Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation ZnO and Al/ZnO Photocatalysts
2.2. Characterization
2.3. Photocatalytic Measurement
3. Results and Discussion
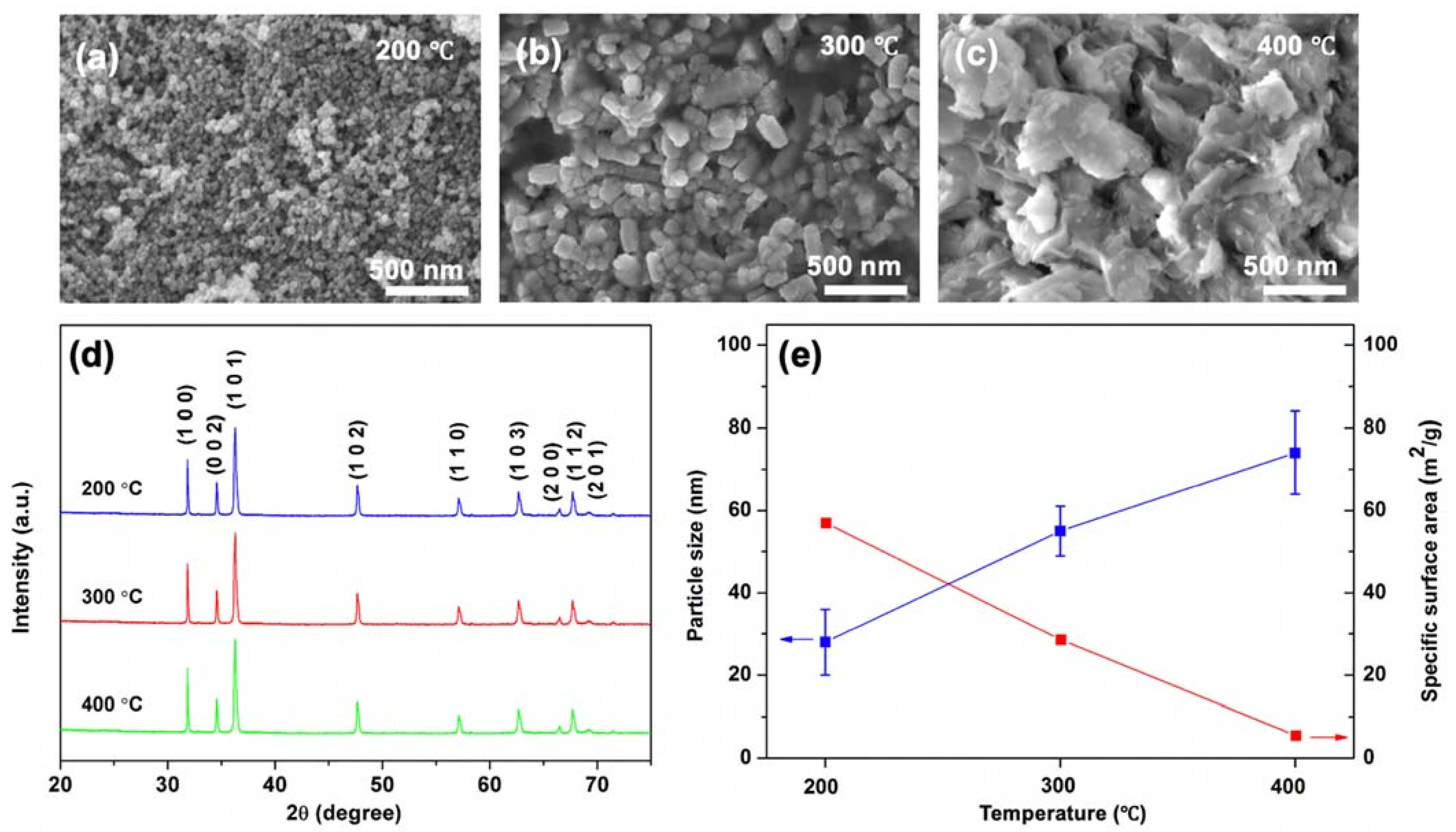
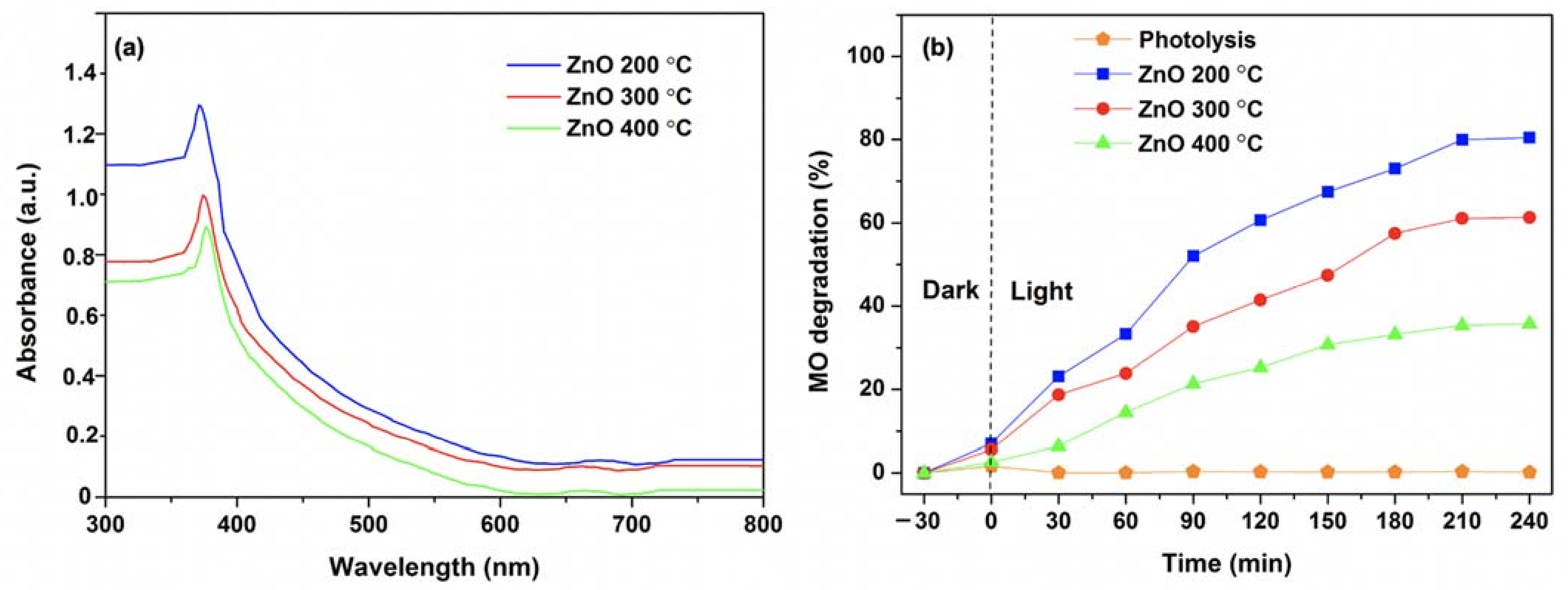
3.1. Effect of Calcination Temperature on the Properties of ZnO NPs
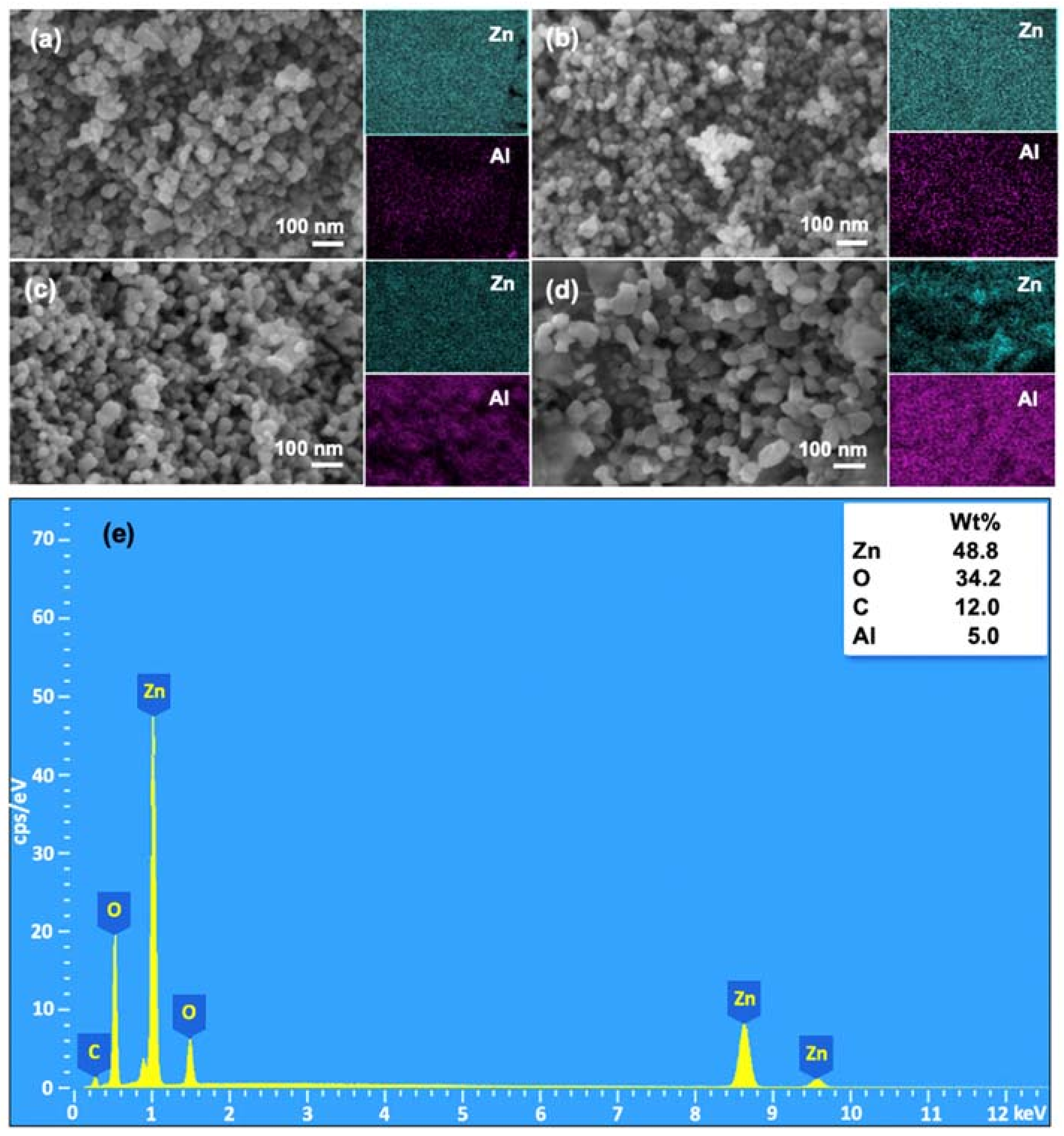
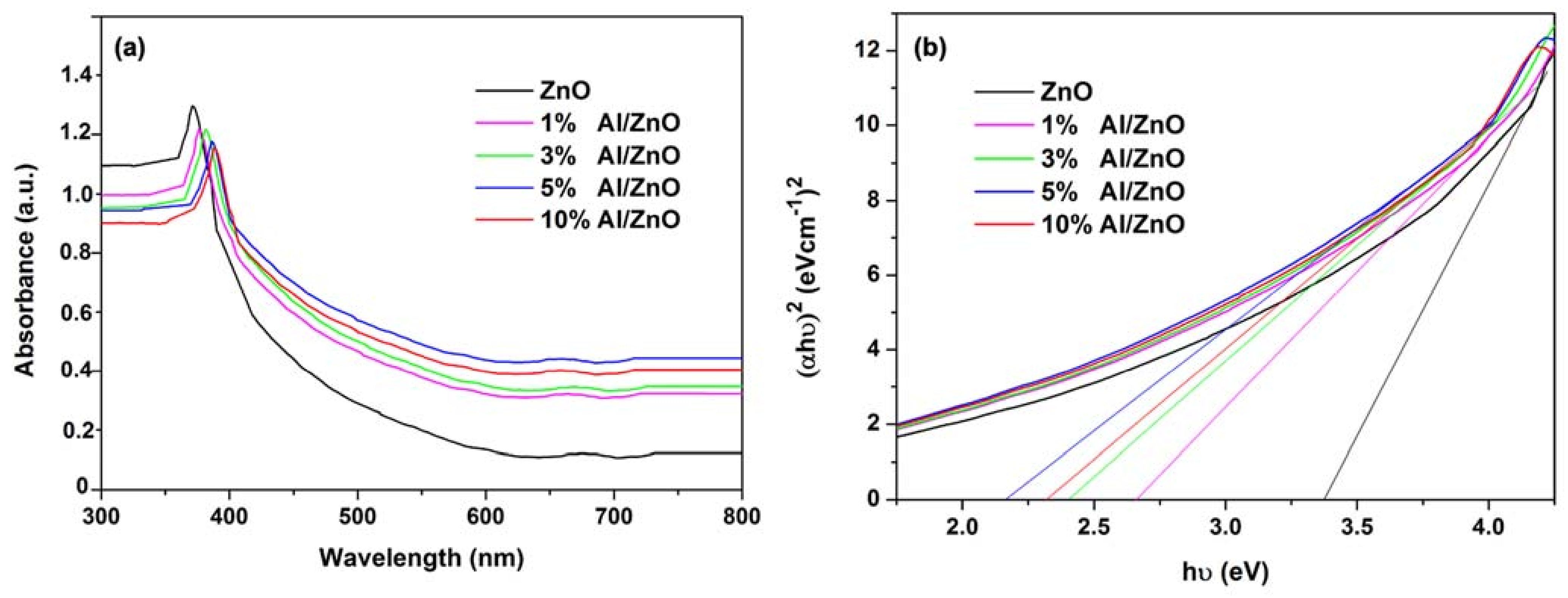
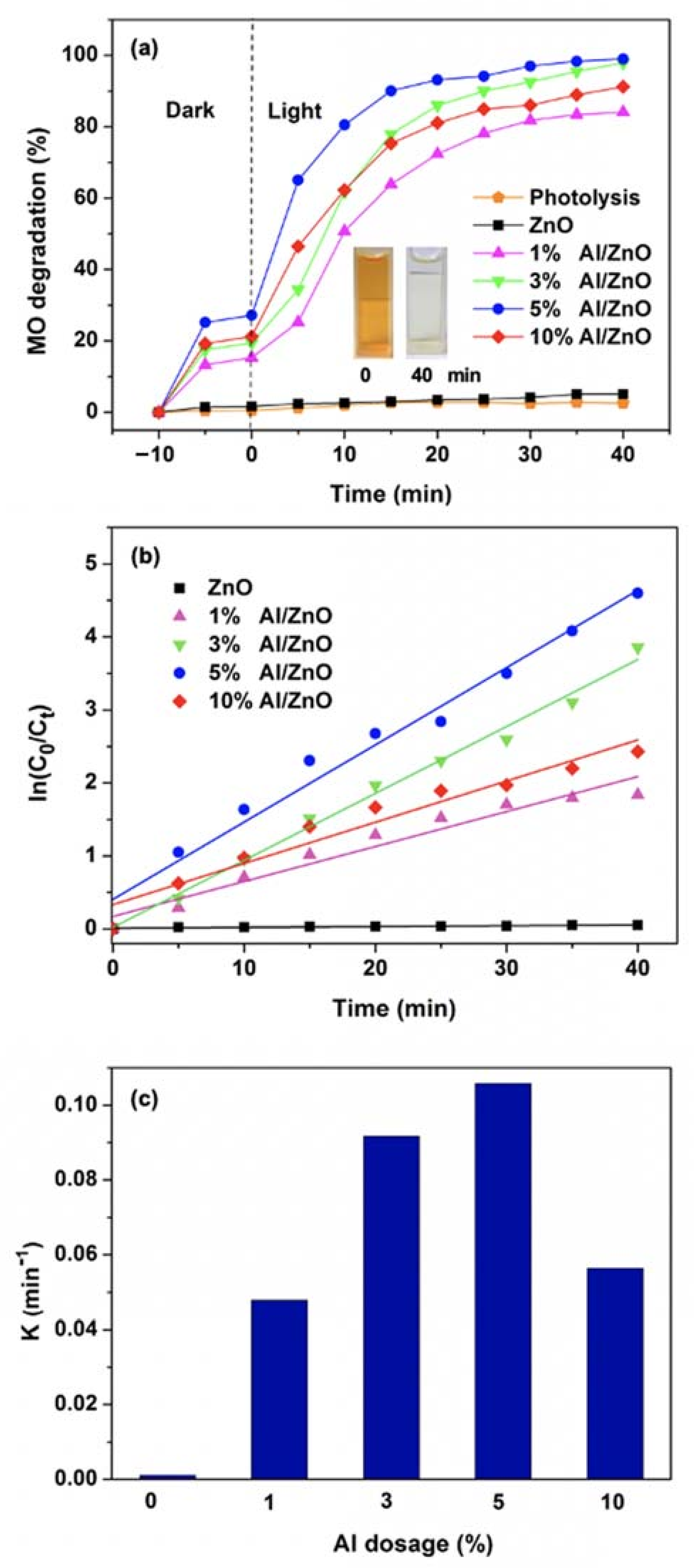
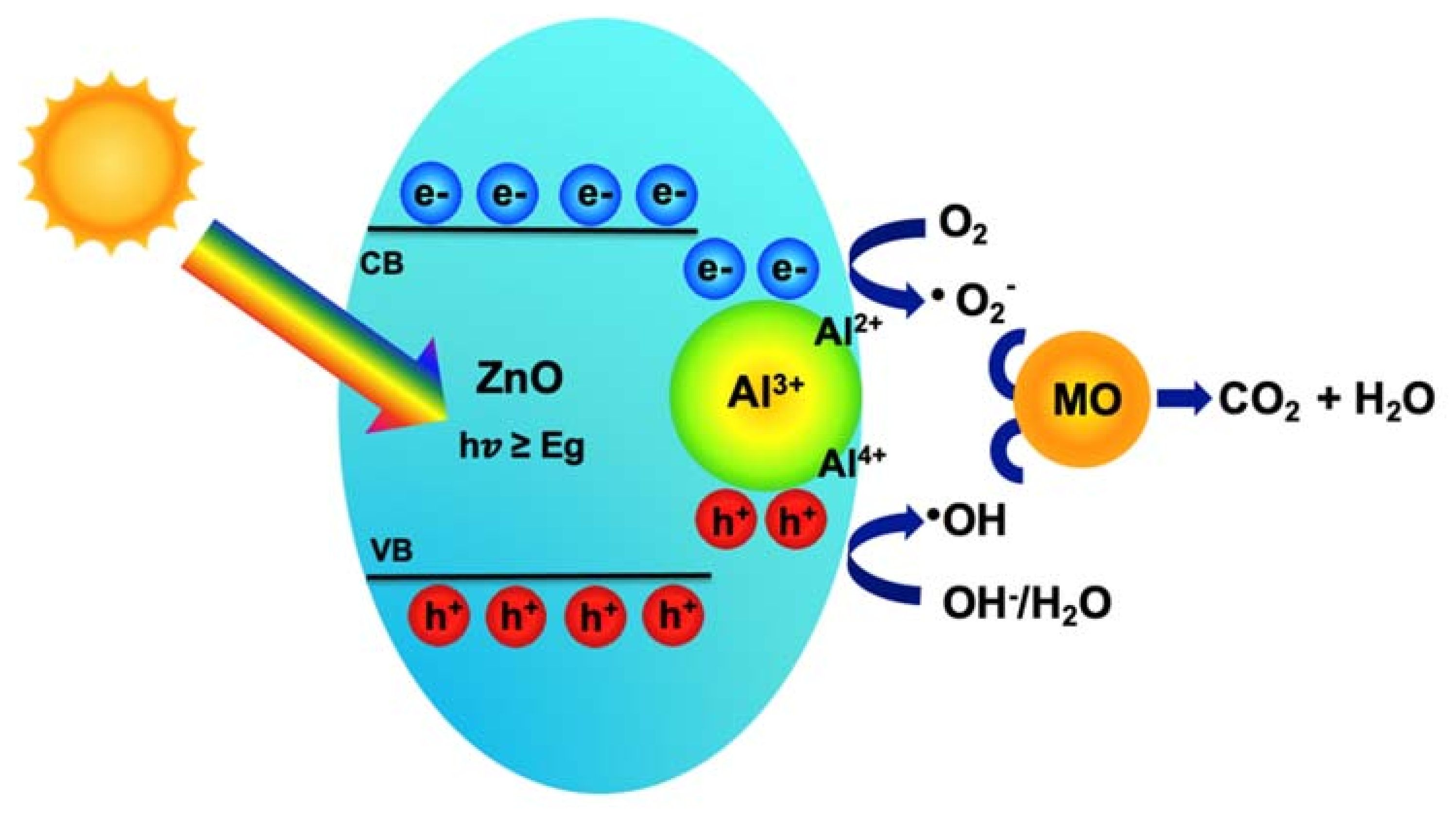
3.2. Effects of Al Dopant on the Properties of ZnO NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, T.; Adachi, Y.; Yamanashi, Y.; Shimada, Y. Long-term natural remediation process in textile dye-polluted river sediment driven by bacterial community changes. Water Res. 2016, 100, 458–465. [Google Scholar] [CrossRef]
- Liu, J.; Peng, G.; Jing, X.; Yi, Z. Treatment of methyl orange by the catalytic wet peroxide oxidation process in batch and continuous fixed bed reactors using Fe-impregnated 13X as catalyst. Water Sci. Technol. 2018, 78, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, V.K.; Shirin, S.; Aman, A.M.; de Solla, S.R.; Mathieu-Denoncourt, J.; Langlois, V.S. Genotoxic and carcinogenic products arising from reductive transformations of the azo dye, Disperse Yellow 7. Chemosphere 2016, 146, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Swarnakar, S.; Ghosh, S.; Majumdar, S.; Banerjee, S. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J. Environ. Chem. Eng. 2019, 7, 102867. [Google Scholar] [CrossRef]
- Neethu, N.; Choudhury, T. Treatment of Methylene Blue and Methyl Orange Dyes in Wastewater by Grafted Titania Pillared Clay Membranes. Recent Pat. Nanotechnol. 2018, 12, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Mirmahalleh, S.-A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Adsorption kinetics of methyl orange from water by pH-sensitive poly(2-(dimethylamino)ethyl methacrylate)/nanocrystalline cellulose hydrogels. Environ. Sci. Pollut Res. 2020, 27, 28091–28103. [Google Scholar] [CrossRef]
