Glass-Ceramic Synthesis of Cr-Substituted Strontium Hexaferrite Nanoparticles with Enhanced Coercivity
Abstract
1. Introduction
2. Materials and Methods
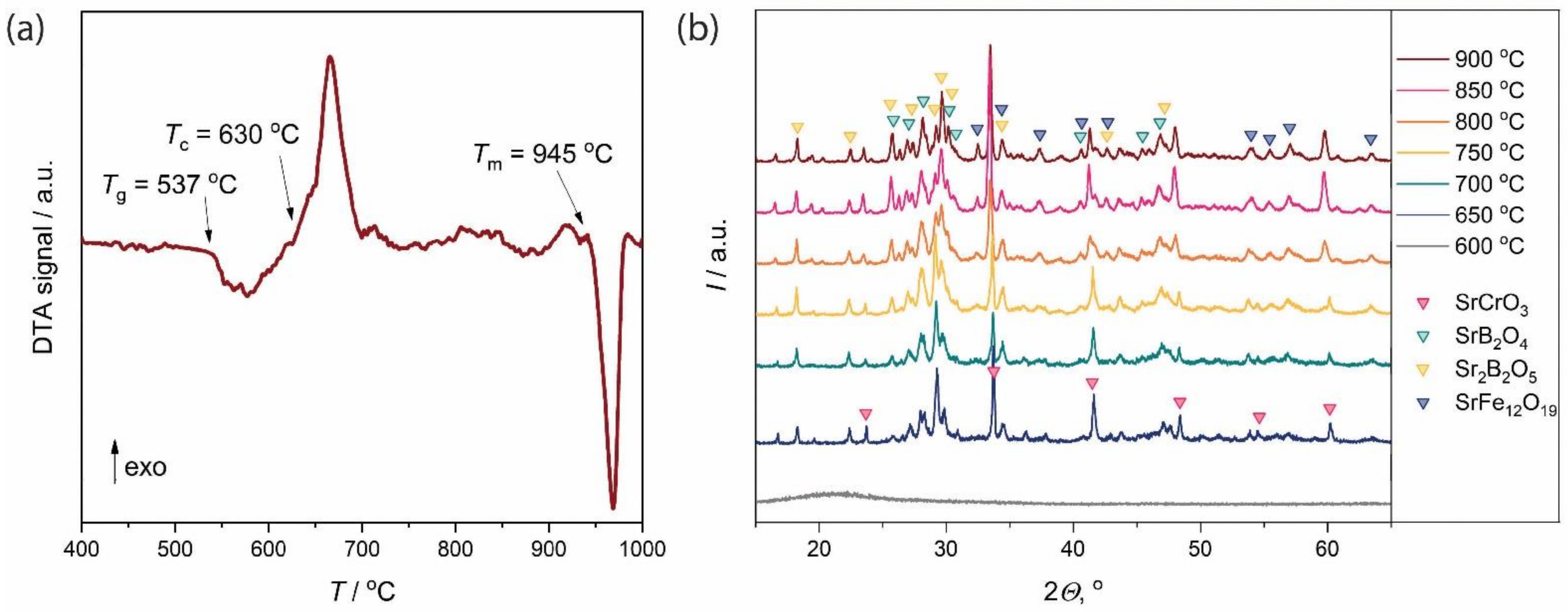
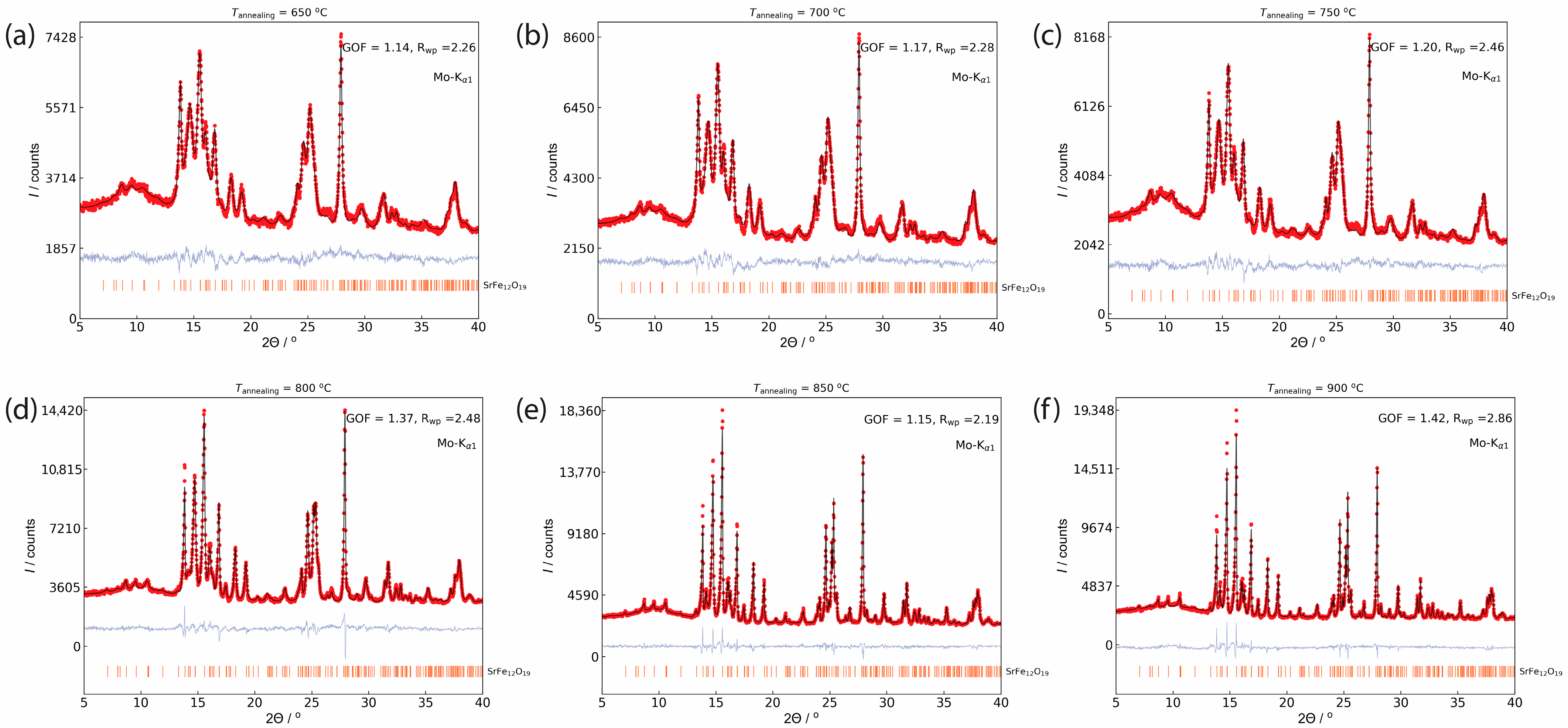
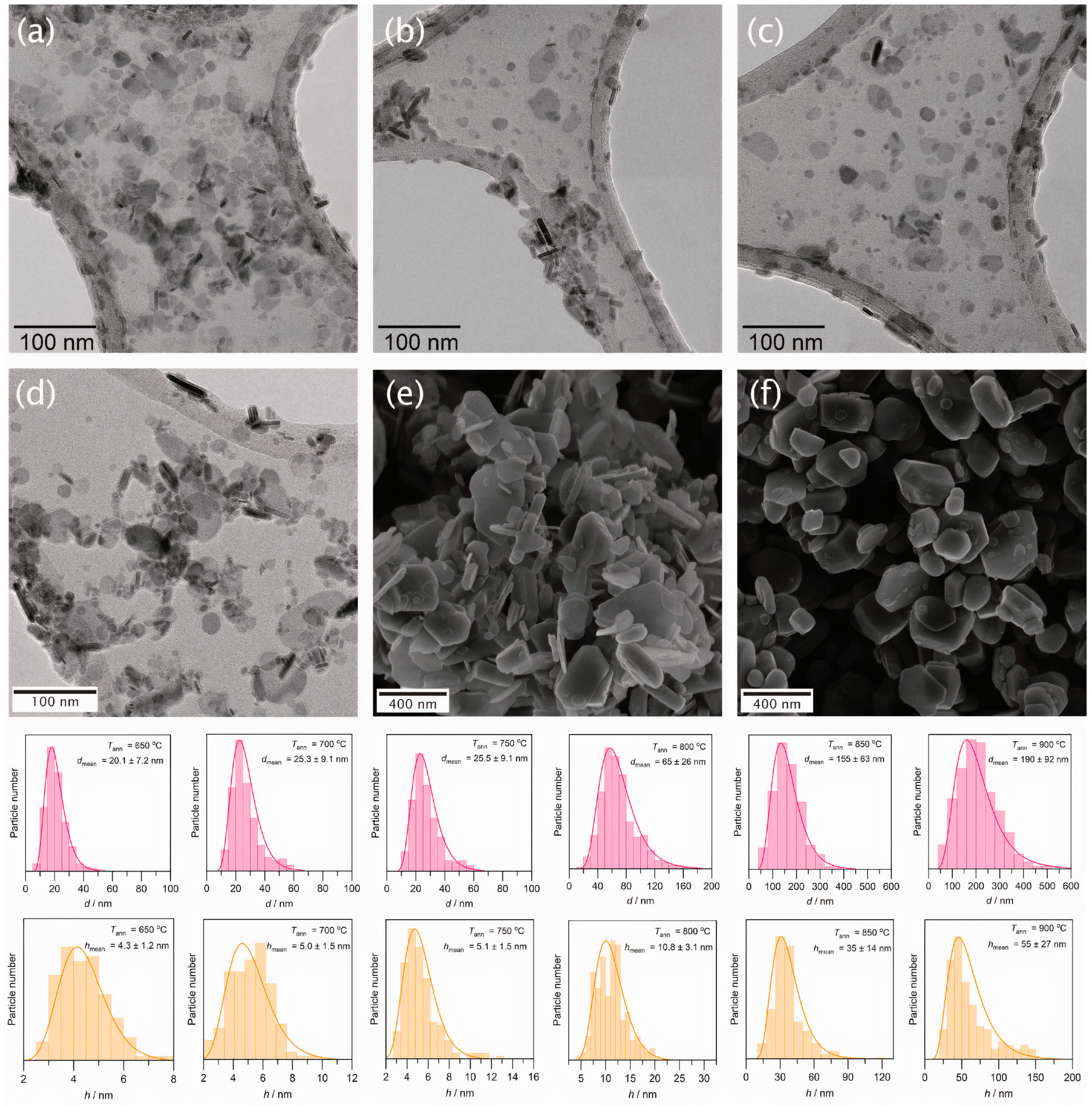
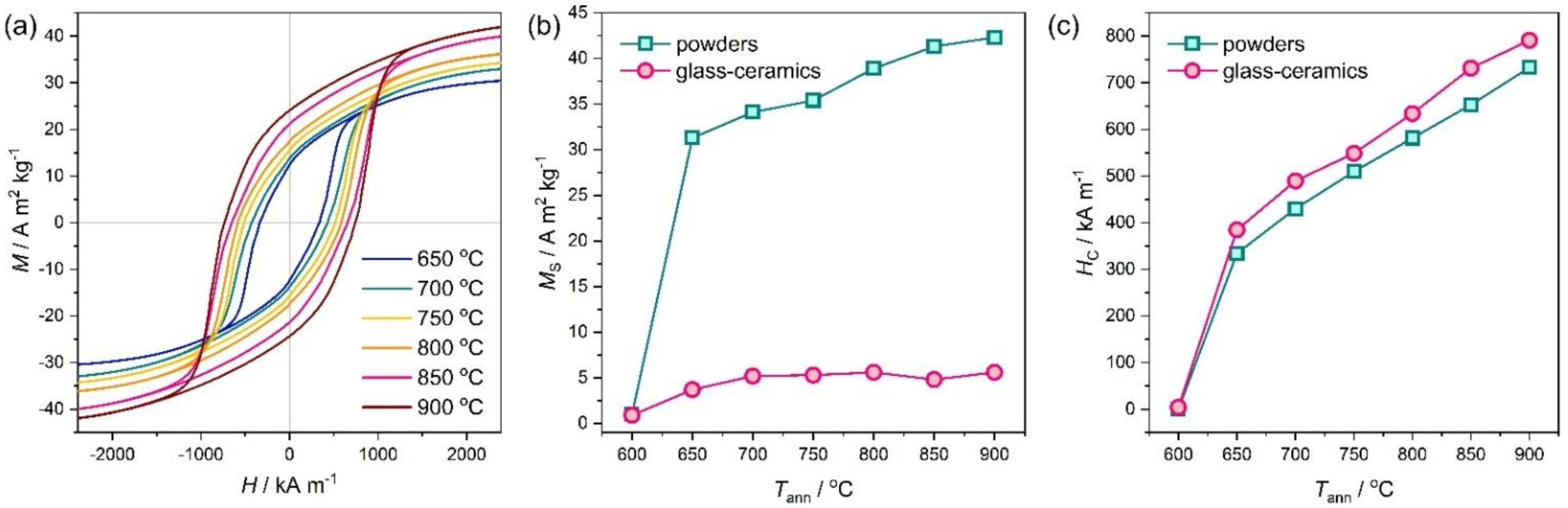
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites; Philips Technical Library: Eindhoven, The Netherlands, 1959. [Google Scholar]
- Gorbachev, E.A.; Kozlyakova, E.S.; Trusov, L.A.; Sleptsova, A.E.; Zykin, M.A.; Kazin, P.E. Design of modern magnetic materials with giant coercivity. Russ. Chem. Rev. 2021, 90. [Google Scholar] [CrossRef]
- Shimizu, O.; Oyanagi, M.; Morooka, A.; Mori, M.; Kurihashi, Y.; Tada, T.; Suzuki, H.; Harasawa, T. Development of advanced barium ferrite tape media. J. Magn. Magn. Mater. 2016, 400, 365–369. [Google Scholar] [CrossRef]
- Lantz, M.A.; Furrer, S.; Engelen, J.B.C.; Pantazi, A.; Rothuizen, H.E.; Cideciyan, R.D.; Cherubini, G.; Haeberle, W.; Jelitto, J.; Eleftheriou, E.; et al. 123 Gbit/in2 Recording Areal Density on Barium Ferrite Tape. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Kashevsky, B.E.; Kashevsky, S.B.; Korenkov, V.S.; Istomin, Y.P.; Terpinskaya, T.I.; Ulashchik, V.S. Magnetic hyperthermia with hard-magnetic nanoparticles. J. Magn. Magn. Mater. 2015, 380, 335–340. [Google Scholar] [CrossRef]
- Trusov, L.A.; Vasiliev, A.V.; Lukatskaya, M.R.; Zaytsev, D.D.; Jansen, M.; Kazin, P.E. Stable colloidal solutions of strontium hexaferrite hard magnetic nanoparticles. Chem. Commun. 2014, 50, 14581–14584. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Eliseev, A.A.; Trusov, L.A.; Chumakov, A.P.; Boesecke, P.; Anokhin, E.O.; Vasiliev, A.V.; Sleptsova, A.E.; Gorbachev, E.A.; Korolev, V.V.; et al. Rotational dynamics of colloidal hexaferrite nanoplates. Appl. Phys. Lett. 2018, 113, 113106. [Google Scholar] [CrossRef]
