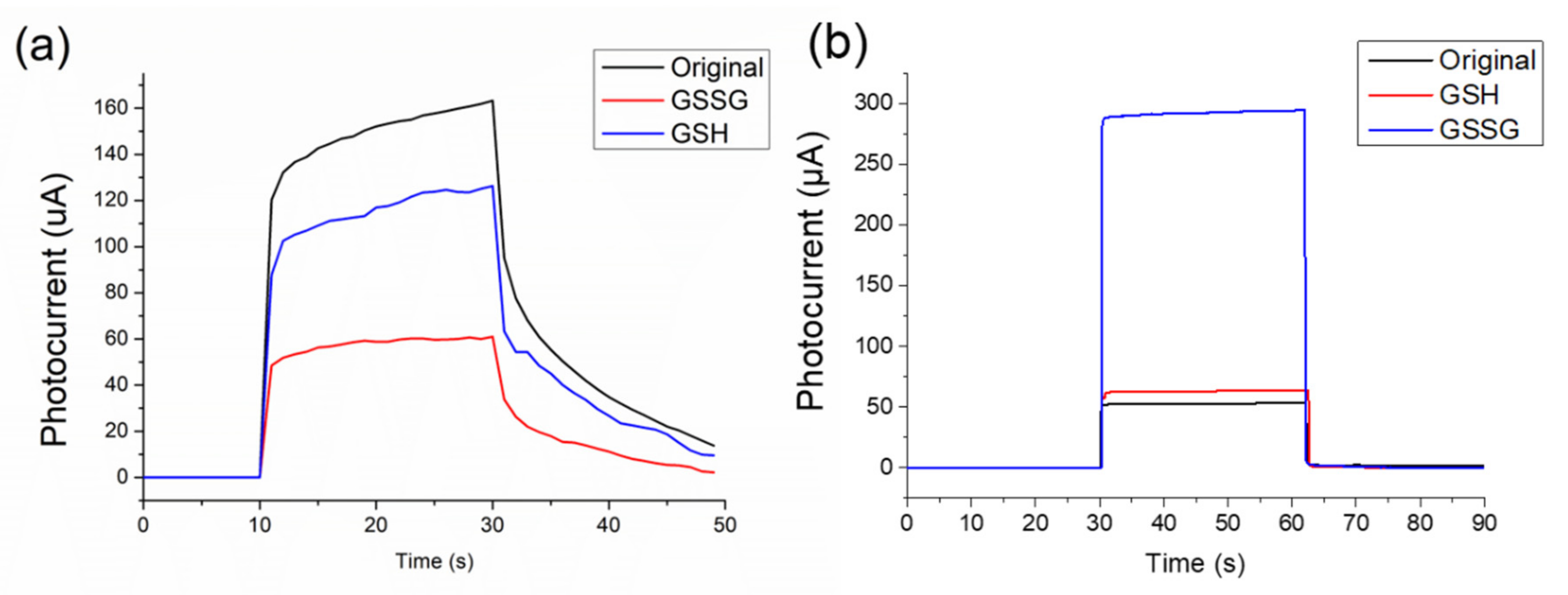
3.2.1. P-Type Structure for Detection of Glutathione
3.2.2. N-Type Structure for Detection of Glutathione
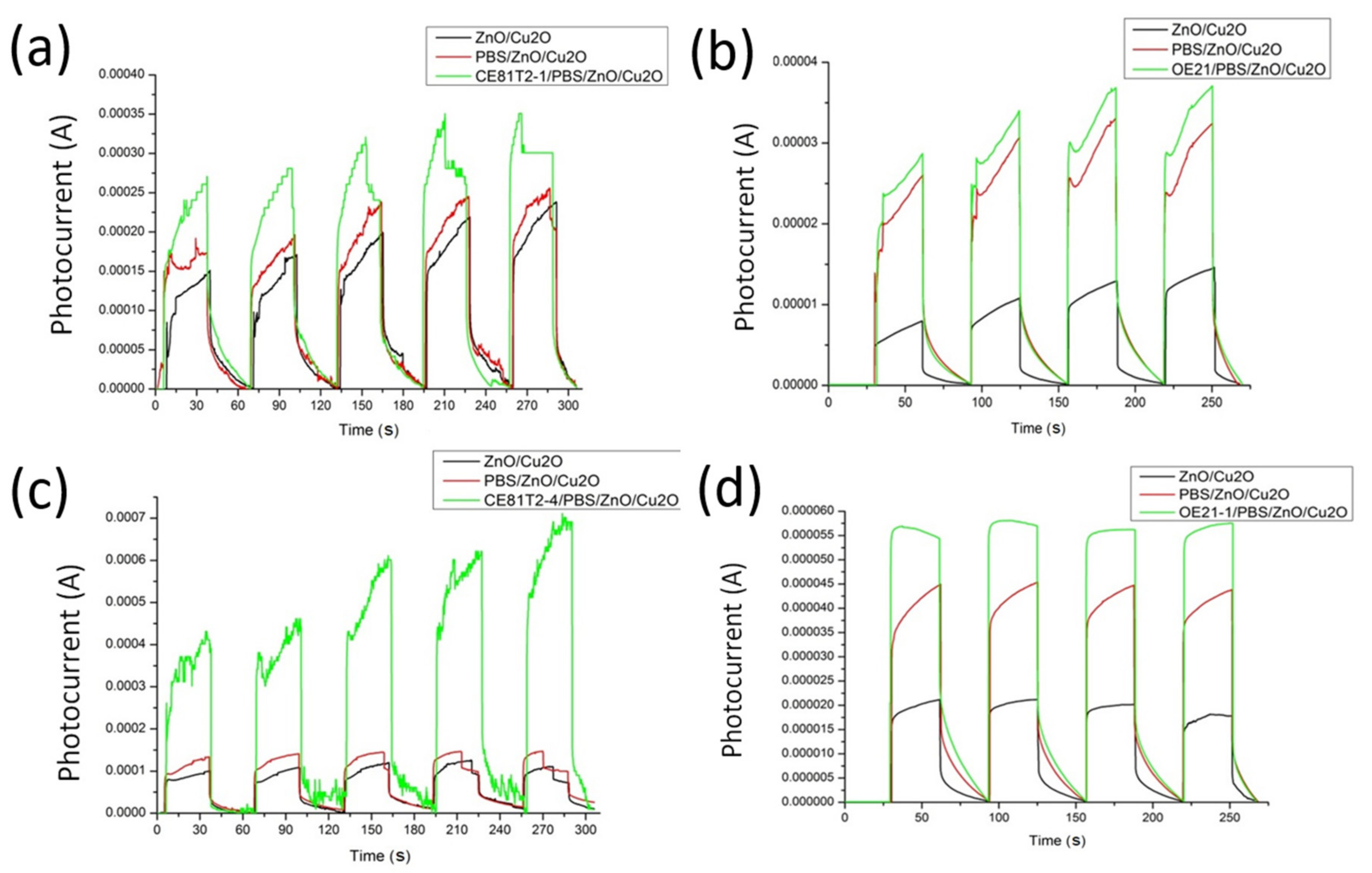
3.2.3. P-Type Structure for Distinguishing Cancer Cell Stage
3.2.4. N-Type Structure for Distinguishing Cancer Cell Stage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Rover, L., Jr.; Kubota, L.T.; Höehr, N.F. Development of an amperometric biosensor based on glutathione peroxidase immobilized in a carbodiimide matrix for the analysis of reduced glutathione from serum. Clin. Chim. Acta 2001, 308, 55–67. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Kalkan, I.H.; Suher, M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak. J. Med. Sci. 2013, 29, 938–942. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef]
- Alghobashy, A.A.; Alkholy, U.M.; Talat, M.A.; Abdalmonem, N.; Zaki, A.; Ahmed, I.A.; Mohamed, R.H. Trace elements and oxidative stress in children with type 1 diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 85–92. [Google Scholar] [CrossRef]
- Ferlita, S.; Yegiazaryan, A.; Noori, N.; Lal, G.; Nguyen, T.; To, K.; Venketaraman, V. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J. Clin. Med. 2019, 8, 2219. [Google Scholar] [CrossRef]
- Shimizu, H.; Kiyohara, Y.; Kato, I.; Kitazono, T.; Tanizaki, Y.; Kubo, M.; Ueno, H.; Ibayashi, S.; Fujishima, M.; Iida, M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: The Hisayama study. Stroke 2004, 35, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kirsch, M.; Khouzami, L.; Caramelle, P.; le Corvoisier, P.; Roudot-Thoraval, F.; Dubois-Randé, J.-L.; Hittinger, L.; Pavoine, C.; Pecker, F. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS ONE 2009, 4, e4871. [Google Scholar] [CrossRef]
- Campolo, J.; Bernardi, S.; Cozzi, L.; Rocchiccioli, S.; Dellanoce, C.; Cecchettini, A.; Tonini, A.; Parolini, M.; de Chiara, B.; Micheloni, G. Medium-term effect of sublingual L-glutathione supplementation on flow-mediated dilation in subjects with cardiovascular risk factors. Nutrition 2017, 38, 41–47. [Google Scholar] [CrossRef]
- Bellanti, F.; Romano, A.D.; Buglio, A.L.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Singh, S.; Khan, A.R.; Gupta, A.K. Role of glutathione in cancer pathophysiology and therapeutic interventions. J. Exp. Ther. Oncol. 2012, 9, 303–316. [Google Scholar] [PubMed]