- Abass, A.K.; Raoof, S.D. Advanced Oxidation Process treatment for azo dyes pollutants using ultra-violet irradiation. J. Phys. Conf. Ser. 2020, 1664, 012066. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Almansoory, A.F.; Ismail, N.I.; Hasan, H.A.; Anuar, N. Role of Salvinia molesta in biodecolorization of methyl orange dye from water. Sci. Rep. 2020, 10, 13980. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Wang, S.; Yun, J.-H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Xiao, M.; Wang, L. Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. J. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef]
- Xu, H.; Xu, L. Preparation of ZnO Nanomaterials and Their Photocatalytic Degradation of Organic Pollutants. J. Donghua University Eng. Ed. 2020, 37, 271–279. [Google Scholar]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Boughelout, A.; Macaluso, R.; Kechouane, M.; Trari, M. Photocatalysis of rhodamine B and methyl orange degradation under solar light on ZnO and Cu2O thin films. React. Kinet. Mech. Catal. 2020, 129, 1115–1130. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Abutaleb, A.; Ashraf Ali, M.; Ahamad, T.; Rahman Ansari, A.; Shariq, M.; Lolla, D.; Khan, A. UV light enabled photocatalytic activity of α-Fe2O3 nanoparticles synthesized via phase transformation. Mater. Lett. 2020, 258, 126748. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Liu, Y.; Zhou, C.; Wu, Y. Photocatalytic Degradation of Methyl Orange by Fe2O3−Fe3O4 Nanoparticles and Fe2O3−Fe3O4−Montmorillonite Nanocomposites. CLEAN–Soil Air Water 2017, 45, 1600472. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.-H.; Chen, H.; Lyu, M.; Butburee, T.; Wang, L. Stable Hematite Nanosheet Photoanodes for Enhanced Photoelectrochemical Water Splitting. Adv. Mater. 2016, 28, 6405–6410. [Google Scholar] [CrossRef] [PubMed]