- Kushnir, S.E.; Gavrilov, A.I.; Kazin, P.E.; Grigorieva, A.V.; Tretyakov, Y.D.; Jansen, M. Synthesis of colloidal solutions of SrFe12O19 plate-like nanoparticles featuring extraordinary magnetic-field-dependent optical transmission. J. Mater. Chem. 2012, 22, 18893. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Trusov, L.A.; Eliseev, A.A.; Lukashin, A.V.; Jansen, M.; Kazin, P.E.; Napolskii, K.S. Controlled way to prepare quasi-1D nanostructures with complex chemical composition in porous anodic alumina. Chem. Commun. 2011, 47, 2396–2398. [Google Scholar] [CrossRef]
- Lisjak, D.; Jenuš, P.; Mertelj, A. Influence of the Morphology of Ferrite Nanoparticles on the Directed Assembly into Magnetically Anisotropic Hierarchical Structures. Langmuir 2014, 30, 6588–6595. [Google Scholar] [CrossRef]
- Gorbachev, E.A.; Trusov, L.A.; Sleptsova, A.E.; Anokhin, E.O.; Zaitsev, D.D.; Vasiliev, A.V.; Eliseev, A.A.; Kazin, P.E. Synthesis and magnetic properties of the exchange-coupled SrFe10.7Al1.3O19/Co composite. Mendeleev Commun. 2018, 28, 401–403. [Google Scholar] [CrossRef]
- Anokhin, E.O.; Trusov, L.A.; Kozlov, D.A.; Chumakov, R.G.; Sleptsova, A.E.; Uvarov, O.V.; Kozlov, M.I.; Petukhov, D.I.; Eliseev, A.A.; Kazin, P.E. Silica coated hard-magnetic strontium hexaferrite nanoparticles. Adv. Powder Technol. 2019, 30, 1976–1984. [Google Scholar] [CrossRef]
