Experimental Study on the Drag Reduction Performance of Clear Fracturing Fluid Using Wormlike Surfactant Micelles and Magnetic Nanoparticles under a Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of FE-NPs
2.3. Preparation of Magnetic WLM Fracturing Fluid
2.4. Characterizations of the FE-NPs and the Magnetic WLM System
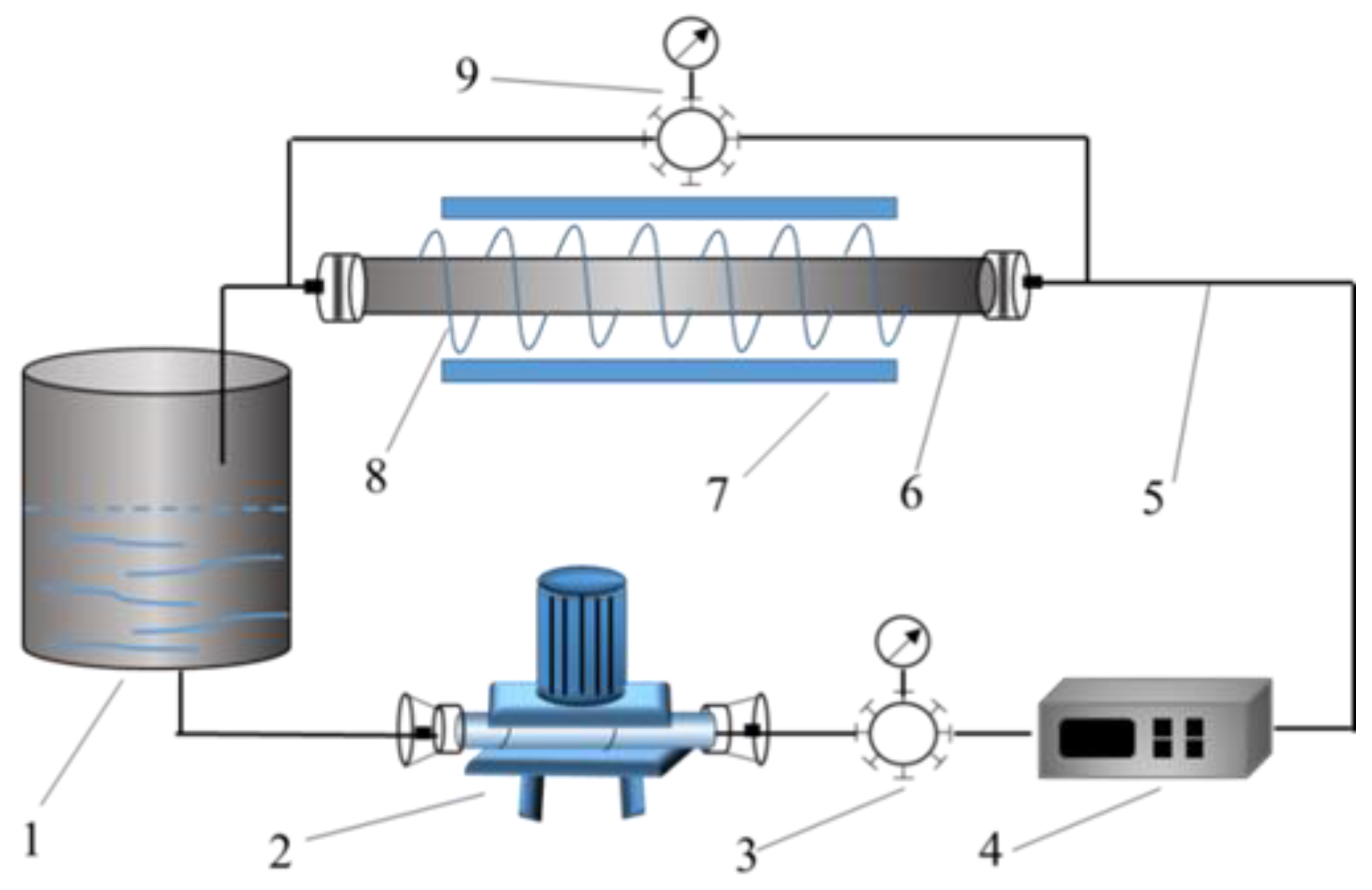
2.5. Test Facility
3. Results and Discussions
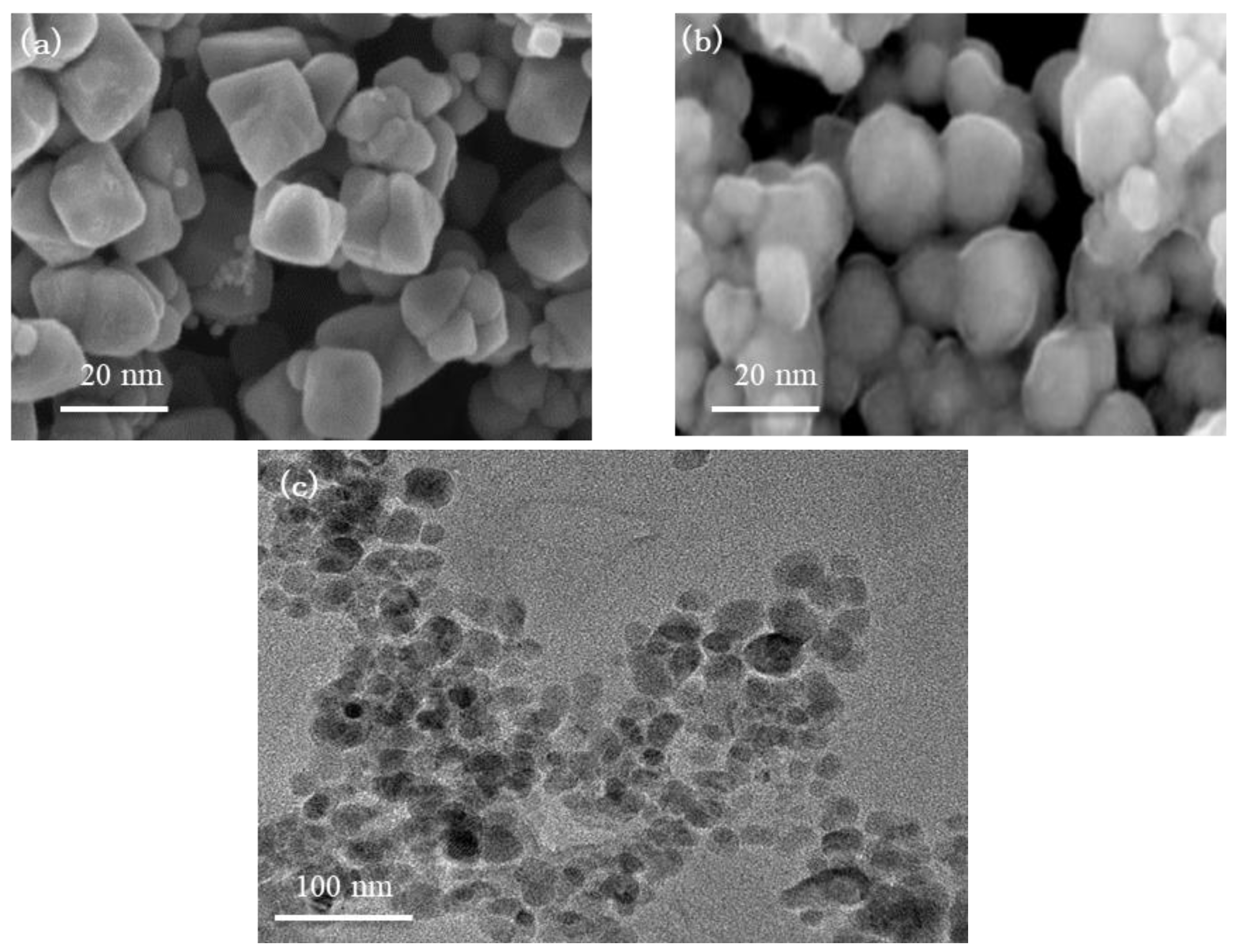
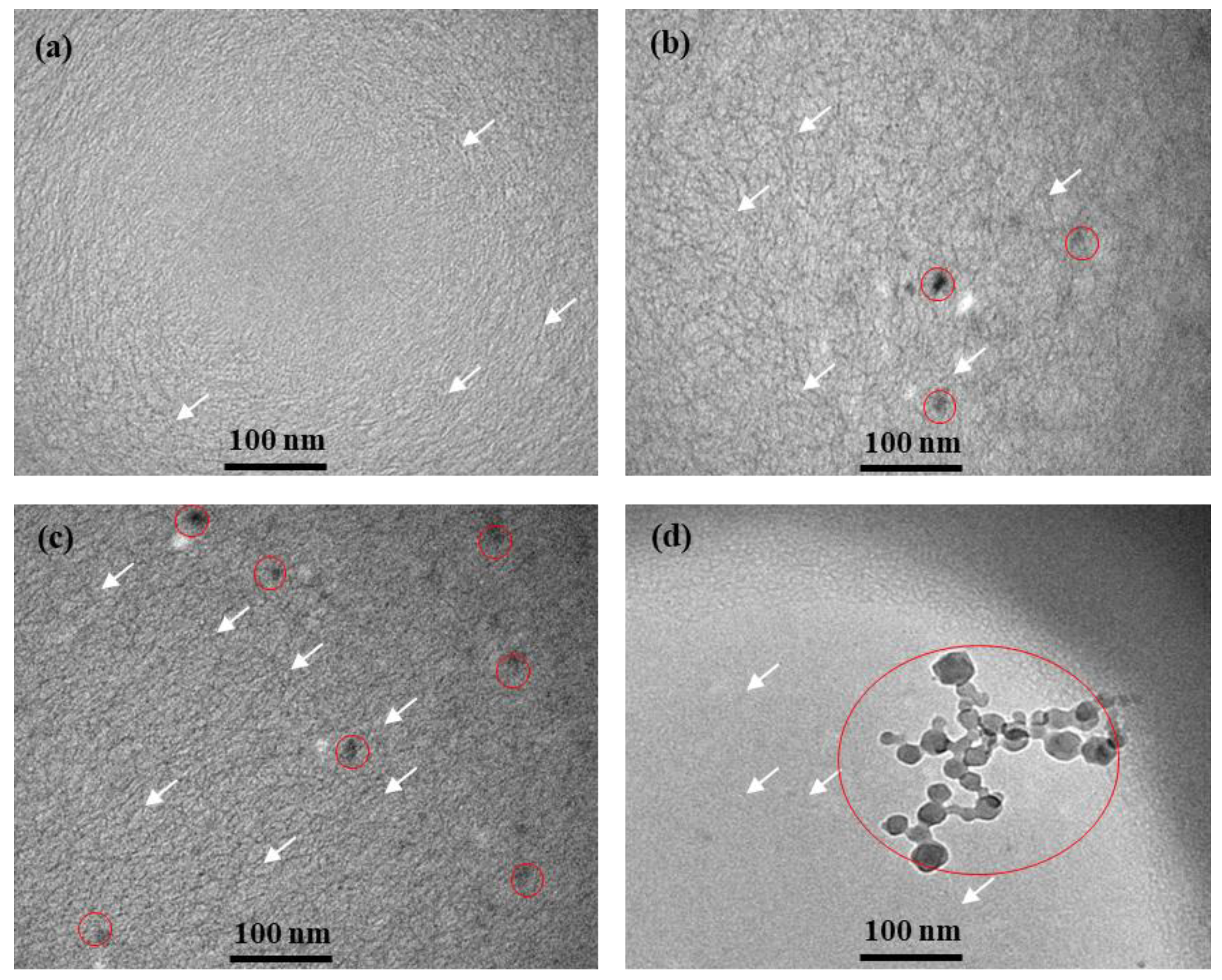
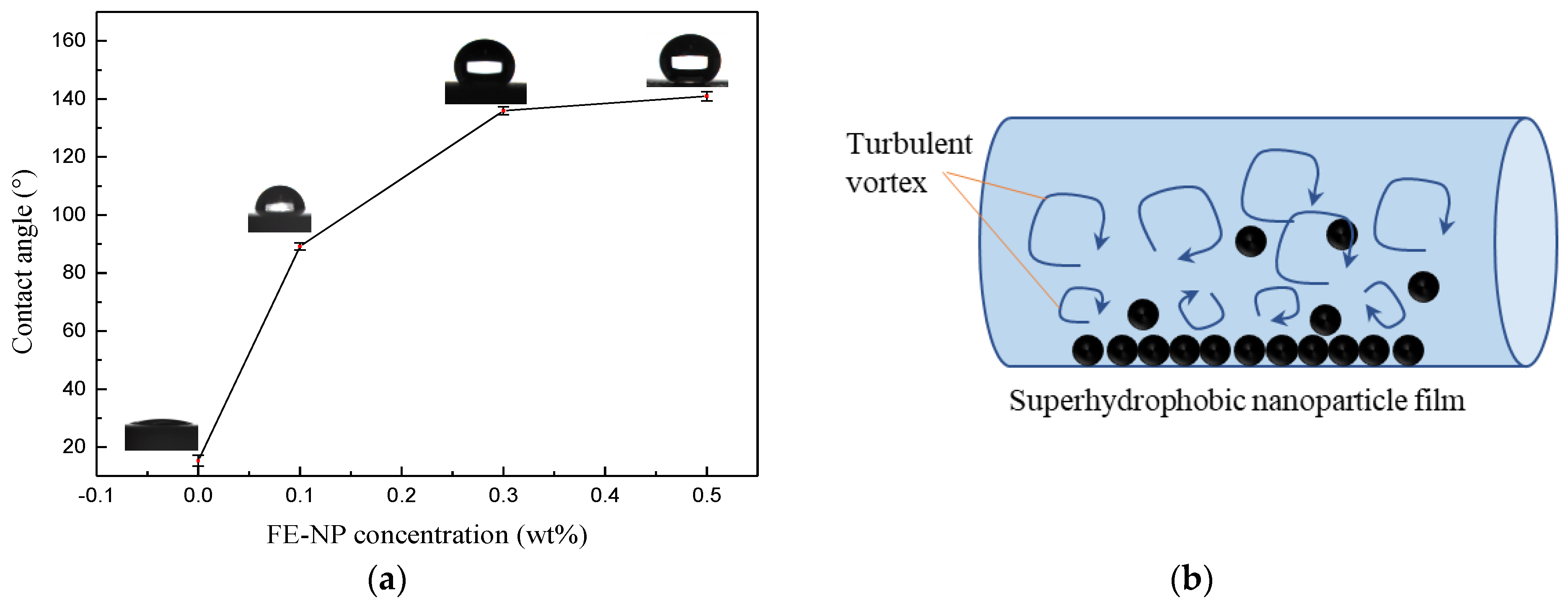
3.1. Chemical Groups and Micro Morphology of the FE-NPs
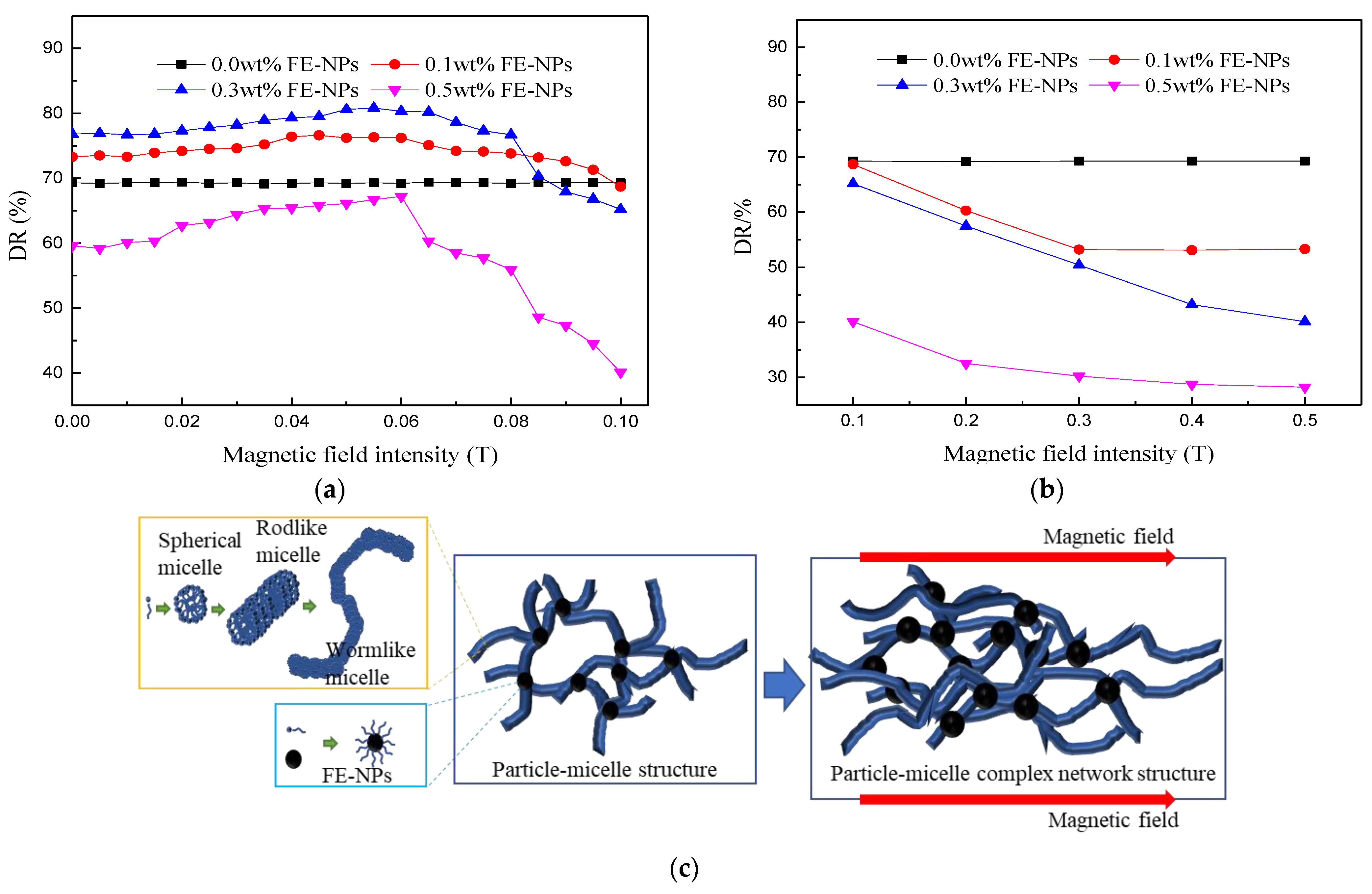
3.2. Drag Reduction Performance of WLMs as Fracturing Fluids
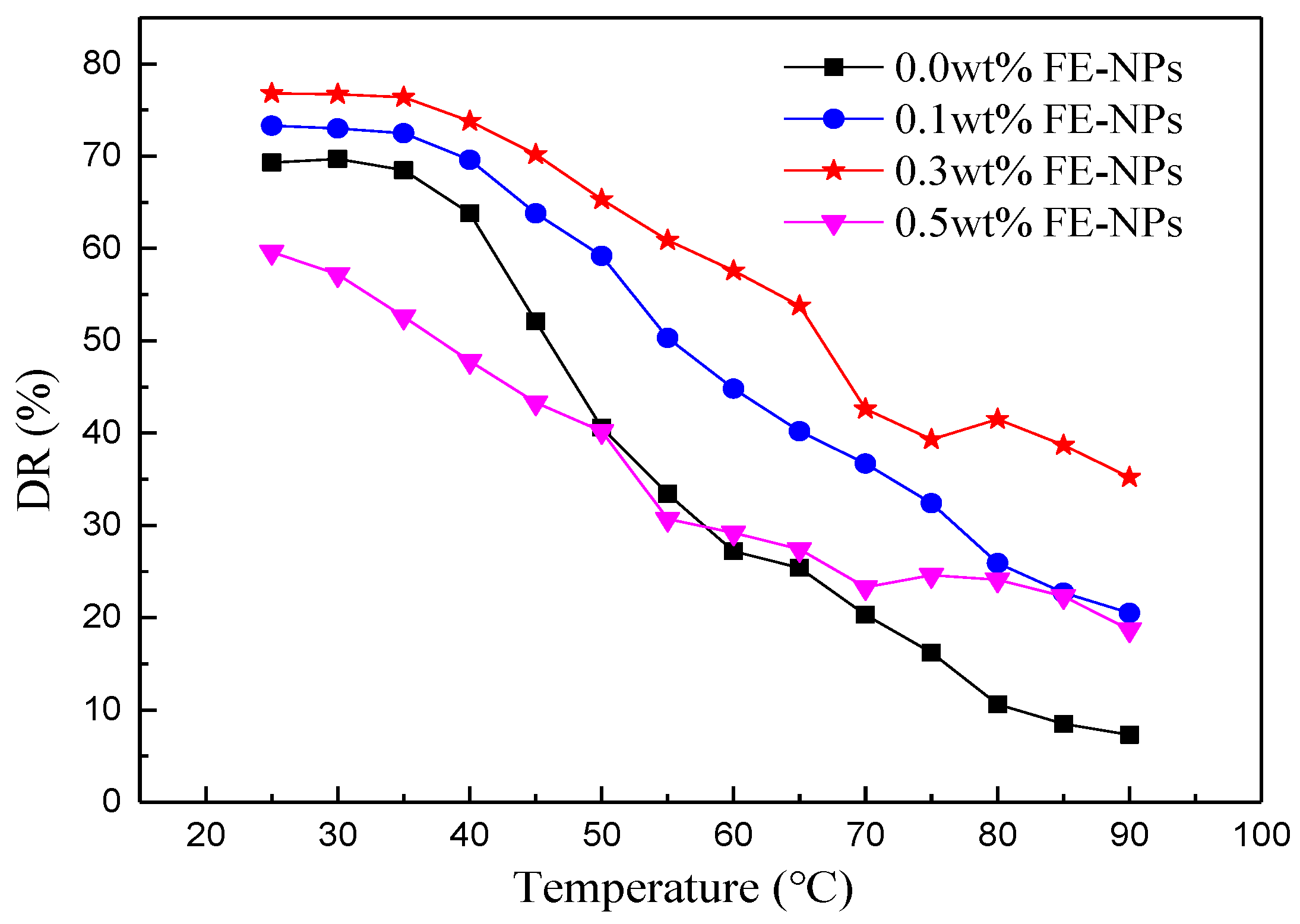
3.2.1. Effects of the Temperature and FE-NP Concentration
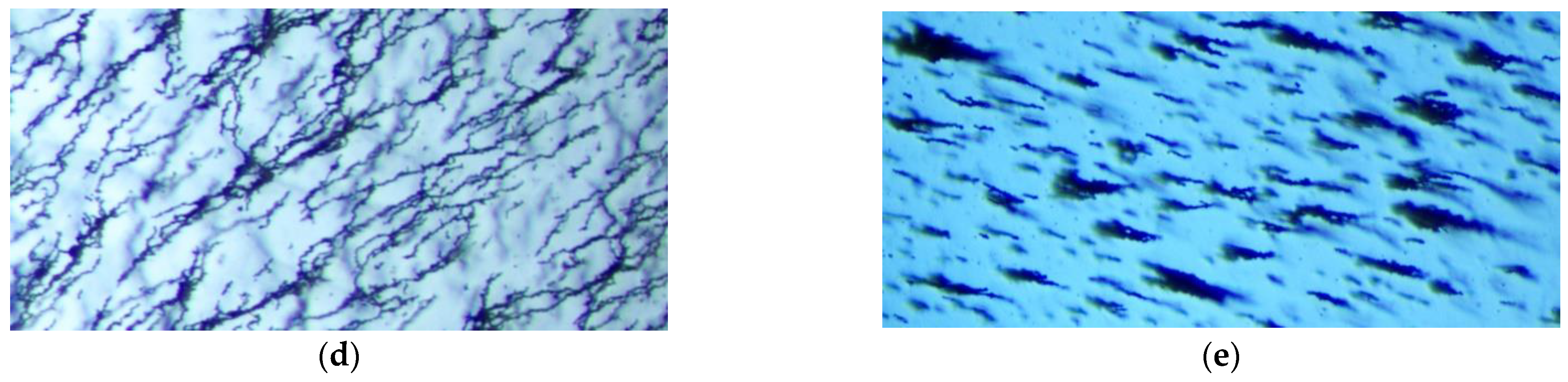
3.2.2. Effect of the Horizontal Magnetic Field Intensity
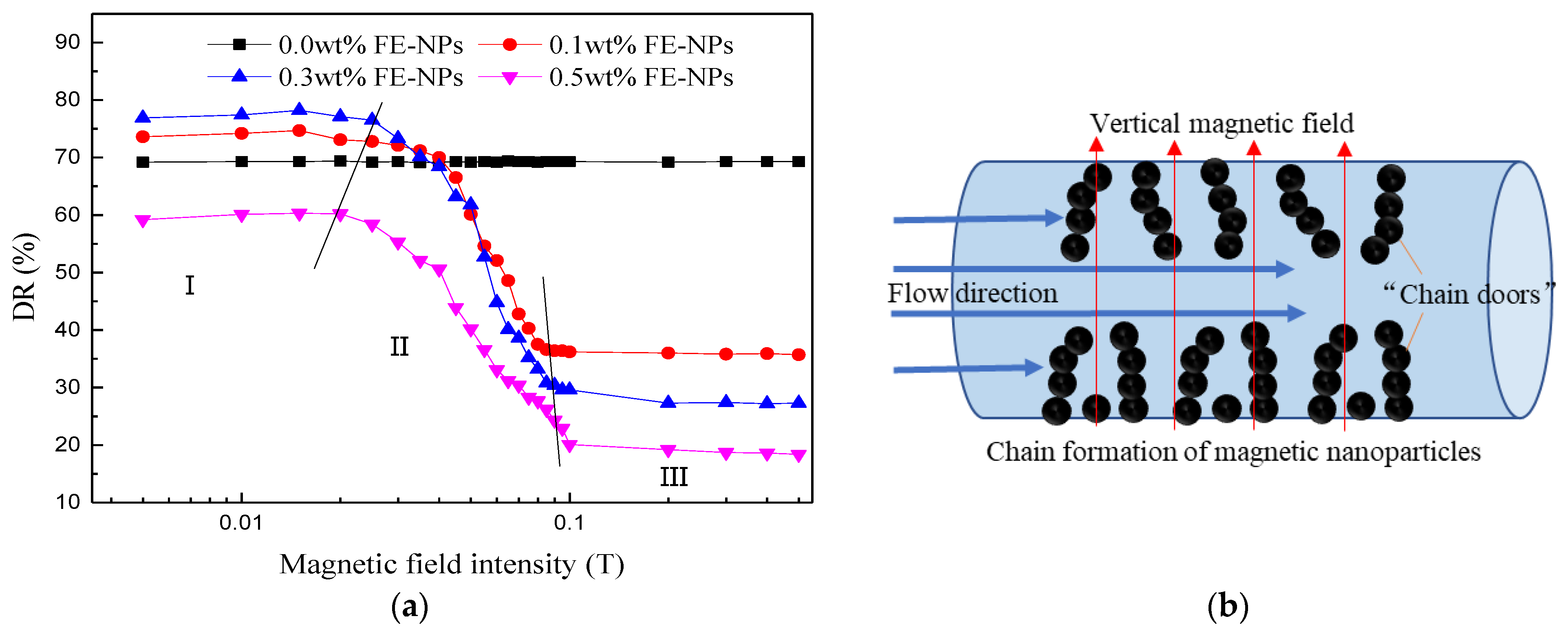
3.2.3. Effect of the Vertical Magnetic Field Intensity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furimsky, E. Properties of tight oils and selection of catalysts for hydroprocessing. Energy Fuels 2015, 29, 2043–2058. [Google Scholar] [CrossRef]
- Brandt, A.R.; Yeskoo, T.; McNally, M.S. Energy intensity and greenhouse gas emissions from tight oil production in the Bakken formation. Energy Fuels 2016, 30, 9613–9621. [Google Scholar] [CrossRef]
- Qian, K.R.; He, Z.L.; Liu, X.W. Intelligent prediction and integral analysis of shale oil and gas sweet spots. Pet. Sci. 2018, 15, 744–755. [Google Scholar] [CrossRef]