- Prado-Chay, D.A.; Cortés-Jácome, M.A.; Angeles-Chávez, C.; Oviedo-Roa, R.; Martínez-Magadán, J.M.; Zuriaga-Monroy, C.; Hernández-Hernández, I.J.; Mayoral, P.R.; Gómora-Herrera, D.R.; Toledo-Antonio, J.A. Synthesis and Photocatalytic Activity of Cu2O Microspheres upon Methyl Orange Degradation. Top. Catal. 2020, 63, 586–600. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Wu, Z.; Liu, Y.; Li, S. Facile hydrothermal synthesis of CuO–Cu2O/GO nanocomposites for the photocatalytic degradation of organic dye and tetracycline pollutants. New J. Chem. 2020, 44, 6420–6427. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.-H.; Butburee, T.; Chen, H.; Thaweesak, S.; Lyu, M.; Wang, S.; Wang, L. Bifunctional photoelectrochemical process for humic acid degradation and hydrogen production using multi-layered p-type Cu2O photoelectrodes with plasmonic Au@TiO2. J. Hazard. Mater. 2021, 402, 123533. [Google Scholar] [CrossRef]
- De Almeida, J.C.; Corrêa, M.T.; Koga, R.H.; Del Duque, D.M.S.; Lopes, O.F.; da Silva, G.T.S.T.; Ribeiro, C.; de Mendonça, V.R. Crystallization time in ZnO: The role of surface OH groups in its photoactivity. New J. Chem. 2020, 44, 18216–18224. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. CrystEngComm 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Boubenia, S.; Dahiya, A.S.; Poulin-Vittrant, G.; Morini, F.; Nadaud, K.; Alquier, D. A facile hydrothermal approach for the density tunable growth of ZnO nanowires and their electrical characterizations. Sci. Rep. 2017, 7, 15187. [Google Scholar] [CrossRef]
- Modwi, A.; Taha, K.K.; Khezami, L.; Al-Ayed, A.S.; Al-Duaij, O.K.; Khairy, M.; Bououdina, M. Structural and Electrical Characterization of Ba/ZnO Nanoparticles Fabricated by Co-precipitation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2633–2644. [Google Scholar] [CrossRef]
