The Influence of Scandium on the Composition and Structure of the Ti-Al Alloy Obtained by “Hydride Technology”
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Alloys
2.2. Research Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, T.; Li, S.; Liang, Y.; Sun, L.; Zhang, Y. Effects of scandium and silicon addition on the microstructure and mechanical properties of Ti-6Al-4V alloy. J. Mater. Res. Technol. 2020, 9, 5676–5688. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys; Science and Technology, Ed.; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2011; pp. 1–745. [Google Scholar]
- Qbau, N.; Nam, N.D.; Ca, N.X.; Hien, N.T. The crack healing effect of scandium in aluminum alloys during laser additive manufacturing. J. Manuf. Process. 2020, 50, 241–246. [Google Scholar] [CrossRef]
- Glazoff, M.; Khvan, V.; Zolotorevsky, V.; Belov, N.; Dinsdale, A. Influence of heat treatment upon microstructure of casting aluminum alloys. In Casting Aluminum Alloys; Butterworth-Heinemann: London, UK, 2019; pp. 235–312. [Google Scholar]
- Songbo, Y.; Boyun, H.; Zhimin, Y. Effect of minor Sc on high temperature mechanical properties of Ti–Al based alloys. Mater. Sci. Eng. A 2000, 280, 204–207. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Stark, A.; Esikov, M.A.; Paul, J.; Bataev, I.A.; Kashimbetova, A.A.; Pyczak, F. Ceramic-reinforced γ-TiAl-based composites: Synthesis, structure, and properties. Materials 2019, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Oh, M.H.; Nakamura, A.; Yamaguchi, M. Ordered domains in TiAl coexisting with Ti3Al in the lamellar structure of Ti-rich TiAl compounds. Philos. Mag. A 1992, 66, 539–555. [Google Scholar] [CrossRef]
- Harada, Y.; Dunand, D. Microstructure of Al3Sc with ternary transition-metal additions. Mater. Sci. Eng. A 2002, 329, 686–695. [Google Scholar] [CrossRef]
- Kurzina, I.A. Ion-implanted nanodimensional intermetallic phases. Inorg. Mater. Appl. Res. 2010, 1, 254–269. [Google Scholar] [CrossRef]