- Palminteri, M.; Dhakar, N.K.; Ferraresi, A.; Caldera, F.; Vidoni, C.; Trotta, F.; Isidoro, C. Cyclodextrin nanosponge for the GSH-mediated delivery of Resveratrol in human cancer cells. Nanotheranostics 2021, 5, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, L.; Jia, J.; Eltayeb, O.; Lu, W.; Tang, Y.; Dong, C.; Shuang, S. Dual photoluminescence emission carbon dots for ratiometric fluorescent GSH sensing and cancer cell recognition. ACS Appl. Mater. Interfaces 2020, 12, 18250–18257. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wang, X.; Wu, Y.; Dong, C.; Shuang, S. Dual role of BSA for synthesis of MnO2 nanoparticles and their mediated fluorescent turn-on probe for glutathione determination and cancer cell recognition. Analyst 2019, 144, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Li, H.; Ning, S.; Ghandi, M.; Kryukov, G.V.; Gopal, S.; Deik, A.; Souza, A.; Pierce, K.; Keskula, P.; Hernandez, D. The landscape of cancer cell line metabolism. Nat. Med. 2019, 25, 850–860. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. A colorimetric sensor array for protein discrimination based on carbon nanodots-induced reversible aggregation of AuNP with GSH as a regulator. Sens. Actuators B Chem. 2019, 296, 126677. [Google Scholar] [CrossRef]
- Niu, L.-Y.; Guan, Y.-S.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef]
- Mandal, P.K.; Tripathi, M.; Sugunan, S. Brain oxidative stress: Detection and mapping of anti-oxidant marker ‘Glutathione’in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem. Biophys. Res. Commun. 2012, 417, 43–48. [Google Scholar] [CrossRef]
- Rae, C.D.; Williams, S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017, 529, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Lagopoulos, J.; Hermens, D.F.; Tobias-Webb, J.; Duffy, S.; Naismith, S.; White, D.; Scott, E.; Hickie, I. In vivo glutathione levels in young persons with bipolar disorder: A magnetic resonance spectroscopy study. J. Psychiatr. Res. 2013, 47, 412–417. [Google Scholar] [CrossRef]
- Hun, X.; Wang, S.; Wang, S.; Zhao, J.; Luo, X. A photoelectrochemical sensor for ultrasensitive dopamine detection based on single-layer NanoMoS2 modified gold electrode. Sens. Actuators B Chem. 2017, 249, 83–89. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Tu, W.; Dong, Y.; Lei, J.; Ju, H. Low-potential photoelectrochemical biosensing using porphyrin-functionalized TiO2 nanoparticles. Anal. Chem. 2010, 82, 8711–8716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, S.; Shen, Q.; Jiang, L.-P.; Zhu, J.-J. Fabrication of glutathione photoelectrochemical biosensor using graphene-CdS nanocomposites. Analyst 2012, 137, 3697–3703. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Wu, C.; Li, C.-Z.; Jiang, H.; Wang, X. Photoelectrocatalytic oxidation of glutathione based on porous TiO2-Pt nanowhiskers. Langmuir 2012, 28, 12393–12399. [Google Scholar] [CrossRef]