- Thambidurai, S.; Gowthaman, P.; Venkatachalam, M.; Suresh, S. Natural sunlight assisted photocatalytic degradation of methylene blue by spherical zinc oxide nanoparticles prepared by facile chemical co-precipitation method. Optik 2020, 207, 163865. [Google Scholar] [CrossRef]
- Ait hssi, A.; Amaterz, E.; Labchir, N.; Atourki, L.; Bouderbala, I.Y.; Elfanaoui, A.; Benlhachemi, A.; Ihlal, A.; Bouabid, K. Electrodeposited ZnO Nanorods as Efficient Photoanodes for the Degradation of Rhodamine B. Phys. Status Solidi A 2020, 217, 2000349. [Google Scholar] [CrossRef]
- Guckan, V.; Altunal, V.; Ozdemir, A.; Tsiumra, V.; Zhydachevskyy, Y.; Yegingil, Z. Calcination effects on europium doped zinc oxide as a luminescent material synthesized via sol-gel and precipitation methods. J. Alloys Compd. 2020, 823, 153878. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Ahmad, M.; Ahmed, E.; Naz, M.Y.; Akhtar, M.S.; Khalid, N.R.; Hussain, A.; Hussain, I. Effect of Al doping on the photocatalytic activity of ZnO nanoparticles decorated on CNTs and graphene: Solvothermal synthesis and study of experimental parameters. Mater. Sci. Semicon. Proc. 2020, 123, 105584. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Zou, Y.; Shen, X.; Zhu, L.; Liao, G. Solvothermal synthesis and photocatalytic properties of ZnO micro/nanostructures. Ceram. Int. 2019, 45, 1724–1729. [Google Scholar] [CrossRef]
- Demirci, S.; Dikici, T.; Tünçay, M.M.; Kaya, N. A study of heating rate effect on the photocatalytic performances of ZnO powders prepared by sol-gel route: Their kinetic and thermodynamic studies. Appl. Surf. Sci. 2020, 507, 145083. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, P.; Yun, J.-H.; Amal, R.; Ng, Y.H. Frequency-regulated pulsed electrodeposition of CuInS2 on ZnO nanorod arrays as visible light photoanodes. J. Mater. Chem. A 2015, 3, 15876–15881. [Google Scholar] [CrossRef]