- Kushnir, S.E.; Koshkodaev, D.S.; Kazin, P.E.; Zuev, D.M.; Zaytsev, D.D.; Jansen, M. Rapid Formation of a Monolayer of Oriented Hard-Magnetic Strontium Hexaferrite Nanoparticles on a Solid Substrate. Adv. Eng. Mater. 2014, 16, 884–888. [Google Scholar] [CrossRef]
- Lisjak, D.; Mertelj, A. Anisotropic magnetic nanoparticles: A review of their properties, syntheses and potential applications. Prog. Mater. Sci. 2018, 95, 286–328. [Google Scholar] [CrossRef]
- Jenuš, P.; Lisjak, D. The influence of material properties on the assembly of ferrite nanoparticles into 3D structures. Mater. Chem. Phys. 2014, 148, 1131–1138. [Google Scholar] [CrossRef]
- Lisjak, D.; Ovtar, S. Directed Assembly of BaFe12O19 Particles and the Formation of Magnetically Oriented Films. Langmuir 2011, 27, 14014–14024. [Google Scholar] [CrossRef]
- Ovtar, S.; Lisjak, D.; Drofenik, M. Preparation of Oriented Barium Hexaferrite Films by Electrophoretic Deposition. J. Am. Ceram. Soc. 2011, 94, 3373–3379. [Google Scholar] [CrossRef]
- Shirk, B.T.; Buessem, W.R. Magnetic Properties of Barium Ferrite Formed by Crystallization of a Glass. J. Am. Ceram. Soc. 1970, 53, 192–196. [Google Scholar] [CrossRef]
- Kazin, P.E.; Trusov, L.A.; Zaitsev, D.D.; Tret’yakov, Y.D. Glass crystallization synthesis of ultrafine hexagonal M-type ferrites: Particle morphology and magnetic characteristics. Russ. J. Inorg. Chem. 2009, 54, 2081–2090. [Google Scholar] [CrossRef]
- Kojima, K. Handbook of Magnetic Materials; Wohlfarth, E.P., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1982; Volume 3, ISBN 9780444863782. [Google Scholar]
- Makovec, D.; Belec, B.; Goršak, T.; Lisjak, D.; Komelj, M.; Dražić, G.; Gyergyek, S. Discrete evolution of the crystal structure during the growth of Ba-hexaferrite nanoplatelets. Nanoscale 2018, 10, 14480–14491. [Google Scholar] [CrossRef]