- Tang, J.; Kong, B.; Wang, Y.; Xu, M.; Wang, Y.; Wu, H.; Zheng, G. Photoelectrochemical detection of glutathione by IrO2-Hemin-TiO2 nanowire arrays. Nano Lett. 2013, 13, 5350–5354. [Google Scholar] [CrossRef]
- Kang, Z.; Gu, Y.; Yan, X.; Bai, Z.; Liu, Y.; Liu, S.; Zhang, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Enhanced photoelectrochemical property of ZnO nanorods array synthesized on reduced graphene oxide for self-powered biosensing application. Biosens. Bioelectron. 2015, 64, 499–504. [Google Scholar] [CrossRef]
- Svitkova, V.; Palchetti, I. Functional polymers in photoelectrochemical biosensing. Bioelectrochemistry 2020, 107590. [Google Scholar] [CrossRef]
- Chen, C.K.; Shen, Y.P.; Chen, H.M.; Chen, C.J.; Chan, T.S.; Lee, J.F.; Liu, R.S. Quantum-dot-sensitized nitrogen-doped ZnO for efficient photoelectrochemical water splitting. Eur. J. Inorg. Chem. 2014, 2014, 773–779. [Google Scholar] [CrossRef]
- Fan, G.-C.; Han, L.; Zhu, H.; Zhang, J.-R.; Zhu, J.-J. Ultrasensitive photoelectrochemical immunoassay for matrix metalloproteinase-2 detection based on CdS: Mn/CdTe cosensitized TiO2 nanotubes and signal amplification of SiO2@ Ab2 conjugates. Anal. Chem. 2014, 86, 12398–12405. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Lim, H.N.; Abou-Zied, O.K.; Huang, N.M.; Estrela, P.; Pandikumar, A. Cadmium sulfide nanoparticles decorated with au quantum dots as ultrasensitive photoelectrochemical sensor for selective detection of copper (II) ions. J. Phys. Chem. C 2016, 120, 22202–22214. [Google Scholar] [CrossRef]
- Hu, C.; Zheng, J.; Su, X.; Wang, J.; Wu, W.; Hu, S. Ultrasensitive all-carbon photoelectrochemical bioprobes for zeptomole immunosensing of tumor markers by an inexpensive visible laser light. Anal. Chem. 2013, 85, 10612–10619. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Tang, J.; Zhang, Y.; Quan, Y.; Gong, X.; Al-Enizi, A.M.; Elzatahry, A.A.; Zhang, L.; Zheng, G. Photoelectrochemical conversion from graphitic C3N4 quantum dot decorated semiconductor nanowires. ACS Appl. Mater. Interfaces 2016, 8, 12772–12779. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Zhuang, Y.-H.; Shan, D.; Su, G.-F.; Cosnier, S.; Zhang, X.-J. Zirconium-based porphyrinic metal-organic framework (PCN-222): Enhanced photoelectrochemical response and its application for label-free phosphoprotein detection. Anal. Chem. 2016, 88, 11207–11212. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-C.; Weng, Y.-H.; Lu, M.-Y.; Jen, C.-P.; Fedorov, V.E.; Chen, W.C.; Wu, M.T.; Kuo, C.-T.; Wang, H.-C. Nano-structure ZnO/Cu2O photoelectrochemical and self-powered biosensor for esophageal cancer cell detection. Opt. Express 2017, 25, 7689–7706. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Yan, X.; Wang, Y.; Bai, Z.; Liu, Y.; Zhang, Z.; Lin, P.; Zhang, X.; Yuan, H.; Zhang, X. Electronic structure engineering of Cu2O film/ZnO nanorods array all-oxide pn heterostructure for enhanced photoelectrochemical property and self-powered biosensing application. Sci. Rep. 2015, 5, 7882. [Google Scholar] [CrossRef]