- Tang, Y.; Yun, J.-H.; Wang, L.; Amal, R.; Ng, Y.H. Complete surface coverage of ZnO nanorod arrays by pulsed electrodeposited CuInS2 for visible light energy conversion. Dalton Trans. 2015, 44, 7127–7130. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zheng, Y.; Kobielusz, M.; Wang, Y.; Bai, Z.; Macyk, W.; Wang, X.; Tang, J. Recent advances in visible light-driven water oxidation and reduction in suspension systems. Mater. Today 2018, 21, 897–924. [Google Scholar] [CrossRef]
- Shu, H.Y.; Chang, M.C.; Tseng, T.H. Solar and Visible Light Illumination on Immobilized Nano Zinc Oxide for the Degradation and Mineralization of Orange G in Wastewater. Catalysts 2017, 7, 16. [Google Scholar]
- Ghanem, A.F.; Badawy, A.A.; Mohram, M.E.; Abdel Rehim, M.H. Synergistic effect of zinc oxide nanorods on the photocatalytic performance and the biological activity of graphene nano sheets. Heliyon 2020, 6, e03283. [Google Scholar] [CrossRef]
- Shah, A.A.; Bhatti, M.A.; Tahira, A.; Chandio, A.D.; Channa, I.A.; Sahito, A.G.; Chalangar, E.; Willander, M.; Nur, O.; Ibupoto, Z.H. Facile synthesis of copper doped ZnO nanorods for the efficient photo degradation of methylene blue and methyl orange. Ceram. Int. 2020, 46, 9997–10005. [Google Scholar] [CrossRef]
- Nguyen, H.T.P.; Nguyen, T.M.T.; Hoang, C.N.; Le, T.K.; Lund, T.; Nguyen, H.K.H.; Huynh, T.K.X. Characterization and photocatalytic activity of new photocatalysts based on Ag, F-modified ZnO nanoparticles prepared by thermal shock method. Arab. J. Chem. 2020, 13, 1837–1847. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Hossienzadeh, K.; Maleki, A.; Ghanbari, R.; Rezaee, R.; Safari, M.; Shahmoradi, B.; Daraei, H.; Jafari, A.; Yetilmezsoy, K.; et al. Effects of doping zinc oxide nanoparticles with transition metals (Ag, Cu, Mn) on photocatalytic degradation of Direct Blue 15 dye under UV and visible light irradiation. J. Environ. Health Sci. Eng. 2019, 17, 479–492. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Synthesis of N-doped ZnO nanoparticles with cabbage morphology as a catalyst for the efficient photocatalytic degradation of methylene blue under UV and visible light. RSC Adv. 2019, 9, 7509–7535. [Google Scholar] [CrossRef]
