Electrografting of 4-Nitrobenzenediazonium Salts on Al-7075 Alloy Surfaces—The Role of Intermetallic Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Preparation of the Al-7075 Surfaces
2.2. Electrografting of 4-NBD on the Al-7075 Substrates
2.3. Characterisation of the Al-7075 Surfaces and 4-NBD Electrografted Films
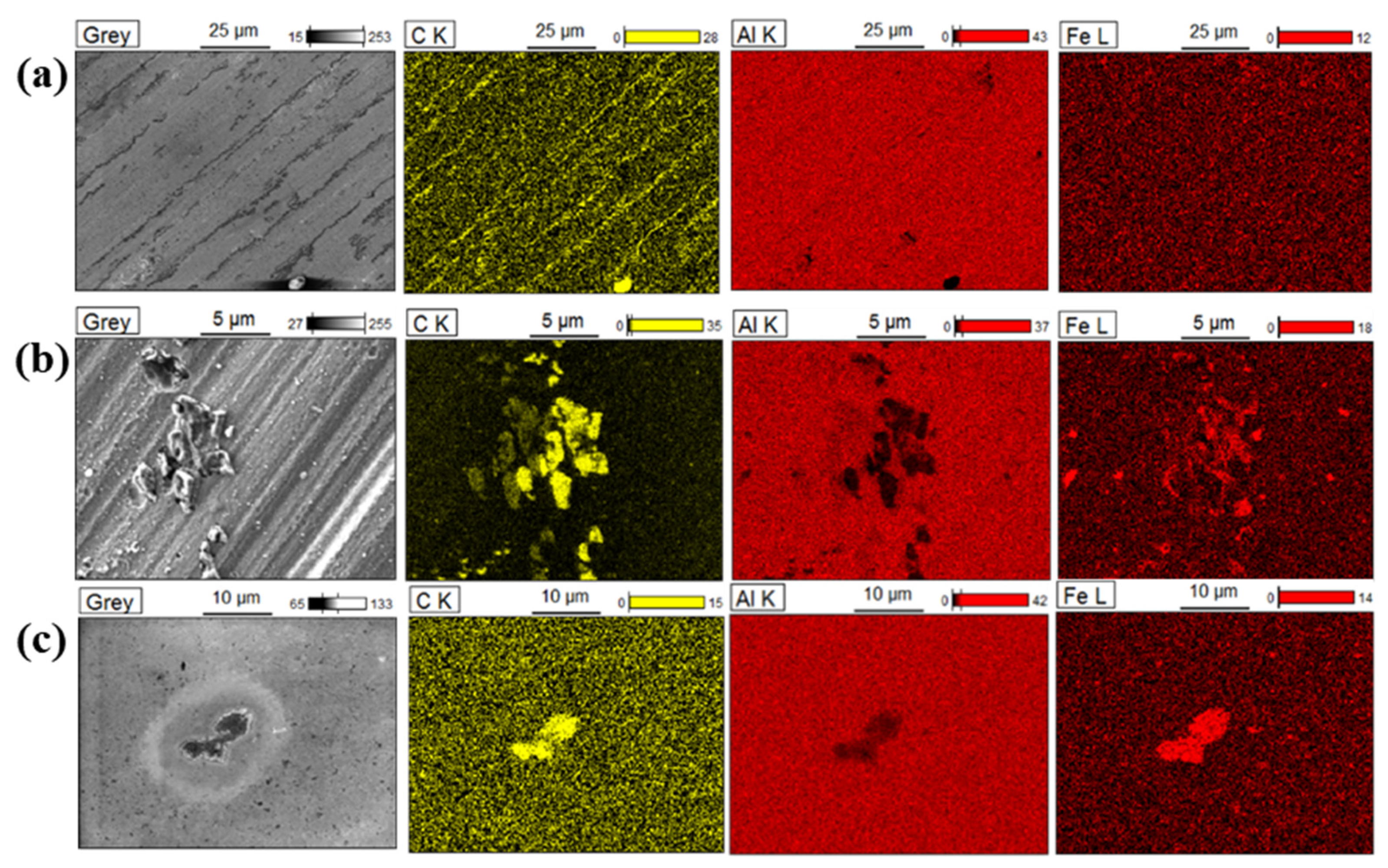
2.3.1. Scanning Electron Microscopy and Energy Dispersive X-ray Mapping (SEM-EDX)
2.3.2. PM-IRRAS Analysis
2.3.3. Raman Spectroscopy
2.3.4. X-ray Photoelectron Spectroscopy
2.3.5. SKPFM Analysis
3. Results
3.1. Physical Characterisation of the Bare Substrates
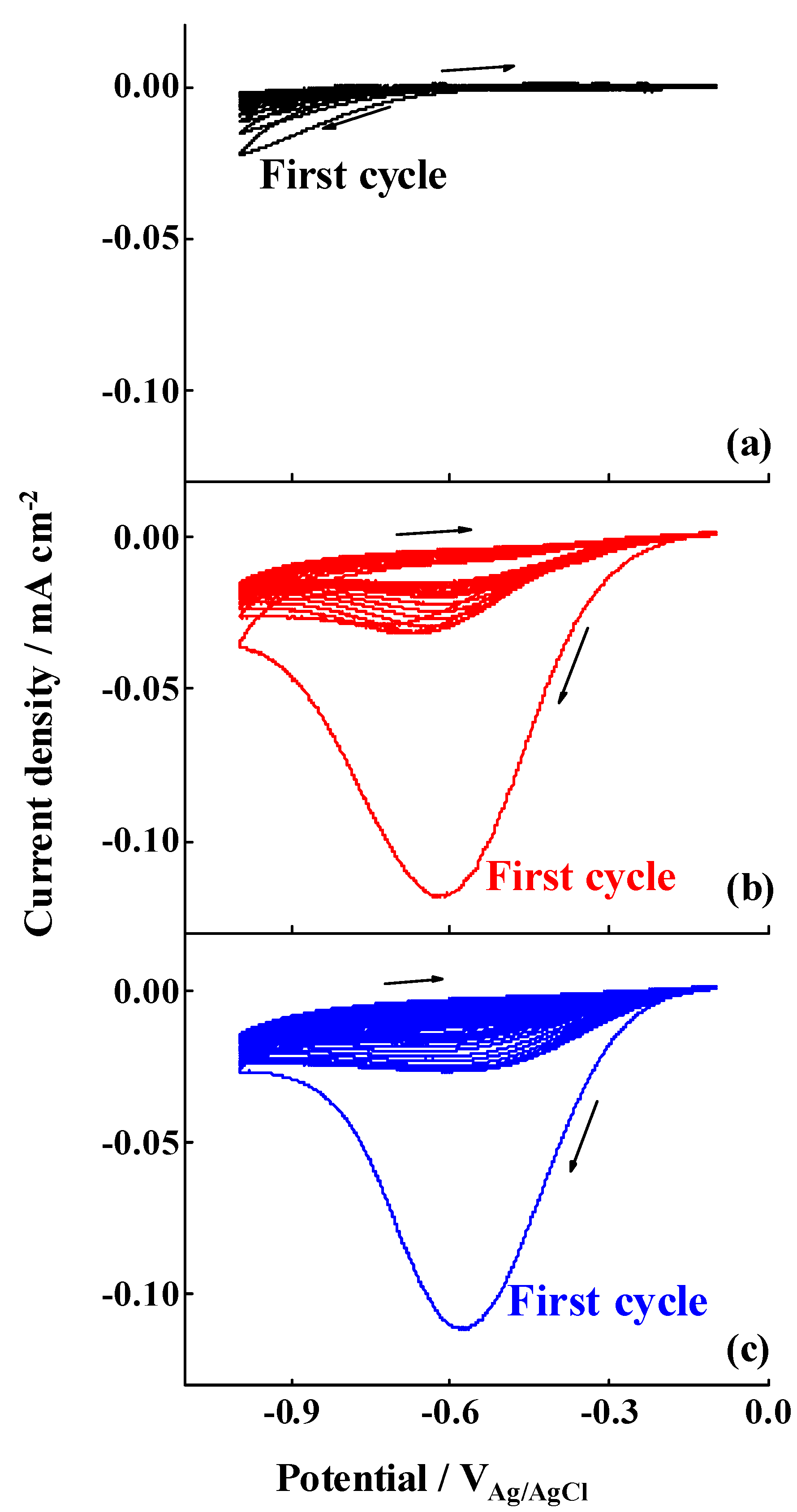
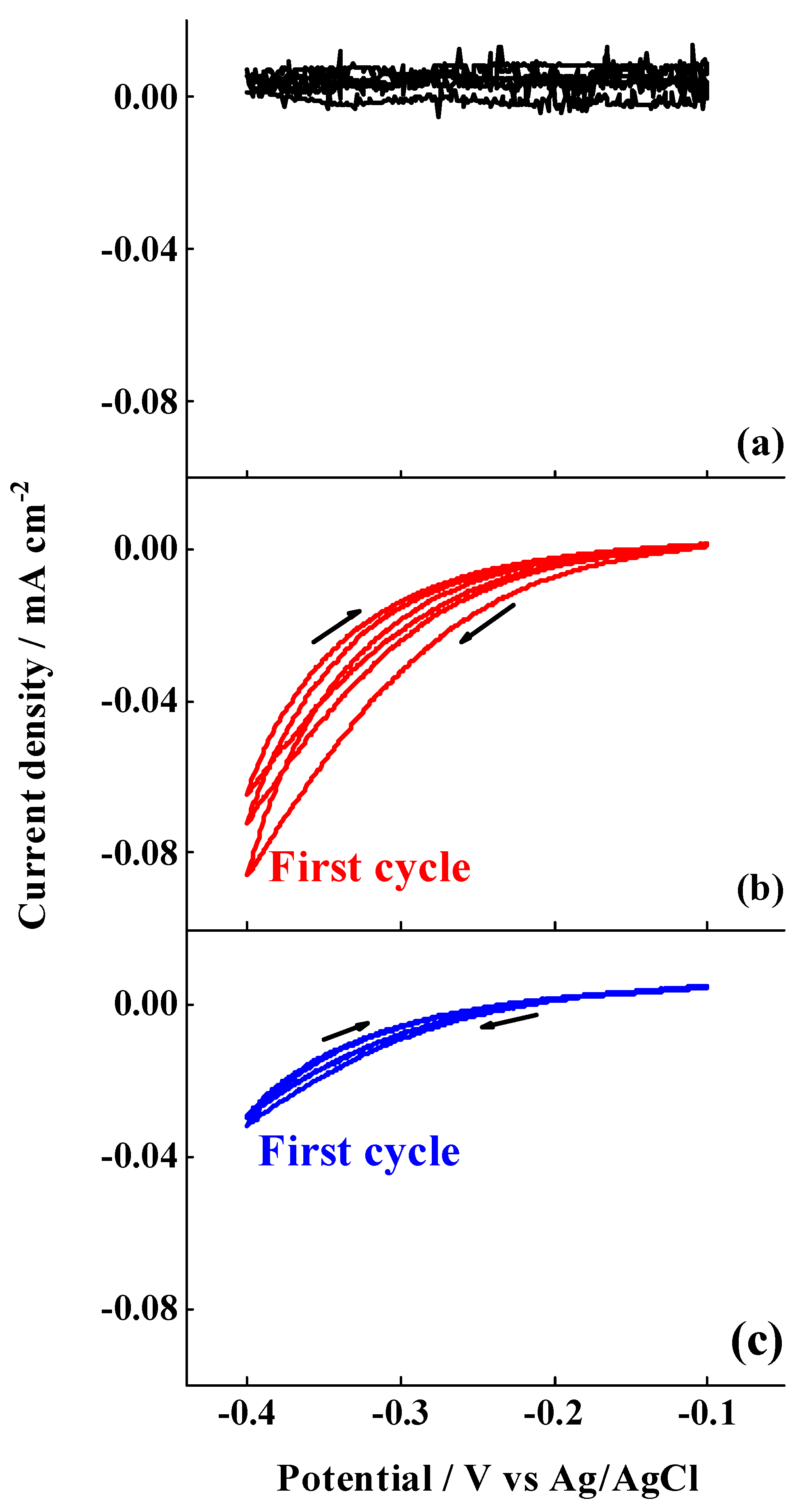
3.2. 4-NBD Electrografting of the Treated Al-7075 Substrates
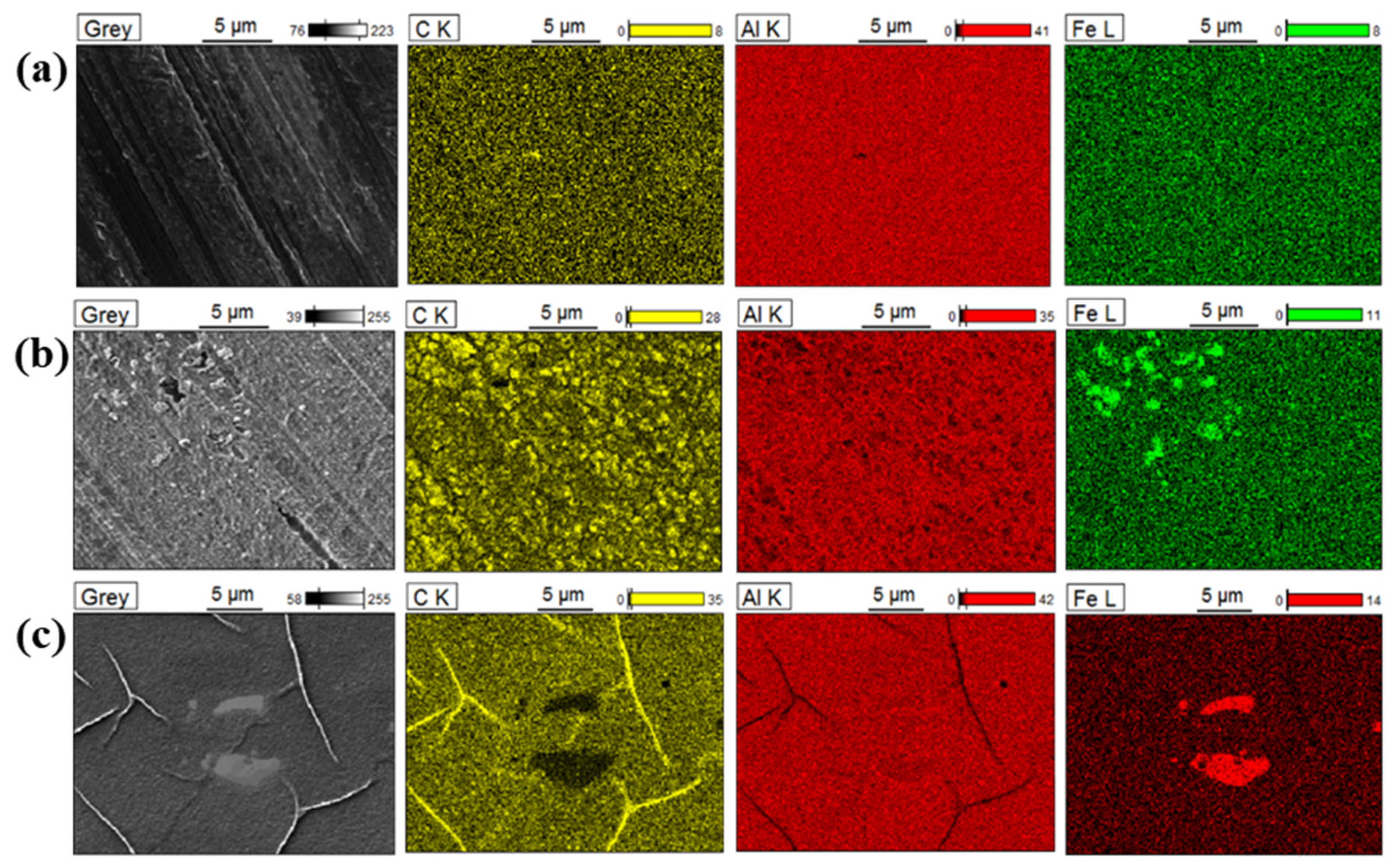
3.3. SEM-EDX Analysis of the 4-NBD-Electrografted Al-7075 Substrates
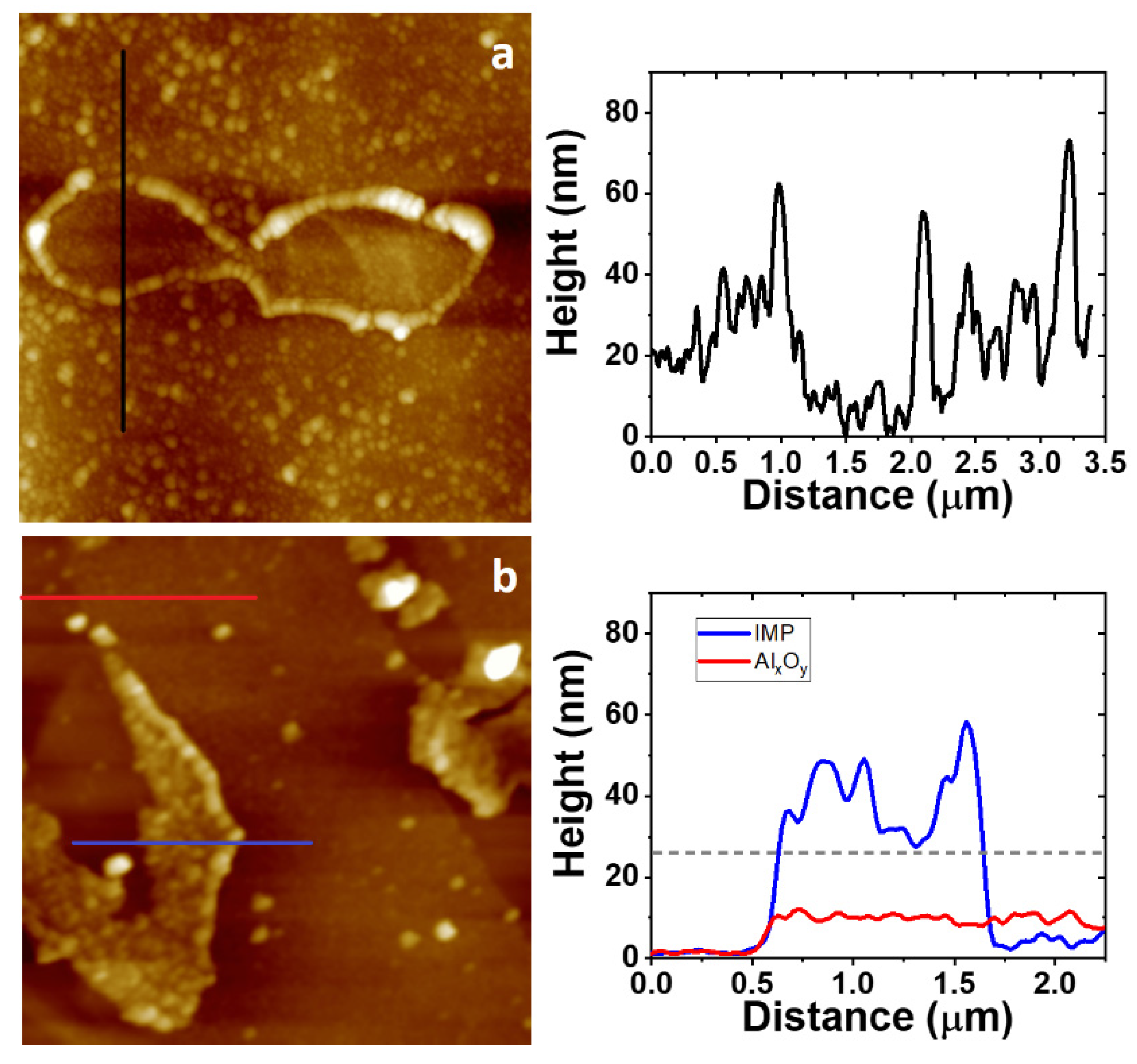
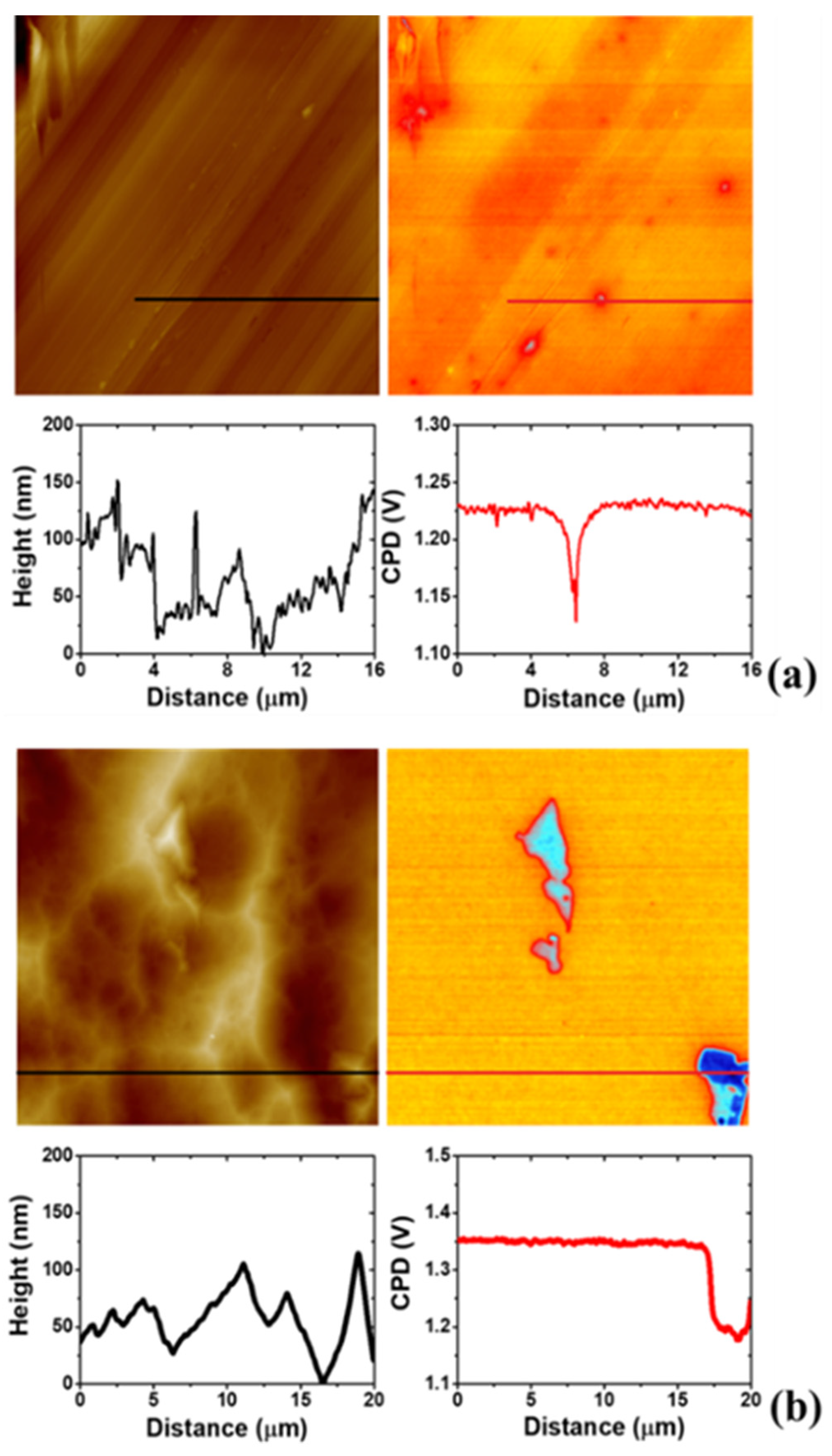
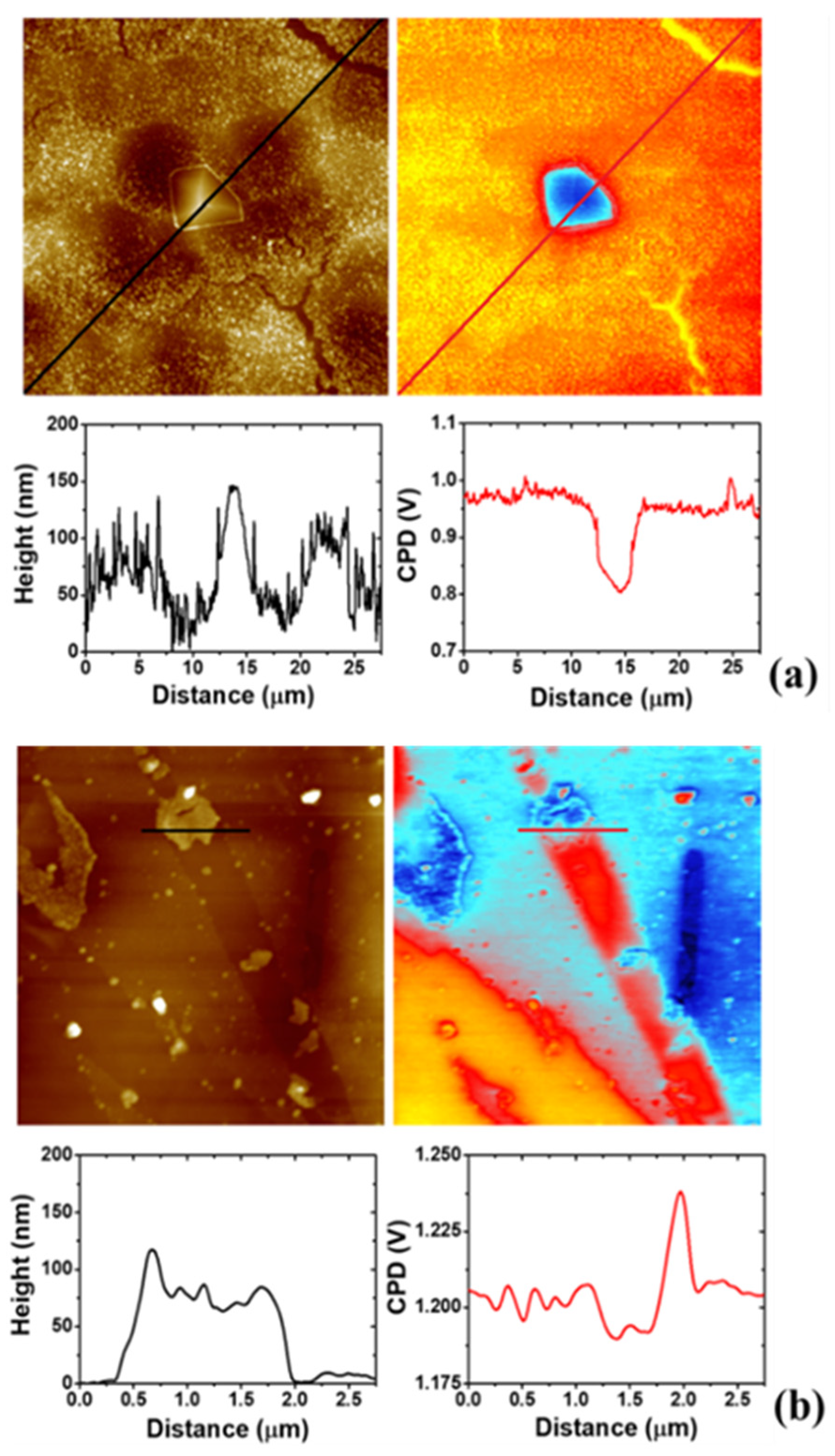
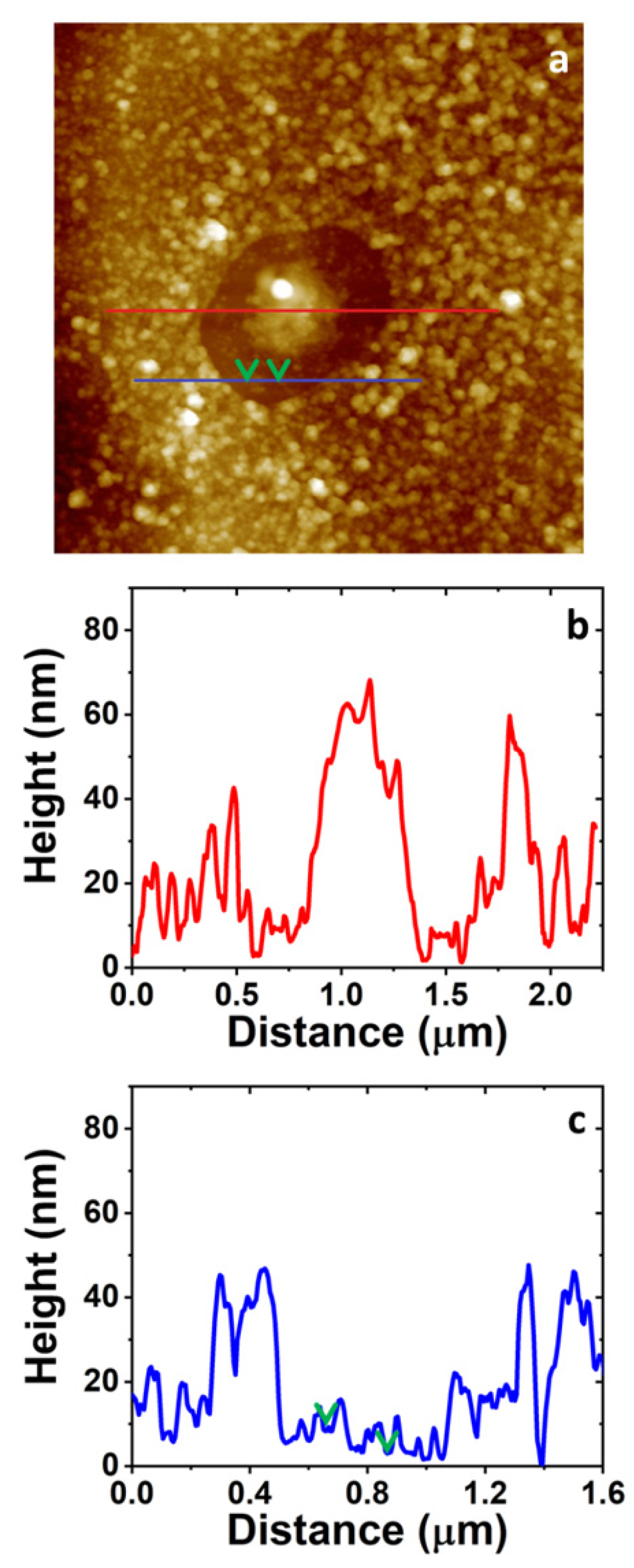
3.4. AFM Study on the Morphology of the Bare Substrates and the 4-NBD Films
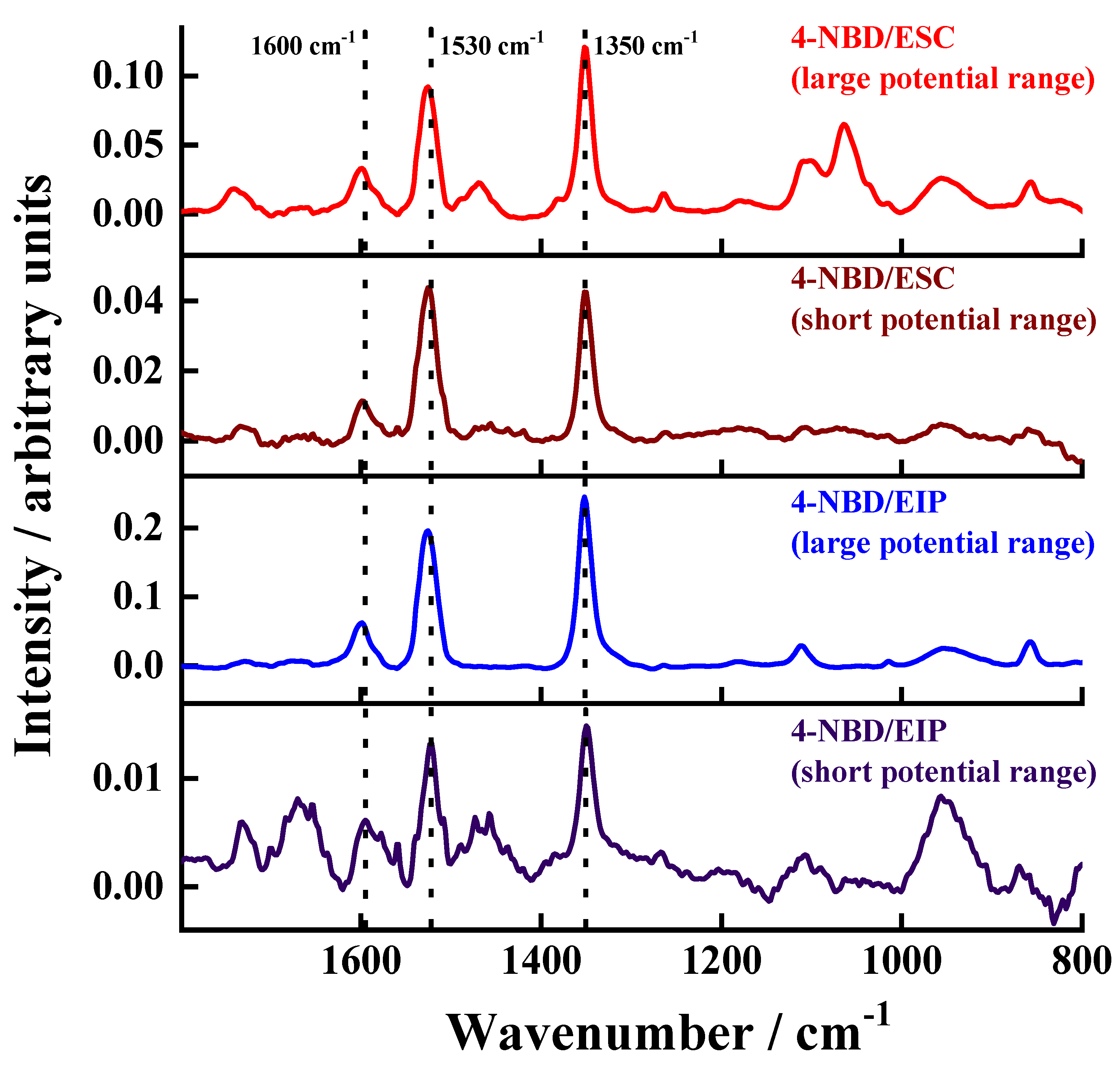
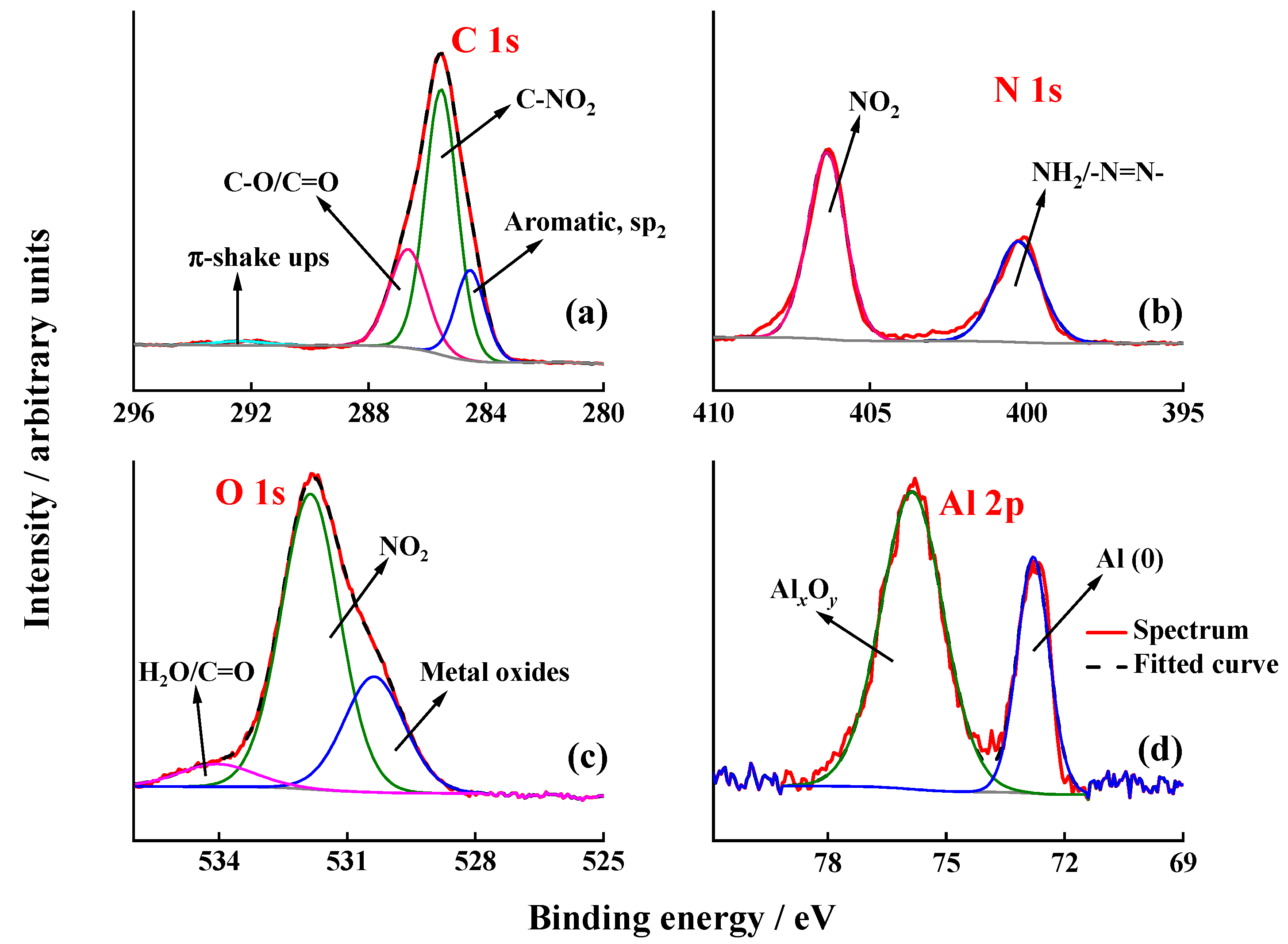
3.5. PM IRRAS, Raman and XPS Characterisation of the Electrografted Films
3.6. Scanning Kelvin Probe Force Microscopy (SKPFM) Analysis of the Modified Surfaces
3.7. Optimisation of the Growth of Nitrophenylene Films on Al-7075
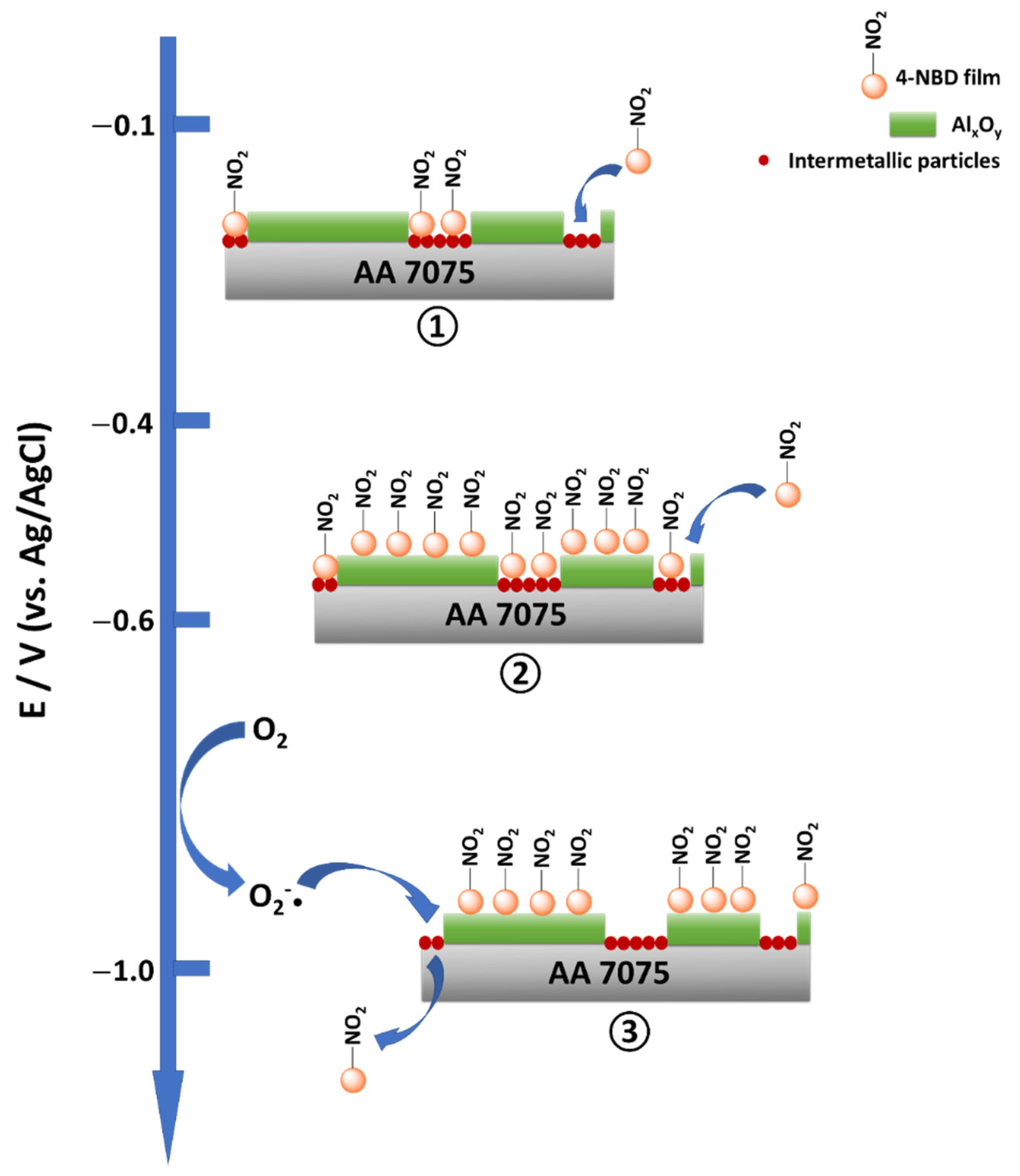
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behrens, B.A.; Hübner, S.; Sunderkötter, C.; Knigge, J.; Weilandt, K.; Voges-Schwieger, K. Local strain hardening of sheet and solid forming components during formation of martensite in metastable austenitic steels. Adv. Mat. Res. 2007, 22, 5–15. [Google Scholar]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic phases in aluminum alloys and their roles in localized corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Hughes, N.B.E.; Mol, J.M.C.; García, S.J.; Zhou, X.; Thompson, G.E. High Strength Al-Alloys: Microstructure, Corrosion and Principles of Protection. In Recent Trends in Processing and Degradation of Aluminium Alloys, 1st ed.; Ahmad, Z., Ed.; InTechOpen: London, UK, 2011; pp. 223–262. [Google Scholar]