- Tiffany, L.; Doug, D.; Shinji, M. Comparison of the degree of fouling at various flux rates and modes of operation using forward osmosis for remediation of produced water from unconventional oil and gas development. Sci. Total Environ. 2019, 675, 73–80. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Etoughe, P.; Siddhamshetty, P.; Cao, K.Y. Incorporation of sustainability in process control of hydraulic fracturing in unconventional reservoirs. Chem. Eng. Res. Des. 2018, 139, 62–76. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K. Nanoparticles applications for hydraulic fracturing of unconventional reservoirs: A comprehensive review of recent advances and prospects. J. Petrol. Sci. Eng. 2019, 178, 41–73. [Google Scholar] [CrossRef]
- Brun, N.L.; Zadrazil, I.; Norman, L. On the drag reduction effect and shear stability of improved acrylamide copolymers for enhanced hydraulic fracturing. Chem. Eng. Sci. 2016, 146, 135–143. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.Z.; Zhang, S. Smart thermoviscosifying polymer for improving drag reduction in slickwater hydrofracking. Fuel 2020, 278, 118408. [Google Scholar] [CrossRef]
- Zadrazil, I.; Bismarck, A.; Hewitt, G.F.; Markides, C.N. Shear layers in the turbulent pipe flow of drag reducing polymer solutions. Chem. Eng. Sci. 2012, 72, 142–154. [Google Scholar] [CrossRef]
- Habibpour, M.; Koteeswaran, S.; Clark, P.E. Drag reduction behavior of hydrolyzed polyacrylamide/polysaccharide mixed polymer solutions—effect of solution salinity and polymer concentration. Rheol. Acta 2017, 56, 1–12. [Google Scholar] [CrossRef]