- Cushing, S.K.; Wu, N. Progress and perspectives of plasmon-enhanced solar energy conversion. J. Phys. Chem. Lett. 2016, 7, 666–675. [Google Scholar] [CrossRef]
- Lin, P.; Chen, X.; Yan, X.; Zhang, Z.; Yuan, H.; Li, P.; Zhao, Y.; Zhang, Y. Enhanced photoresponse of Cu2O/ZnO heterojunction with piezo-modulated interface engineering. Nano Res. 2014, 7, 860–868. [Google Scholar] [CrossRef]
- Tseng, K.-W.; Hsiao, Y.-P.; Jen, C.-P.; Chang, T.-S.; Wang, H.-C. Cu2O/PEDOT: PSS/ZnO Nanocomposite Material Biosensor for Esophageal Cancer Detection. Sens. Actuators B Chem. 2020, 20, 2455. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Liao, C.-H.; Chueh, Y.-L.; Lai, C.-C.; Chen, L.-Y.; Chu, A.-K.; Kuo, C.-T.; Wang, H.-C. High performance Cu2O/ZnO core-shell nanorod arrays synthesized using a nanoimprint GaN template by the hydrothermal growth technique. Opt. Mater. Express 2014, 4, 1473–1486. [Google Scholar] [CrossRef]
- Zang, Y.; Hu, X.; Zhou, H.; Xu, Q.; Huan, P.; Xue, H. Enhanced photoelectrochemical behavior of CdS/WS2 heterojunction for sensitive glutathione biosensing in human serum. Int. J. Electrochem. Sci. 2018, 13, 7558–7570. [Google Scholar] [CrossRef]
- Liu, Q.; Bao, J.; Yang, M.; Wang, X.; Lan, S.; Hou, C.; Wang, Y.; Fa, H. A core-shell MWCNT@ rGONR heterostructure modified glassy carbon electrode for ultrasensitive electrochemical detection of glutathione. Sens. Actuators B Chem. 2018, 274, 433–440. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Gao, Y.; Chen, F.-Z.; Fan, G.-C.; Han, D.-M.; Wang, C.; Zhao, W.-W. Fast electrochemical deposition of CuO/Cu2O heterojunction photoelectrode: Preparation and application for rapid cathodic photoelectrochemical detection of L-cysteine. Sens. Actuators B Chem. 2019, 290, 312–317. [Google Scholar] [CrossRef]
- Li, H.; Wen, Y.; Zhu, X.; Wang, J.; Zhang, L.; Sun, B. Novel heterostructure of a MXene@ NiFe-LDH nanohybrid with superior peroxidase-like activity for sensitive colorimetric detection of glutathione. ACS Sustain. Chem. Eng. 2019, 8, 520–526. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef]
- Ranjan, P.; Parihar, A.; Jain, S.; Kumar, N.; Dhand, C.; Murali, S.; Mishra, D.; Sanghi, S.K.; Chaurasia, J.; Srivastava, A.K. Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: A comprehensive review. Anal. Biochem. 2020, 113996. [Google Scholar] [CrossRef]