- Birbilis, N.; Cavanaugh, M.K.; Buchheit, R.G. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651. Corros. Sci. 2006, 48, 4202–4215. [Google Scholar] [CrossRef]
- Mallinson, C.F.; Yates, P.M.; Baker, M.A.; Castle, J.E.; Harvey, A.; Watts, J.F. The localised corrosion associated with individual second phase particles in AA7075-T6: A study by SEM, EDX, AES, SKPFM and FIB-SEM. Mater. Corros. 2017, 68, 748–763. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Hu, J.; Tang, A.Q.; He, J.; Mao, J.H. A facile method for fabrication of superhydrophobic coating on aluminum alloy. Surf. Interface Anal. 2012, 44, 439–444. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, F.; Hao, J.; Liu, W. Effects of system parameters on making aluminum alloy lotus. J. Colloid Interface Sci. 2006, 303, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Revilla, R.I.; Terryn, H.; De Graeve, I. On the use of SKPFM for in situ studies of the re-passivation of the native oxide film on aluminium in air. Electrochem. Commun. 2018, 93, 162–165. [Google Scholar] [CrossRef]
- Alexander, M.R.; Thompson, G.E.; Beamson, G. Characterization of the oxide/hydroxide surface of aluminium using x-ray photoelectron spectroscopy: A procedure for curve fitting the O 1s core level. Surf. Interface Anal. 2000, 29, 468–477. [Google Scholar] [CrossRef]
- Giza, M.; Thissen, P.; Grundmeier, G. Adsorption kinetics of organophosphonic acids on plasma-modified oxide-covered aluminum surfaces. Langmuir 2008, 24, 8688–8694. [Google Scholar] [CrossRef]
- Thissen, P.; Valtiner, M.; Grundmeier, G. Stability of phosphonic acid self-assembled monolayers on amorphous and single-crystalline aluminum oxide surfaces in aqueous solution. Langmuir 2010, 26, 156–164. [Google Scholar] [CrossRef]
- Lewington, T.A.; Alexander, M.R.; Thompson, G.E.; McAlpine, E. Bodycote Prize Paper: Characterisation of alkyl phosphonic acid monolayers self assembled on hydrated surface of aluminium. Surf. Eng. 2002, 18, 228–232. [Google Scholar] [CrossRef]