- Makovec, D.; Dražić, G.; Gyergyek, S.; Lisjak, D. A new polymorph of strontium hexaferrite stabilized at the nanoscale. CrystEngComm 2020, 22, 7113–7122. [Google Scholar] [CrossRef]
- Makovec, D.; Gyergyek, S.; Goršak, T.; Belec, B.; Lisjak, D. Evolution of the microstructure during the early stages of sintering barium hexaferrite nanoplatelets. J. Eur. Ceram. Soc. 2019, 39, 4831–4841. [Google Scholar] [CrossRef]
- Gorbachev, E.A.; Trusov, L.A.; Sleptsova, A.E.; Kozlyakova, E.S.; Alyabyeva, L.N.; Yegiyan, S.R.; Prokhorov, A.S.; Lebedev, V.A.; Roslyakov, I.V.; Vasiliev, A.V.; et al. Hexaferrite materials displaying ultra-high coercivity and sub-terahertz ferromagnetic resonance frequencies. Mater. Today 2020, 32, 13–18. [Google Scholar] [CrossRef]
- Trusov, L.A.; Gorbachev, E.A.; Lebedev, V.A.; Sleptsova, A.E.; Roslyakov, I.V.; Kozlyakova, E.S.; Vasiliev, A.V.; Dinnebier, R.E.; Jansen, M.; Kazin, P.E. Ca-Al double-substituted strontium hexaferrites with giant coercivity. Chem. Commun. 2018, 54, 479–482. [Google Scholar] [CrossRef]
- Sleptsova, A.E.; Alyabyeva, L.N.; Gorbachev, E.A.; Kozlyakova, E.S.; Karpov, M.A.; Xinming, C.; Vasiliev, A.V.; Gorshunov, B.P.; Prokhorov, A.S.; Kazin, P.E.; et al. Tuning the morphology and magnetic properties of single-domain SrFe8Al4O19 particles prepared by citrate auto-combustion route. Mendeleev Commun. 2021, 31, 221–223. [Google Scholar] [CrossRef]
- Zaitsev, D.D.; Kazin, P.E.; Trusov, L.A.; Vishnyakov, D.A.; Tretyakov, Y.D.; Jansen, M. Synthesis of magnetic glass-ceramics in the system SrO–Fe2O3–Al2O3–B2O3. J. Magn. Magn. Mater. 2006, 300, e473–e475. [Google Scholar] [CrossRef]