- Quan, Q.; Wang, S.; Wang, L.; Shi, Y.; Xie, J. Experimental study on the effect of high-molecular polymer as drag reducer on drag reduction rate of pipe flow. J. Petrol. Sci. Eng. 2019, 178, 852–856. [Google Scholar] [CrossRef]
- Wu, J.J.; Yu, W.C.; Ding, F. A Breaker-Free, Non-Damaging Friction Reducer for All-Brine Field Conditions. J. Nanosci. Nanotechnol. 2017, 17, 6919–6925. [Google Scholar] [CrossRef]
- Novelli, G.L.; Ferrari, L.A.; Vargas, G.G. A synergistic analysis of drag reduction on binary polymer mixtures containing guar gum. Int. J. Biol. Macromol. 2019, 137, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Al-Wahaibi, T.; Al-Wahaibi, Y. Roles of drag reducing polymers in single- and multi-phase flows. Chem. Eng. Res. Des. 2014, 92, 2153–2181. [Google Scholar] [CrossRef]
- Liu, D.J.; Wang, Q.H.; Wei, J.J. Experimental study on drag reduction performance of mixed polymer and surfactant solutions. Chem. Eng. Res. Des. 2018, 132, 460–469. [Google Scholar] [CrossRef]
- Reis, L.G.; Oliveira, I.P.; Lucas, F.E. Influence of structure and composition of poly(acrylamide-g-propylene oxide) copolymers on drag reduction of aqueous dispersions. Colloid Surf. A 2016, 502, 121–129. [Google Scholar] [CrossRef]
- Mohammadtabar, M.; Sanders, R.S.; Ghaemi, S. Viscoelastic properties of flexible and rigid polymers for turbulent drag reduction. J. Non-Newton. Fluid Mech. 2020, 283, 104347. [Google Scholar] [CrossRef]
- Sokhal, K.S.; Gangacharyulu, D.; Bulasara, V.K. Effect of guar gum and salt concentrations on drag reduction and shear degradation properties of turbulent flow of water in a pipe. Carbohyd. Polym. 2018, 181, 1017–1025. [Google Scholar] [CrossRef]