- Nikonenko, A.V.; Popova, N.A.; Nikonenko, E.L.; Kurzina, I.A. The effect of aluminum ion implantation on the grain size and structure of UFG titanium. Surf. Coat. Tech. 2020, 393, 125750. [Google Scholar] [CrossRef]
- Hyde, K.B.; Norman, A.F.; Prangnell, P.B. The effect of cooling rate on the morphology of primary Al3Sc intermetallic particles in Al–Sc alloys. Acta Mater. 2001, 49, 1327–1337. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zu, Q.; Han, B.; Han, X.; Cui, C. Significantly improved particle strengthening of Al–Sc alloy by high Sc composition design and rapid solidification. Mater. Sci. Eng. A 2021, 800, 140304. [Google Scholar] [CrossRef]
- Glezer, A.M.; Kozlov, E.V.; Koneva, N.A.; Popova, N.A.; Kurzina, I.A. Plastic Deformation of Nanostructured Materials; CISP CRC, Ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A Review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2018, 35, 270–284. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.R.; Zhang, Y.; Xia, Y.; Free, M. Powder metallurgy of titanium—Past, present, and future. Int. Mater. Rev. 2018, 63, 1–53. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Stock, H.R.; Köhler, B.; Bomas, H.; Zoch, H.W. Characteristics of aluminium–scandium alloy thin sheets obtained by physical vapour deposition. Mater. Des. 2010, 31, 76–81. [Google Scholar] [CrossRef]
- Yener, T.; Okumus, S.C.; Zeytin, S. In Situ Formation of Ti-TiAl3 metallic-intermetallic composite by electric current activated sintering method. Acta Phys. Pol. A 2015, 127, 917–920. [Google Scholar] [CrossRef]
- Belgibayeva, A.; Abzaev, Y.; Karakchieva, N.; Erkasov, R.; Sachkov, V.; Kurzina, I. The Structural and phase state of the TiAl system alloyed with rare-earth metals of the controlled composition synthesized by the “Hydride technology”. Metals 2020, 859, 1–17. [Google Scholar]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Process for scandium recovery from Jamaican bauxite residue: A probabilistic economic assessment. Mater. Today-Proc. 2019, 9, 578–586. [Google Scholar] [CrossRef]