- Kazin, P.E.; Trusov, L.A.; Zaitsev, D.D.; Tretyakov, Y.D.; Jansen, M. Formation of submicron-sized SrFe12-xAlxO19 with very high coercivity. J. Magn. Magn. Mater. 2008, 320, 1068–1072. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Haneda, K.; Kojima, H. Intrinsic coercivity of substituted bafeo12o19. Jpn. J. Appl. Phys. 1973, 12, 355–360. [Google Scholar] [CrossRef]
- Lutterotti, L.; Chateigner, D.; Ferrari, S.; Ricote, J. Texture, residual stress and structural analysis of thin films using a combined X-ray analysis. Thin Solid Films 2004, 450, 34–41. [Google Scholar] [CrossRef]
- Zaitsev, D.D.; Kushnir, S.E.; Kazin, P.E.; Tretyakov, Y.D.; Jansen, M. Preparation of the SrFe12O19-based magnetic composites via boron oxide glass devitrification. J. Magn. Magn. Mater. 2006, 301, 489–494. [Google Scholar] [CrossRef]
- Obradors, X.; Solans, X.; Collomb, A.; Samaras, D.; Rodriguez, J.; Pernet, M.; Font-Altaba, M. Crystal structure of strontium hexaferrite SrFe12O19. J. Solid State Chem. 1988, 72, 218–224. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A Mechanism of Magnetic Hysteresis in Heterogeneous Alloys. Philos. Trans. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Koutzarova, T.; Kolev, S.; Ghelev, C.; Grigorov, K.; Nedkov, I. Structural and Magnetic Properties and Preparation Techniques of Nanosized M-type Hexaferrite Powders. In Advances in Nanoscale Magnetism; Springer: Berlin/Heidelberg, Germany, 2009; pp. 183–203. [Google Scholar]
- Trusov, L.A.; Babarkina, O.V.; Anokhin, E.O.; Sleptsova, A.E.; Gorbachev, E.A.; Eliseev, A.A.; Filippova, T.V.; Vasiliev, A.V.; Kazin, P.E. Crystallization of magnetic particles in nNa2O-9SrO-6Fe2O3-8B2O3 (n = 1 and 4) glasses. J. Magn. Magn. Mater. 2019, 476, 311–316. [Google Scholar] [CrossRef]