- Tamano, S.; Ikarashi, H.; Morinishi, Y.; Taga, K. Drag reduction and degradation of nonionic surfactant solutions with organic acid in turbulent pipe flow. J. Non-Newton. Fluid Mech. 2015, 215, 1–7. [Google Scholar] [CrossRef]
- Habibpour, M.; Clark, P.E. Drag reduction behavior of hydrolyzed polyacrylamide/xanthan gum mixed polymer solutions. Pet. Sci. 2017, 14, 412–423. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.; Mao, J. Review of friction reducers used in slickwater fracturing fluids for shale gas reservoirs. J. Nat. Gas. Sci. Eng. 2019, 62, 302–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, C.L.; Qian, Y. Rheological properties and formation dynamic filtration damage evaluation of a novel nanoparticle-enhanced VES fracturing system constructed with wormlike micelles. Colloid Surf. A 2018, 553, 244–252. [Google Scholar] [CrossRef]
- Li, P.; Kawaguchi, Y.; Daisaka, H. Heat transfer enhancement to the drag-reducing flow of surfactant solution in two-dimensional channel with mesh-screen inserts at the inlet. J. Heat Transf. 2001, 123, 779–789. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A. A critical review of hydraulic-fracturing fluids for moderate-to ultralow-permeability formations over the last decade. SPE Prod. Oper. 2014, 29, 243–260. [Google Scholar] [CrossRef]
- Samuel, M.M.; Card, R.J.; Nelson, E.B. Polymer-free fluid for fracturing applications. SPE Drill Complet. 1999, 14, 240–246. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Liang, F.; Hull, K.L. Nanoparticle-enhanced hydraulic-fracturing fluids: A review. SPE Prod. Oper. 2017, 32, 186–195. [Google Scholar] [CrossRef]