- Hauffman, T.; Blajiev, O.; Snauwaert, J.; van Haesendonck, C.; Hubin, A.; Terryn, H. Study of the self-assembling of n-octylphosphonic acid layers on aluminum oxide. Langmuir 2008, 24, 13450–13456. [Google Scholar] [CrossRef]
- Telegdi, J.; Luciano, G.; Mahanty, S.; Abohalkuma, T. Inhibition of aluminum alloy corrosion in electrolytes by self-assembled fluorophosphonic acid molecular layer. Mater. Corros. 2016, 67, 1027–1033. [Google Scholar] [CrossRef][Green Version]
- Van den Brand, J.; Blajiev, O.; Beentjes, P.C.J.; Terryn, H.; de Wit, J.H.W. Interaction of ester functional groups with aluminum oxide surfaces studied using infrared reflection absorption spectroscopy. Langmuir 2004, 20, 6318–6326. [Google Scholar] [CrossRef]
- Liascukiene, I.; Aissaoui, N.; Asadauskas, S.J.; Landoulsi, J.; Lambert, J.F. Ordered nanostructures on a hydroxylated aluminum surface through the self-assembly of fatty acids. Langmuir 2012, 28, 5116–5124. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering regimes of porous alumina: The 10 porosity rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.; Paynter, R.; Chen, X. Superhydrophobic aluminum alloy surfaces by a novel one-step process. ACS Appl. Mater. Interfaces 2010, 2, 2500–2502. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interfaces 2011, 3, 4775–4781. [Google Scholar] [CrossRef]
- Hurley, B.L.; McCreery, R.L. Covalent bonding of organic molecules to Cu and Al alloy 2024 T3 surfaces via diazonium ion reduction. J. Electrochem. Soc. 2004, 151, B252–B259. [Google Scholar] [CrossRef]
- Doublet, A.; Kjellberg, M.; Jousselme, B.; Pinault, M.; Deniau, G.; Cornut, R.; Charrier, G. Bifunctional coatings: Coupling an organic adhesion promoter with an anticorrosion inorganic layer. RSC Adv. 2019, 9, 24043–24049. [Google Scholar] [CrossRef]
- Pinson, J.; Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 2005, 34, 429–439. [Google Scholar] [CrossRef]
- Delamar, M.; Hitmi, R.; Pinson, J.; Saveant, J.M. Covalent modification of carbon surfaces by grafting of functionalized aryl radicals produced from electrochemical reduction of diazonium salts. J. Am. Chem. Soc. 1992, 114, 5883–5884. [Google Scholar] [CrossRef]