- Obradors, X.; Isalgue, A.; Collomb, A.; Pernet, M.; Pannetier, J.; Roctriguez, J.; Tejada, J.; Joubert, J.C. Cation distribution and high field magnetization studies on SrFe12-xCrxO19. IEEE Trans. Magn. 1984, 20, 1636–1638. [Google Scholar] [CrossRef]
- de Julian Fernandez, C.; Sangregorio, C.; de la Figuera, J.; Belec, B.; Makovec, D.; Quesada, A. Topical Review: Progress and Prospects of Hard Hexaferrites for Permanent Magnet Applications. J. Phys. D. Appl. Phys. 2020. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Namai, A.; Imoto, K.; Yoshikiyo, M.; Tarora, W.; Nakagawa, K.; Komine, M.; Miyamoto, Y.; Nasu, T.; Oka, S.; et al. Nanometer-size hard magnetic ferrite exhibiting high optical-transparency and nonlinear optical-magnetoelectric effect. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Haneda, K.; Kojima, H. Intrinsic Coercivity of BaFe12−xCrxO19. Phys. Status Solidi 1971, 6, 259–264. [Google Scholar] [CrossRef]
| Tann °C | Lattice parameters | Mean size1 | Mean size2 | MS A m2 kg−1 | HC kA m−1 | TC | |||
|---|---|---|---|---|---|---|---|---|---|
| a, Å | c, Å | d, nm | h, nm | d, nm | h, nm | K | |||
| 650 | 5.8749(4) | 23.037(3) | 19.9 | 3.8 | 20.1 | 4.3 | 31.3 | 334 | 622 |
| 700 | 5.8747(3) | 23.014(3) | 23.6 | 4.8 | 25.3 | 5.0 | 34.1 | 430 | 637 |
| 750 | 5.8744(4) | 22.998(3) | 24.2 | 4.8 | 25.5 | 5.1 | 35.4 | 509 | 653 |
| 800 | 5.8745(2) | 22.990(1) | 61.7 | 12.0 | 65.0 | 10.8 | 38.9 | 581 | 661 |
| 850 | 5.8745(1) | 22.9901(5) | / | 155 | 35 | 41.3 | 653 | 658 | |
| 900 | 5.8746(1) | 22.9908(5) | / | 190 | 55 | 42.3 | 732 | 658 | |