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2011, 4. [Google Scholar] [CrossRef]
- Li, K.-C.; Lu, M.-Y.; Nguyen, H.T.; Feng, S.-W.; Artemkina, S.B.; Fedorov, V.E.; Wang, H.-C. Intelligent Identification of MoS2 Nanostructures with Hyperspectral Imaging by 3D-CNN. Nanomaterials 2020, 10, 1161. [Google Scholar] [CrossRef]
- Wang, J.-P.; WD, C. Oxidation behavior of pure copper in oxygen and/or water vapor at intermediate temperature. ISIJ Int. 2009, 49, 1926–1931. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Su, S.-H.; Chang, J.-K.; Tsai, D.-S.; Chen, C.-H.; Wu, C.-I.; Li, L.-J.; Chen, L.-J.; He, J.-H. Monolayer MoS2 heterojunction solar cells. ACS Nano 2014, 8, 8317–8322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Xue, Y.; Fang, H.; Wang, W. Facile electrodeposition of environment-friendly Cu2O/ZnO heterojunction for robust photoelectrochemical biosensing. Sens. Actuators B Chem. 2014, 191, 619–624. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. Single-phase Cu2O and CuO thin films obtained by low-temperature oxidation processes. J. Alloy. Compd. 2018, 737, 718–724. [Google Scholar] [CrossRef]
- Shen, Z.-Y.; Shen, W.-Y.; Chen, M.-H.; Shen, J.; Zeng, Y. Reactive oxygen species and antioxidants in apoptosis of esophageal cancer cells induced by As2O3. Int. J. Mol. Med. 2003, 11, 479–484. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, J.-H.; Nguyen, H.-T.; Feng, S.-W.; Artemkina, S.B.; Fedorov, V.E.; Hsieh, S.-C.; Wang, H.-C. Characteristics of P-Type and N-Type Photoelectrochemical Biosensors: A Case Study for Esophageal Cancer Detection. Nanomaterials 2021, 11, 1065. https://doi.org/10.3390/nano11051065
Leung J-H, Nguyen H-T, Feng S-W, Artemkina SB, Fedorov VE, Hsieh S-C, Wang H-C. Characteristics of P-Type and N-Type Photoelectrochemical Biosensors: A Case Study for Esophageal Cancer Detection. Nanomaterials. 2021; 11(5):1065. https://doi.org/10.3390/nano11051065
Chicago/Turabian StyleLeung, Joseph-Hang, Hong-Thai Nguyen, Shih-Wei Feng, Sofya B. Artemkina, Vladimir E. Fedorov, Shang-Chin Hsieh, and Hsiang-Chen Wang. 2021. "Characteristics of P-Type and N-Type Photoelectrochemical Biosensors: A Case Study for Esophageal Cancer Detection" Nanomaterials 11, no. 5: 1065. https://doi.org/10.3390/nano11051065
APA StyleLeung, J.-H., Nguyen, H.-T., Feng, S.-W., Artemkina, S. B., Fedorov, V. E., Hsieh, S.-C., & Wang, H.-C. (2021). Characteristics of P-Type and N-Type Photoelectrochemical Biosensors: A Case Study for Esophageal Cancer Detection. Nanomaterials, 11(5), 1065. https://doi.org/10.3390/nano11051065