- Leroux, Y.R.; Hapiot, P. Nanostructured monolayers on carbon substrates prepared by electrografting of protected aryldiazonium salts. Chem. Mater. 2013, 25, 489–495. [Google Scholar] [CrossRef]
- Pichereau, L.; López, I.; Cesbron, M.; Dabos-Seignon, S.; Gautier, C.; Breton, T. Controlled diazonium electrografting driven by overpotential reduction: A general strategy to prepare ultrathin layers. Chem. Commun. 2019, 55, 455–457. [Google Scholar] [CrossRef]
- González, M.C.R.; González-Orive, A.; Salvarezza, R.C.; Creus, A.H. Electrodeposition of gold nanoparticles on aryl diazonium monolayer functionalized HOPG surfaces. Phys. Chem. Chem. Phys. 2016, 18, 1953–1960. [Google Scholar] [CrossRef]
- González, M.C.R.; Carro, P.; Creus, A.H. Morphological changes in electrografted aryl-based thin films induced by using diazonium salts or aryl iodides. ChemElectroChem 2018, 5, 464–470. [Google Scholar] [CrossRef]
- González, M.C.R.; Rivera, L.M.; Pastor, E.; Creus, A.H.; García, G. A facile method for the fabrication of hierarchical nanosized metal catalysts. J. Catal. 2018, 366, 1–7. [Google Scholar] [CrossRef]
- Lee, L.; Ma, H.; Brooksby, P.A.; Brown, S.A.; Leroux, Y.R.; Hapiot, P.; Downard, A.J. Covalently anchored carboxyphenyl monolayer via aryldiazonium ion grafting: A well-defined reactive tether layer for on-surface chemistry. Langmuir 2014, 30, 7104–7111. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, M.; Bousquet, A.; Hinge, M.; Pedersen, S.U.; Daasbjerg, K. Using a mediating effect in the electroreduction of aryldiazonium salts to prepare conducting organic films of high thickness. Chem. Mater. 2011, 23, 1551–1557. [Google Scholar] [CrossRef]
- Betelu, S.; Tijunelyte, I.; Boubekeur-Lecaque, L.; Ignatiadis, I.; Ibrahim, J.; Gaboreau, S.; Berho, C.; Toury, T.; Guenin, E.; Lidgi-Guigui, N.; et al. Evidence of the grafting mechanisms of diazonium salts on gold nanostructures. J. Phys. Chem. C 2016, 120, 18158–18166. [Google Scholar] [CrossRef]