- Fu, L.; Li, Y.; Jiang, F.; Huang, J.; Xu, G.; Yin, Z. On the role of Sc or Er micro-alloying in the microstructure evolution of Al-Mg alloy sheets during annealing. Mater. Charact. 2019, 157, 109918. [Google Scholar] [CrossRef]
- Zakharov, V.V. Prospects of Creation of Aluminum Alloys Sparingly Alloyed with Scandium. Met. Sci. Heat Treat. 2018, 60, 172–176. [Google Scholar] [CrossRef]
- Zakharov, V.V. Effect of scandium on the structure and properties of aluminum alloys. Met. Sci. Heat Treat. 2003, 45, 246–253. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Vahid, A.; Langan, T. Aluminium scandium alloys. In Fundamentals of Aluminium Metallurgy; Woodhead Publishing: New York, NY, USA, 2018; pp. 439–494. [Google Scholar]
- Zuo, X.; Cui, H. Effect of Minor Sc, Zr and Ti Additions on the microstructures and the mechanical properties of pure aluminium. Adv. Mat. Res. 2011, 152, 1071–1078. [Google Scholar]
- Feng, J.; Ye, B.; Zuo, L.; Qi, R.; Wang, Q.; Jiang, H.; Wang, C. Effects of Zr, Ti and Sc additions on the microstructure and mechanical properties of Al-0.4Cu-0.14Si-0.05Mg-0.2Fe alloys. J. Mater. Sci. Technol. 2018, 34, 2316–2324. [Google Scholar] [CrossRef]
- Lathabai, S.; Lloyd, P.G. The effect of scandium on the microstructure, mechanical properties and weldability of a cast Al–Mg alloy. Acta Mater. 2002, 50, 4275–4292. [Google Scholar] [CrossRef]
- Zhang, W.G.; He, L.J.; Li, P.J.; Ye, Y.C.; Xue, F.E.N.G.; Novikov, L.S. Dynamic response and numerical simulation of Al-Sc and Al-Ti alloys under high-speed impact. Trans. Nonferr. Metal. Soc. 2015, 25, 559–570. [Google Scholar] [CrossRef]
- Tong, Y.X.; Fan, X.M.; Shuitcev, A.V.; Chen, F.; Tian, B.; Li, L.; Zheng, Y.F. Effects of Sc addition and aging on microstructure and martensitic transformation of Ni-rich NiTiHfSc high temperature shape memory alloys. J. Alloy. Compd. 2020, 845, 156331. [Google Scholar] [CrossRef]