| Tann °C | ICP-MS, at. Ratio | ||
|---|---|---|---|
| Sr | Fe | Cr | |
| 650 | 0.86 | 9.88 | 2.12 |
| 700 | 0.84 | 9.73 | 2.27 |
| 750 | 0.83 | 9.68 | 2.32 |
| 800 | 0.90 | 10.28 | 1.72 |
| 850 | 0.90 | 10.20 | 1.80 |
| 900 | 0.91 | 10.24 | 1.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trusov, L.A.; Sleptsova, A.E.; Duan, J.; Gorbachev, E.A.; Kozlyakova, E.S.; Anokhin, E.O.; Eliseev, A.A.; Karpov, M.A.; Vasiliev, A.V.; Brylev, O.A.; et al. Glass-Ceramic Synthesis of Cr-Substituted Strontium Hexaferrite Nanoparticles with Enhanced Coercivity. Nanomaterials 2021, 11, 924. https://doi.org/10.3390/nano11040924
Trusov LA, Sleptsova AE, Duan J, Gorbachev EA, Kozlyakova ES, Anokhin EO, Eliseev AA, Karpov MA, Vasiliev AV, Brylev OA, et al. Glass-Ceramic Synthesis of Cr-Substituted Strontium Hexaferrite Nanoparticles with Enhanced Coercivity. Nanomaterials. 2021; 11(4):924. https://doi.org/10.3390/nano11040924
Chicago/Turabian StyleTrusov, Lev A., Anastasia E. Sleptsova, Jingtong Duan, Evgeny A. Gorbachev, Ekaterina S. Kozlyakova, Evgeny O. Anokhin, Artem A. Eliseev, Maxim A. Karpov, Alexander V. Vasiliev, Oleg A. Brylev, and et al. 2021. "Glass-Ceramic Synthesis of Cr-Substituted Strontium Hexaferrite Nanoparticles with Enhanced Coercivity" Nanomaterials 11, no. 4: 924. https://doi.org/10.3390/nano11040924
APA StyleTrusov, L. A., Sleptsova, A. E., Duan, J., Gorbachev, E. A., Kozlyakova, E. S., Anokhin, E. O., Eliseev, A. A., Karpov, M. A., Vasiliev, A. V., Brylev, O. A., & Kazin, P. E. (2021). Glass-Ceramic Synthesis of Cr-Substituted Strontium Hexaferrite Nanoparticles with Enhanced Coercivity. Nanomaterials, 11(4), 924. https://doi.org/10.3390/nano11040924