- Adenier, A.; Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Formation of polyphenylene films on metal electrodes by electrochemical reduction of benzenediazonium salts. Chem. Mater. 2006, 18, 2021–2029. [Google Scholar] [CrossRef]
- Adenier, A.; Bernard, M.C.; Chehimi, M.M.; Cabet-Deliry, E.; Desbat, B.; Fagebaume, O.; Pinson, J.; Podvorica, F. Covalent modification of iron surfaces by electrochemical reduction of aryldiazonium salts. J. Am. Chem. Soc. 2001, 123, 4541–4549. [Google Scholar] [CrossRef]
- Adenier, A.; Cabet-Deliry, E.; Lalot, T.; Pinson, J.; Podvorica, F. Attachment of polymers to organic moieties covalently bonded to iron surfaces. Chem. Mater. 2002, 14, 4576–4585. [Google Scholar] [CrossRef]
- Chaussé, A.; Chehimi, M.M.; Karsi, N.; Pinson, J.; Podvorica, F.; Vautrin-Ul, C. The electrochemical Reduction of diazonium salts on iron electrodes. The formation of covalently bonded organic layers and their effect on corrosion. Chem. Mater. 2002, 14, 392–400. [Google Scholar] [CrossRef]
- Wang, C.; Huang, N.; Zhuang, H.; Jiang, X. Enhanced performance of nanocrystalline ZnO DNA biosensor via introducing electrochemical covalent biolinkers. ACS Appl. Mater. Interfaces 2015, 7, 7605–7612. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Delhalle, J.; Mekhalif, Z. Hybrid coating on steel: ZnNi electrodeposition and surface modification with organothiols and diazonium salts. Electrochim. Acta 2008, 53, 2852–2861. [Google Scholar] [CrossRef]
- Kullapere, M.; Matisen, L.; Saar, A.; Sammelselg, V.; Tammeveski, K. Electrochemical behaviour of nickel electrodes modified with nitrophenyl groups. Electrochem. Commun. 2007, 9, 2412–2417. [Google Scholar] [CrossRef]
- Hicks, J.M.; Wong, Z.Y.; Scurr, D.J.; Silman, N.; Jackson, S.K.; Mendes, P.M.; Aylott, J.W.; Rawson, F.J. Tailoring the electrochemical properties of carbon nanotube modified indium tin oxide via in situ grafting of aryl diazonium. Langmuir 2017, 33, 4924–4933. [Google Scholar] [CrossRef]
- Lo, M.; Pires, R.; Diaw, K.; Gningue-Sall, D.; Oturan, M.A.; Aaron, J.J.; Chehimi, M.M. Diazonium salts: Versatile molecular glues for sticking conductive polymers to flexible electrodes. Surfaces 2018, 1, 43–58. [Google Scholar] [CrossRef]