- Cai, C.; Sang, N.; Teng, S. Super-hydrophobic surface fabricated by spraying hydrophobic R974 nanoparticles and the drag reduction in water. Surf. Coat. Technol. 2016, 307, 366–373. [Google Scholar] [CrossRef]
- Taghvaei, E.; Moosavi, A. Super-hydrophobic surfaces with a dual-layer micro and nanoparticle coating for drag reduction. Energy 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Q.; Xu, D. SiO2 nanoparticle assisted low-concentration viscoelastic cationic surfactant fracturing fluid. J. Mol. Liq. 2018, 266, 864–869. [Google Scholar] [CrossRef]
- Nettesheim, F.; Liberatore, M.W.; Hodgdon, T.K. Influence of nanoparticle addition on the properties of wormlike micellar solutions. Langmuir 2008, 24, 7718–7726. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Crews, J.B. Nanotechnology applications in viscoelastic surfactant stimulation fluids. SPE Prod. Oper. 2008, 23, 512–517. [Google Scholar] [CrossRef]
- Luo, M.L.; Jia, Z.L.; Sun, H. Rheological behavior and microstructure of an anionic surfactant micelle solution with pyroelectric nanoparticle. Colloid Surf. A 2012, 395, 267–275. [Google Scholar] [CrossRef]
- Philippova, O.E.; Molchanov, V.S. Enhanced rheological properties and performance of viscoelastic surfactant fluids with embedded nanoparticles. Curr. Opin. Colloid Interface Sci. 2019, 43, 52–62. [Google Scholar] [CrossRef]
- Wu, X.P.; Song, Z.H.; Zhen, J.W. A smart recyclable VES fluid for high temperature and high pressure fracturing. J. Petrol. Sci. Eng. 2020, 190, 107097. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Magnetic fluids. Sci. Am. 1982, 247, 136–145. [Google Scholar] [CrossRef]