- Thaweesak, S.; Wang, S.; Lyu, M.; Xiao, M.; Peerakiatkhajohn, P.; Wang, L. Boron-doped graphitic carbon nitride nanosheets for enhanced visible light photocatalytic water splitting. Dalton Trans. 2017, 46, 10714–10720. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Z.; Qi, S.; Yin, Z.; Deng, S.; Zhang, M.; Sun, Z. Construction of Ag-modified TiO2/ZnO heterojunction nanotree arrays with superior photocatalytic and photoelectrochemical properties. RSC Adv. 2020, 10, 34702–34711. [Google Scholar] [CrossRef]
- Munawar, T.; Iqbal, F.; Yasmeen, S.; Mahmood, K.; Hussain, A. Multi metal oxide NiO-CdO-ZnO nanocomposite–synthesis, structural, optical, electrical properties and enhanced sunlight driven photocatalytic activity. Ceram. Int. 2020, 46, 2421–2437. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Yun, J.-H.; Chen, H.; Richards, R.M.; Wang, L. A hybrid photoelectrode with plasmonic Au@TiO2 nanoparticles for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2015, 3, 20127–20133. [Google Scholar] [CrossRef]
- Khayatian, S.A.; Kompany, A.; Shahtahmassebi, N.; Khorsand Zak, A. Enhanced Photocatalytic Performance of Al-Doped ZnO NPs-Reduced Graphene Oxide Nanocomposite for Removing of Methyl Orange Dye from Water Under Visible-Light Irradiation. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2677–2688. [Google Scholar] [CrossRef]
- Shaziman, S.; Ismail@rosdi, A.S.; Mamat, M.H.; Zoolfakar, A.S. Influence of Growth Time and Temperature on the Morphology of ZnO Nanorods via Hydrothermal. IOP Conf. Mater. Sci. Eng. 2015, 99, 012016. [Google Scholar] [CrossRef]
- Sugihartono, I.; Retnoningtyas, A.; Rustana, C.; Umiatin; Yudasari, N.; Isnaeni; Imawan, C.; Kurniadewi, F. The influence of calcination temperature on optical properties of ZnO nanoparticles. AIP Conf. Proc. 2019, 2169, 060010. [Google Scholar]