- Bakas, I.; Yilmaz, G.; Ait-Touchente, Z.; Lamouri, A.; Lang, P.; Battaglini, N.; Carbonnier, B.; Chehimi, M.M.; Yagci, Y. Diazonium salts for surface-confined visible light radical photopolymerization. J. Polym. Sci. Polym. Chem. 2016, 54, 3506–3515. [Google Scholar] [CrossRef]
- Hinge, M.; Ceccato, M.; Kingshott, P.; Besenbacher, F.; Pedersen, S.U.; Daasbjerg, K. Electrochemical modification of chromium surfaces using 4-nitro-and 4-fluorobenzenediazonium salts. New J. Chem. 2009, 33, 2405–2408. [Google Scholar] [CrossRef]
- Atmane, Y.A.; Sicard, L.; Lamouri, A.; Pinson, J.; Sicard, M.; Masson, C.; Nowak, S.; Decorse, P.; Piquemal, J.Y.; Galtayries, A.; et al. Functionalization of aluminum nanoparticles using a combination of aryl diazonium salt chemistry and iniferter method. J. Phys. Chem. C 2013, 117, 26000–26006. [Google Scholar] [CrossRef]
- Berisha, A.; Hazimeh, H.; Galtayries, A.; Decorse, P.; Kanoufi, F.; Combellas, C.; Pinson, J.; Podvorica, F.I. Grafting of an aluminium surface with organic layers. RSC Adv. 2016, 6, 78369–78377. [Google Scholar] [CrossRef]
- Quinton, D.; Galtayries, A.; Prima, F.; Griveau, S. Functionalization of titanium surfaces with a simple electrochemical strategy. Surf. Coat. Technol. 2012, 206, 2302–2307. [Google Scholar] [CrossRef]
- Jacques, A.; Chehimi, M.M.; Poleunis, C.; Delcorte, A.; Delhalle, J.; Mekhalif, Z. Grafting of 4-pyrrolyphenyldiazonium in situ generated on NiTi, an adhesion promoter for pyrrole electropolymerisation? Electrochim. Acta 2016, 211, 879–890. [Google Scholar] [CrossRef]
- Hinge, M.; Gonçalves, E.S.; Pedersen, S.U.; Daasbjerg, K. On the electrografting of stainless steel from para-substituted aryldiazonium salts and the thermal stability of the grafted layer. Surf. Coat. Technol. 2010, 205, 820–827. [Google Scholar] [CrossRef]