- Costa, S.; Puga, H.; Barbosa, J.; Pinto, A.M.P. The effect of Sc additions of the microstructure and age hardening behaviour of as cast Al-Sc alloys. Mater. Des. 2012, 42, 347–352. [Google Scholar] [CrossRef]
- Van Dalen, M.E.; Seidman, D.N.; Dunand, D.C. Creep- and coarsening properties of Al–0.06at.% Sc–0.06at.% Ti at 300–450 °C. Acta Mater. 2008, 56, 369–4377. [Google Scholar] [CrossRef]
- Davydov, V.G.; Rostova, T.D.; Zakharov, V.V.; Filatov, Y.A.; Yelagin, V.I. Scientific principles of making an alloying addition of scandium to aluminium alloys. Mat. Sci. Eng. A-Struct. 2000, 280, 30–36. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary Aluminum-Titanium phase diagram. J. Phase Equilib. Diff. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Pecharsky, V.; Zavalij, P. Fundamentals of Powder Diffraction and Structural Characterization of Materials, 2nd ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2005. [Google Scholar]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder. Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Young, R.A. The Ritveld Method; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Guptaa, R.K.; Panta, B. Titanium aluminides. In Intermetallic Matrix Composites; Mitra, R., Ed.; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Belgibayeva, A.F.; Erkasov, R.S.; Kurzina, I.A.; Karakchieva, N.I.; Sachkov, V.I.; Abzaev, Y.A. Influence of microalloying with scandium on the structure of alloys based on titanium aluminides, Bulletin of the LN Gumilyov Eurasian National University. Chemistry. Geography. Ecol. Ser. 2000, 131, 23–30. [Google Scholar]
- Dettenwanger, F.; Schumann, E.; RuÈhle, M.; Rakowski, J.; Meier, G.H. Microstructural Study of Oxidized –TiAl. Oxid. Met. 1998, 50, 269–307. [Google Scholar] [CrossRef]
- Weinert, K.; Biermann, D.; Bergmann, S. Machining of High Strength Light Weight Alloys for Engine Applications. CIRP Ann. 2007, 56, 105–108. [Google Scholar] [CrossRef]