- Sharma, H.K.; Archana, R.; Sankar ganesh, R.; Singh, B.P.; Ponnusamy, S.; Hayakawa, Y.; Muthamizhchelvan, C.; Raji, P.; Kim, D.Y.; Sharma, S.K. Substitution of Al3+ to Zn2+ sites of ZnO enhanced the photocatalytic degradation of methylene blue under irradiation of visible light. Solid State Sci. 2019, 94, 45–53. [Google Scholar] [CrossRef]
- Zak, A.K.; Hashim, A.M.; Darroudi, M. Optical properties of ZnO/BaCO3 nanocomposites in UV and visible regions. Nanoscale Res. Lett. 2014, 9, 399. [Google Scholar] [CrossRef]
- Narayana, A.; Bhat, S.A.; Fathima, A.; Lokesh, S.V.; Surya, S.G.; Yelamaggad, C.V. Green and low-cost synthesis of zinc oxide nanoparticles and their application in transistor-based carbon monoxide sensing. RSC Adv. 2020, 10, 13532–13542. [Google Scholar] [CrossRef]
- Debanath, M.K.; Karmakar, S. Study of blueshift of optical band gap in zinc oxide (ZnO) nanoparticles prepared by low-temperature wet chemical method. Mater. Lett. 2013, 111, 116–119. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band Gap Narrowing and Widening of ZnO Nanostructures and Doped Materials. Nanoscale Res. Lett. 2015, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Venu Gopal, V.R.; Kamila, S. Effect of temperature on the morphology of ZnO nanoparticles: A comparative study. Appl. Nanosci. 2017, 7, 75–82. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef]
- Yu, F.; Wang, B.; Hu, H.; Li, H.; Song, T.; Xu, B.; He, L.; Duan, H.; Wang, S. The Photocatalytic Properties of Al-doped ZnO Nanotubes Decorated with Cu2O Nanoparticles. Phys. Status Solidi. A 2019, 216, 1900386. [Google Scholar] [CrossRef]
- Robles-Aguila, M.J.; Luna-Lopez, J.A.; de la Luz, A.D.H.; Martinez-Juarez, J.; Rabanal, M.E. Synthesis and Characterization of Nanocrystalline ZnO Doped with Al3+ and Ni2+ by a Sol-Gel Method Coupled with Ultrasound Irradiation. Crystals 2018, 8, 406. [Google Scholar] [CrossRef]