- Oweis, Y.; Alageel, O.; Kozak, P.; Abdallah, M.N.; Retrouvey, J.M.; Cerruti, M.; Tamimi, F. Metal-composite adhesion based on diazonium chemistry. Dent. Mater. 2017, 33, e393–e404. [Google Scholar] [CrossRef] [PubMed]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Role of intermetallics in corrosion of aluminum alloys. Smart corrosion protection. In Intermetallic Matrix Composites, 1st ed.; Mitra, R., Ed.; Woodhead Publishing: Duxford, UK, 2018; Chapter 6; pp. 425–462. [Google Scholar]
- Örnek, C.; Leygraf, C.; Pan, J. On the Volta potential measured by SKPFM–fundamental and practical aspects with relevance to corrosion science. Corros. Eng. Sci. Technol. 2019, 54, 185–198. [Google Scholar] [CrossRef]
- Örnek, C.; Liu, M.; Pan, J.; Jin, Y.; Leygraf, C. Volta potential evolution of intermetallics in aluminum alloy microstructure under thin aqueous adlayers: A combined DFT and experimental study. Top. Catal. 2018, 61, 1169–1182. [Google Scholar] [CrossRef]
- Wysocka, J.; Cieslik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; David, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy, 1st ed.; Perkin-Elmer Corporation: Eden Praire, MA, USA, 1978. [Google Scholar]
- King, D.E.; Swartz, W.E. Variable-angle X-ray photoelectron spectroscopic determination of the thickness of the oxide layer on aluminum metal: An advanced undergraduate laboratory experiment. J. Chem. Educ. 1987, 64, 981–983. [Google Scholar] [CrossRef]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminum alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths for 31 materials. Surf. Interface Anal. 1988, 11, 577–589. [Google Scholar] [CrossRef]
- Hoppe, C.; Ebbert, C.; Grothe, R.; Schmidt, H.C.; Hordych, I.; Homberg, W.; Maier, H.J.; Grundmeier, G. Influence of the surface and heat treatment on the bond strength of galvanized steel/aluminum composites joined by plastic deformation. Adv. Eng. Mater. 2016, 18, 1371–1380. [Google Scholar] [CrossRef]
- Adenier, A.; Cabet-Deliry, E.; Chaussé, A.; Griveau, S.; Mercier, F.; Pinson, J.; Vautrin-Ul, C. Grafting of nitrophenyl groups on carbon and metallic surfaces without electrochemical induction. Chem. Mater. 