- Raj, K.; Moskowitz, R. Commercial applications of ferrofluids. J. Magn. Magn. Mater. 1990, 85, 233–245. [Google Scholar] [CrossRef]
- Zhu, L.; Li, C.; Wang, J. A simple method to synthesize modified Fe3O4 for the removal of organic pollutants on water surface. Appl. Surf. Sci. 2012, 258, 6320–6330. [Google Scholar] [CrossRef]
- Sengupta, S. An Innovative Approach to Image Fracture Dimensions by Injecting Ferrofluids. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11 November 2012. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Bryant, S.L.; Huh, C. Crosswell magnetic sensing of superparamagnetic nanoparticles for subsurface applications. SPE J. 2015, 20, 1067–1082. [Google Scholar] [CrossRef]
- Divandari, H.; Hemmati, S.A.; Schaffie, M. Integrating synthesized citric acid-coated magnetite nanoparticles with magnetic fields for enhanced oil recovery: Experimental study and mechanistic understanding. J. Petrol. Sci. Eng. 2019, 174, 425–436. [Google Scholar] [CrossRef]
- Ali, A.M.; Yahya, N.; Qureshi, S. Interactions of ferro-nanoparticles (hematite and magnetite) with reservoir sandstone: Implications for surface adsorption and interfacial tension reduction. Pet. Sci. 2020, 17, 1037–1055. [Google Scholar] [CrossRef]
- Betancur, S.; Olmos, C.M.; Pérez, M. A microfluidic study to investigate the effect of magnetic iron core-carbon shell nanoparticles on displacement mechanisms of crude oil for chemical enhanced oil recovery. J. Petrol. Sci. Eng. 2020, 184, 106589. [Google Scholar] [CrossRef]