- Ahammed, N.; Hassan, M.S.; Hassan, M. Effects of aluminum (Al) incorporation on structural, optical and thermal properties of ZnO nanoparticles. Mater. Sci.-Poland 2018, 36, 419–426. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S.A. Sol-gel synthesis, structural and enhanced photocatalytic performance of Al doped ZnO nanoparticles. Adv. Powder Technol. 2017, 28, 1418–1425. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, M.; Farhad, S.F.U.; Podder, J. Structural, optical and photocatalysis properties of sol–gel deposited Al-doped ZnO thin films. Surf. Interfaces 2019, 16, 120–126. [Google Scholar] [CrossRef]
- Saber, O.; El-Brolossy, T.A.; Al Jaafari, A.A. Improvement of Photocatalytic Degradation of Naphthol Green B Under Solar Light Using Aluminum Doping of Zinc Oxide Nanoparticles. Water Air Soil Pollut. 2012, 223, 4615–4626. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.H.; Park, S.S.; Hong, S.S.; Lee, G.D. Degradation kinetics for photocatalytic reaction of methyl orange over Al-doped ZnO nanoparticles. J. Ind. Eng. Chem. 2015, 25, 199–206. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peerakiatkhajohn, P.; Butburee, T.; Sul, J.-H.; Thaweesak, S.; Yun, J.-H. Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanomaterials 2021, 11, 1059. https://doi.org/10.3390/nano11041059
Peerakiatkhajohn P, Butburee T, Sul J-H, Thaweesak S, Yun J-H. Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanomaterials. 2021; 11(4):1059. https://doi.org/10.3390/nano11041059
Chicago/Turabian StylePeerakiatkhajohn, Piangjai, Teera Butburee, Jung-Hoon Sul, Supphasin Thaweesak, and Jung-Ho Yun. 2021. "Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles" Nanomaterials 11, no. 4: 1059. https://doi.org/10.3390/nano11041059
APA StylePeerakiatkhajohn, P., Butburee, T., Sul, J.-H., Thaweesak, S., & Yun, J.-H. (2021). Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanomaterials, 11(4), 1059. https://doi.org/10.3390/nano11041059