2005, 17, 491–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Yang, B.; Ma, L. Synergistic effect of ferrous ion and copper oxide on the oxidative degradation of aqueous acetaminophen at acid conditions: A mechanism investigation. Chem. Eng. J. 2017, 326, 612–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Yang, B.; Huang, W.; Ma, L. Copper–catalyzed activation of molecular oxygen for oxidative destruction of acetaminophen: The mechanism and superoxide-mediated cycling of copper species. Chemosphere 2017, 166, 89–95. [Google Scholar] [CrossRef]
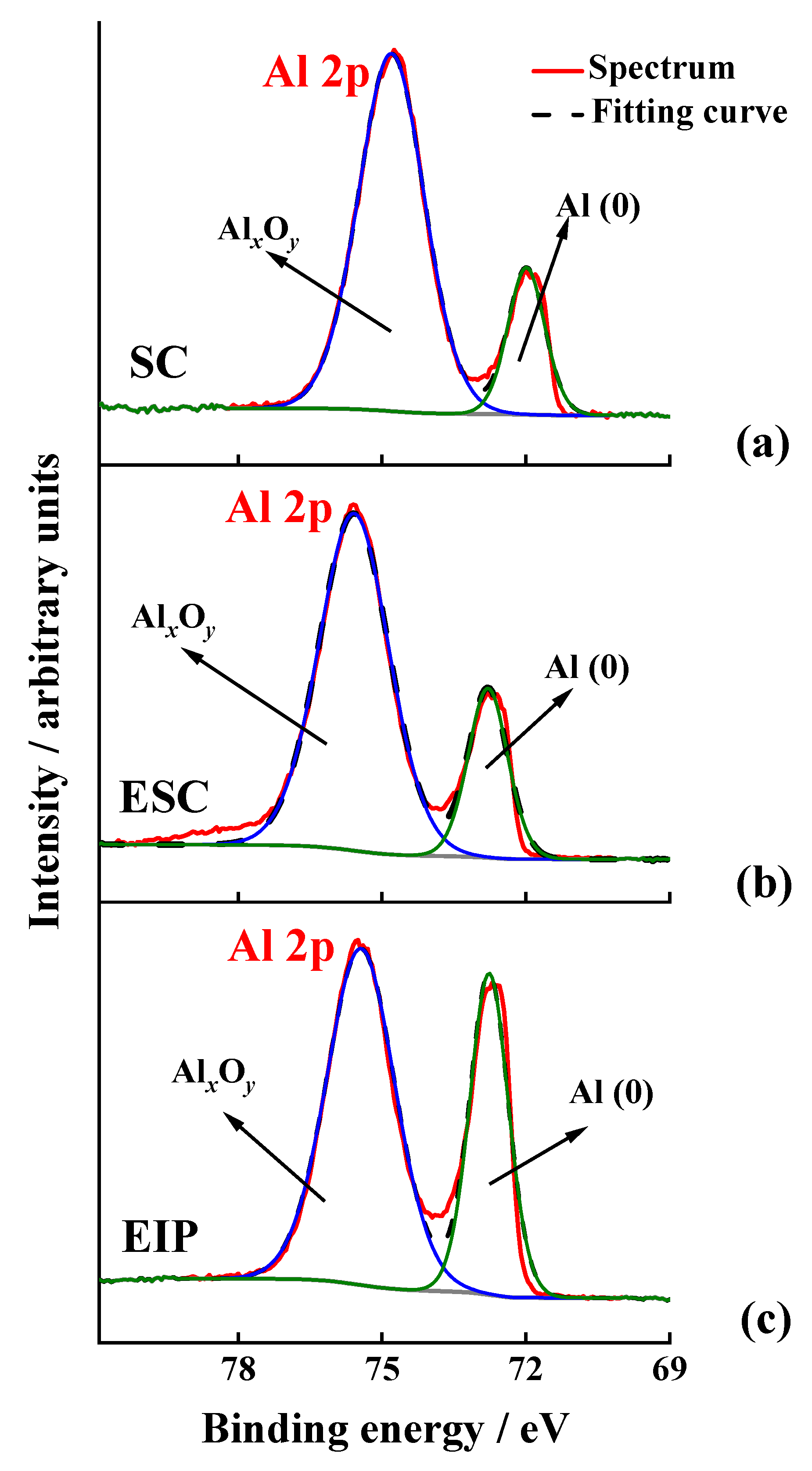
| Substrate | Atomic Percentage per Element (at-%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C 1s | N 1s | O 1s | Al 2p | Zn 2p3/2 | Fe 2p3/2 | Cu 2p3/2 | Mg 2s | |
| SC | 44.8 | 1.3 | 32.3 | 21.5 | 0.2 | - | - | - |
| ESC | 15.9 | 0.3 | 45.7 | 37.2 | 0.5 | - | 0.2 | 0.1 |
| EIP | 19.4 | 0.2 | 41.1 | 38.7 | 0.1 | 0.2 | 0.2 | 0.1 |
| Substrate | IOx/Area% | IM/Area% | λOx/nm | λM/nm | NOx | NM | sin θ | d/nm |
|---|---|---|---|---|---|---|---|---|
| SC | 81.3 | 18.7 | 2.8 | 2.6 | 1.0 | 1.5 | 0.5 | 2.7 |
| ESC | 77.9 | 22.1 | 2.8 | 2.6 | 1.0 | 1.5 | 0.5 | 2.5 |
| EIP | 65.1 | 34.9 | 2.8 | 2.6 | 1.0 | 1.5 | 0.5 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Calderón Gómez, J.C.; Grundmeier, G.; González Orive, A. Electrografting of 4-Nitrobenzenediazonium Salts on Al-7075 Alloy Surfaces—The Role of Intermetallic Particles. Nanomaterials 2021, 11, 894. https://doi.org/10.3390/nano11040894
Su J, Calderón Gómez JC, Grundmeier G, González Orive A. Electrografting of 4-Nitrobenzenediazonium Salts on Al-7075 Alloy Surfaces—The Role of Intermetallic Particles. Nanomaterials. 2021; 11(4):894. https://doi.org/10.3390/nano11040894
Chicago/Turabian StyleSu, Jiangling, Juan Carlos Calderón Gómez, Guido Grundmeier, and Alejandro González Orive. 2021. "Electrografting of 4-Nitrobenzenediazonium Salts on Al-7075 Alloy Surfaces—The Role of Intermetallic Particles" Nanomaterials 11, no. 4: 894. https://doi.org/10.3390/nano11040894
APA StyleSu, J., Calderón Gómez, J. C., Grundmeier, G., & González Orive, A. (2021). Electrografting of 4-Nitrobenzenediazonium Salts on Al-7075 Alloy Surfaces—The Role of Intermetallic Particles. Nanomaterials, 11(4), 894. https://doi.org/10.3390/nano11040894