- Amrouche, F.; Gomari, S.R.; Islam, M.; Xu, D. A novel hybrid technique to enhance oil production from oil-wet carbonate reservoirs by combining a magnetic field with alumina and iron oxide nanoparticles. J. Clean Prod. 2021, 281, 124891. [Google Scholar] [CrossRef]
- Sun, M.L.; Wang, B. Study on stability of magnetic fluid viscous drag reducing coating. In Proceedings of the China National Conference on Functional Materials and Applications, Changsha, China, 15–18 October 2010. [Google Scholar]
- Rosa, A.P.; Gontijo, R.G.; Cunha, F.R. Laminar pipe flow with drag reduction induced by a magnetic field gradient. Appl. Math. Model. 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Nadia, E.; Reza, Z.; Zahra, H. The investigation and optimization of drag reduction in turbulent flow of newtonian fluid passing through horizontal pipelines using functionalized magnetic nano-photocatalysts and lecithin. Chin. J. Chem. Eng. 2020, 28, 67–75. [Google Scholar] [CrossRef]
- Peng, D.F.; Beysen, S.; Li, Q. Hydrothermal growth of octahedral Fe3O4 crystals. Particuology 2009, 7, 35–38. [Google Scholar] [CrossRef]
- Wang, Z.L. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 2012, 104, 1153–1175. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Medrzycka, K.B. The effect of particle concentration on zeta potential in extremely dilute solutions. Colloid Polym. Sci. 1991, 269, 85–90. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Liu, F.; Liu, D.J.; Zhou, W.J.; Wang, S.; Chen, F.; Wei, J.J. Weakening or losing of surfactant drag reduction ability: A coarse-grained molecular dynamics study. Chem. Eng. Sci. 2020, 219, 115610. [Google Scholar] [CrossRef]