- Mandal, P.K. Heat Treatment and Friction Stir Processing Effects on Mechanical Properties and Microstructural Evolution of Sc Inoculated Al-Zn-Mg Alloys. Mater. Sci. Metal. Eng. 2017, 4, 16–28. [Google Scholar]
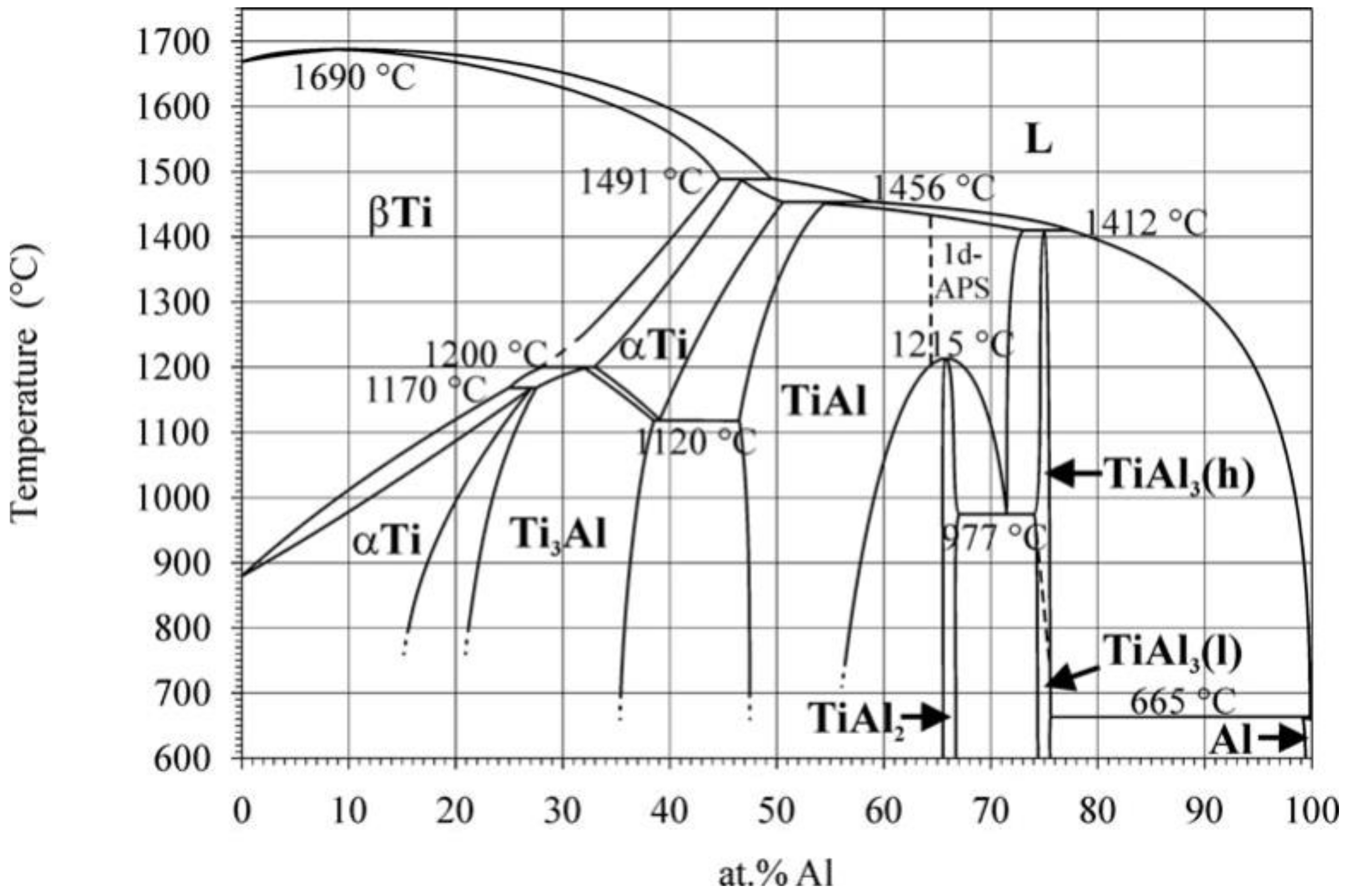
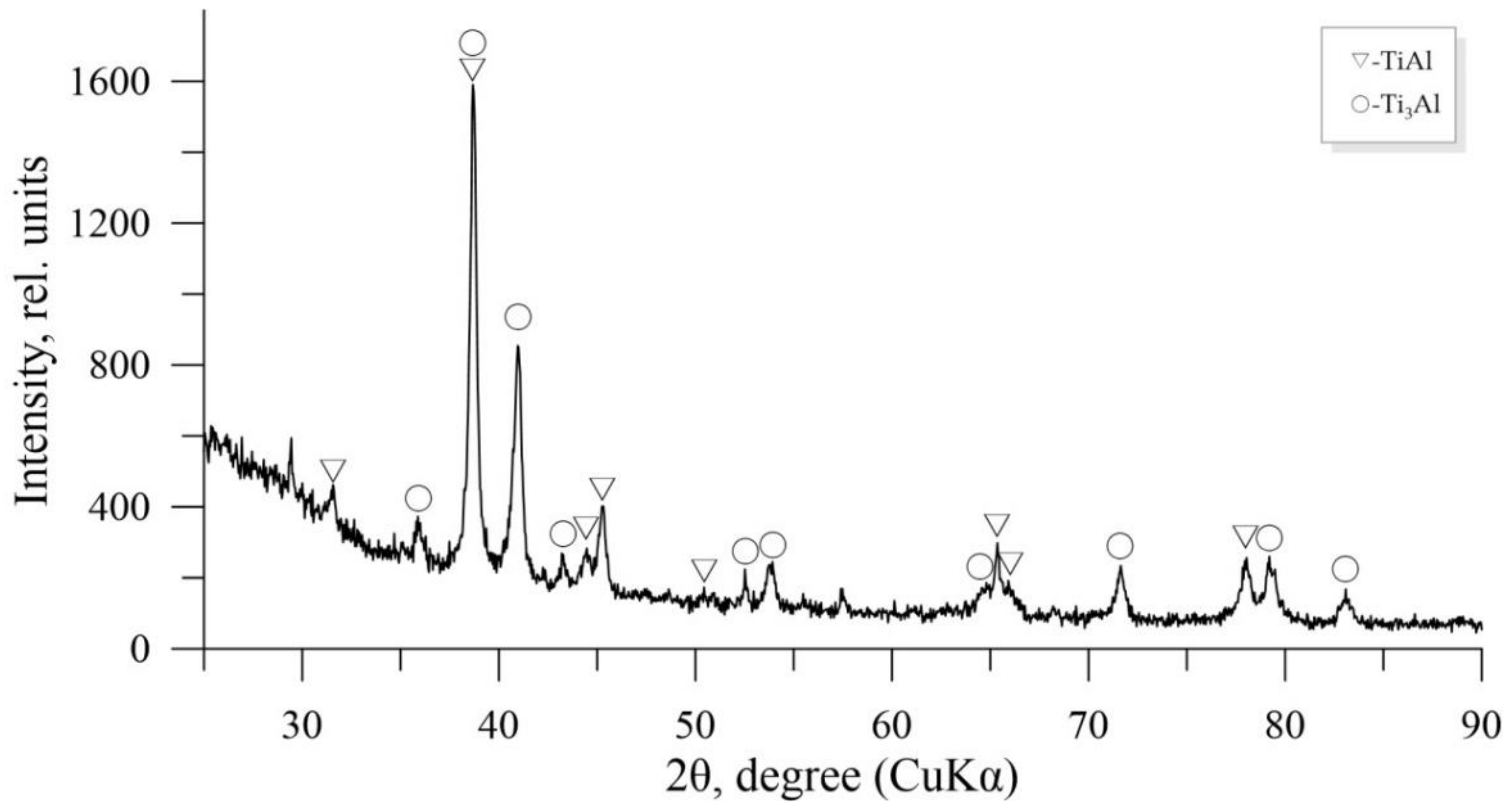








| Phase. | ΔH° (Formation), kJ/mole [33] | Lattice Type | Ti-Al [19] | Ti-Al-Sc [38] |
|---|---|---|---|---|
| Proportion, % | Proportion, % | |||
| TiAl | −40.0 ± 1.0 | P4/mmm | 31 | 42 |
| Ti3Al | −20.3 ± 1.9 | P63/mmc | 19 | 26 |
| Ti1.5Al2.5 | - | Pmmm | 3 | 11 |
| Ti2Al5 | - | P4/mmm | 3 | 4 |
| Ti5Al11 | - | I4/mmm | 8 | 4 |
| TiAl2 | −38.6 ± 2.6 | Cmmm | 9 | 3 |
| (TiAl2)1.33 | - | P4/mmm | 2 | - |
| Al | - | Fm-3m | 1 | 2 |
| α-Ti | −9.5 ± 1.0 | Im-3m | 19 | 6 |
| β-Ti | - | Im-3m | 2 | 2 |
| Total | 100 | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakchieva, N.; Lepakova, O.; Abzaev, Y.; Sachkov, V.; Kurzina, I. The Influence of Scandium on the Composition and Structure of the Ti-Al Alloy Obtained by “Hydride Technology”. Nanomaterials 2021, 11, 918. https://doi.org/10.3390/nano11040918
Karakchieva N, Lepakova O, Abzaev Y, Sachkov V, Kurzina I. The Influence of Scandium on the Composition and Structure of the Ti-Al Alloy Obtained by “Hydride Technology”. Nanomaterials. 2021; 11(4):918. https://doi.org/10.3390/nano11040918
Chicago/Turabian StyleKarakchieva, Natalia, Olga Lepakova, Yuri Abzaev, Victor Sachkov, and Irina Kurzina. 2021. "The Influence of Scandium on the Composition and Structure of the Ti-Al Alloy Obtained by “Hydride Technology”" Nanomaterials 11, no. 4: 918. https://doi.org/10.3390/nano11040918
APA StyleKarakchieva, N., Lepakova, O., Abzaev, Y., Sachkov, V., & Kurzina, I. (2021). The Influence of Scandium on the Composition and Structure of the Ti-Al Alloy Obtained by “Hydride Technology”. Nanomaterials, 11(4), 918. https://doi.org/10.3390/nano11040918