- Zhou, W.J.; Liu, F.; Liu, D.J.; Chen, F.; Wei, J.J. Energy analysis of a surfactant micelle’s deformation by coarse-grained molecular dynamics simulations. Chem. Eng. Sci. 2019, 202, 138–145. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.-L.; Si, X.-D.; Li, M.-Z.; Jia, X.-H.; Yang, Y.-L.; Zhan, Y.-P. Experimental Study on the Drag Reduction Performance of Clear Fracturing Fluid Using Wormlike Surfactant Micelles and Magnetic Nanoparticles under a Magnetic Field. Nanomaterials 2021, 11, 885. https://doi.org/10.3390/nano11040885
Luo M-L, Si X-D, Li M-Z, Jia X-H, Yang Y-L, Zhan Y-P. Experimental Study on the Drag Reduction Performance of Clear Fracturing Fluid Using Wormlike Surfactant Micelles and Magnetic Nanoparticles under a Magnetic Field. Nanomaterials. 2021; 11(4):885. https://doi.org/10.3390/nano11040885
Chicago/Turabian StyleLuo, Ming-Liang, Xiao-Dong Si, Ming-Zhong Li, Xiao-Han Jia, Yu-Ling Yang, and Yong-Ping Zhan. 2021. "Experimental Study on the Drag Reduction Performance of Clear Fracturing Fluid Using Wormlike Surfactant Micelles and Magnetic Nanoparticles under a Magnetic Field" Nanomaterials 11, no. 4: 885. https://doi.org/10.3390/nano11040885
APA StyleLuo, M.-L., Si, X.-D., Li, M.-Z., Jia, X.-H., Yang, Y.-L., & Zhan, Y.-P. (2021). Experimental Study on the Drag Reduction Performance of Clear Fracturing Fluid Using Wormlike Surfactant Micelles and Magnetic Nanoparticles under a Magnetic Field. Nanomaterials, 11(4), 885. https://doi.org/10.3390/nano11040885





