Dual-Cation Electrolytes Crosslinked with MXene for High-Performance Electrochromic Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrolytes
2.2.1. LiTFSI-Based Electrolytes (MXene/LiTFSI)
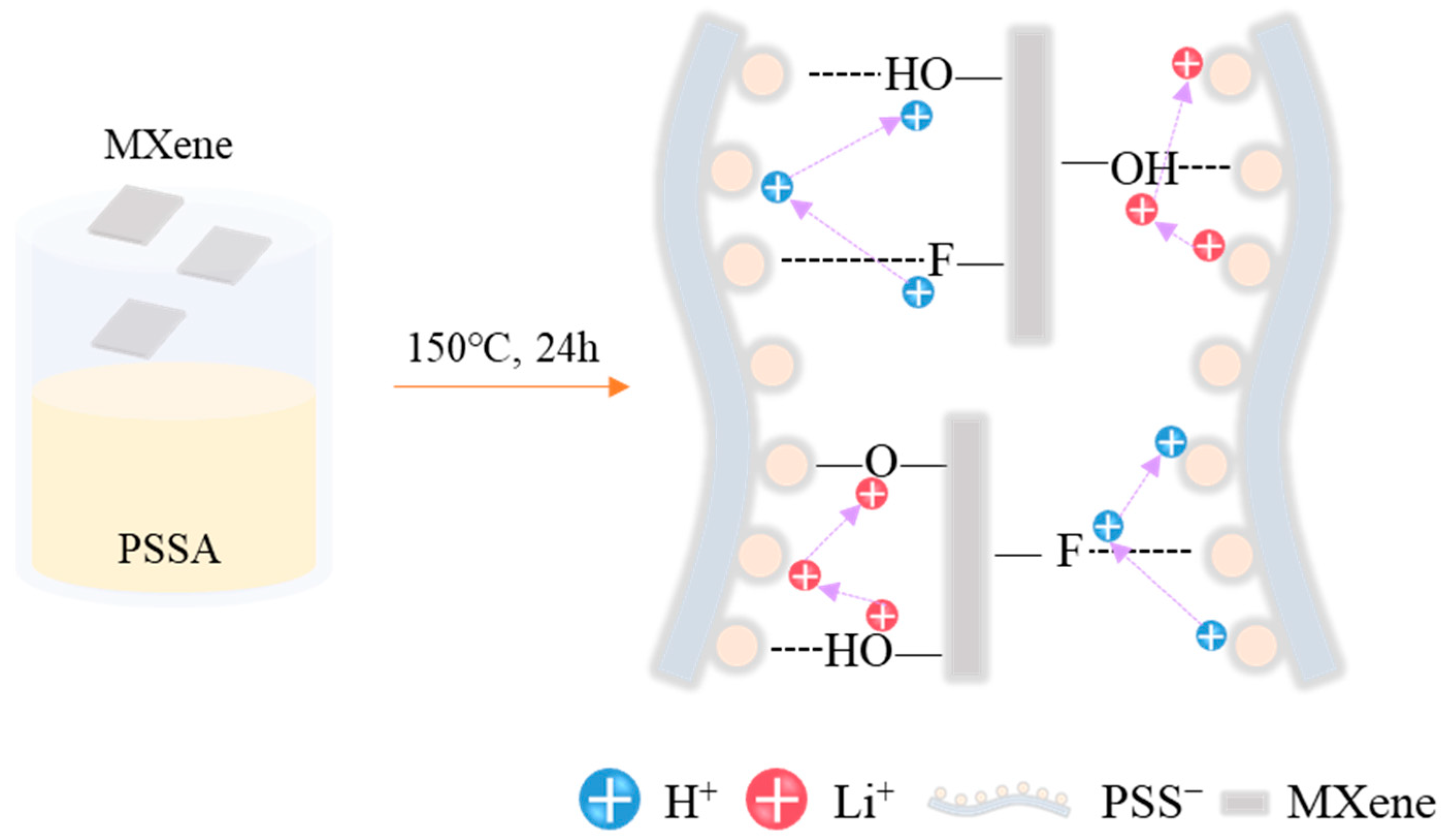
2.2.2. Preparation of PSSA-Based Electrolytes Crosslinked with MXene (M-PSSA)
2.2.3. Preparation of Dual-Cation Electrolytes Crosslinked with MXene (M-PSSA/LiTFSI)
2.3. Preparation of Electrochromic Electrodes
2.3.1. Synthesis of PEDOT:PSS
2.3.2. Synthesis of PANI:PSS
2.3.3. Fabrication of Electrochromic Electrodes
2.4. Fabrication of Flexible ECDs
2.5. Sample Characterization
3. Results and Discussion
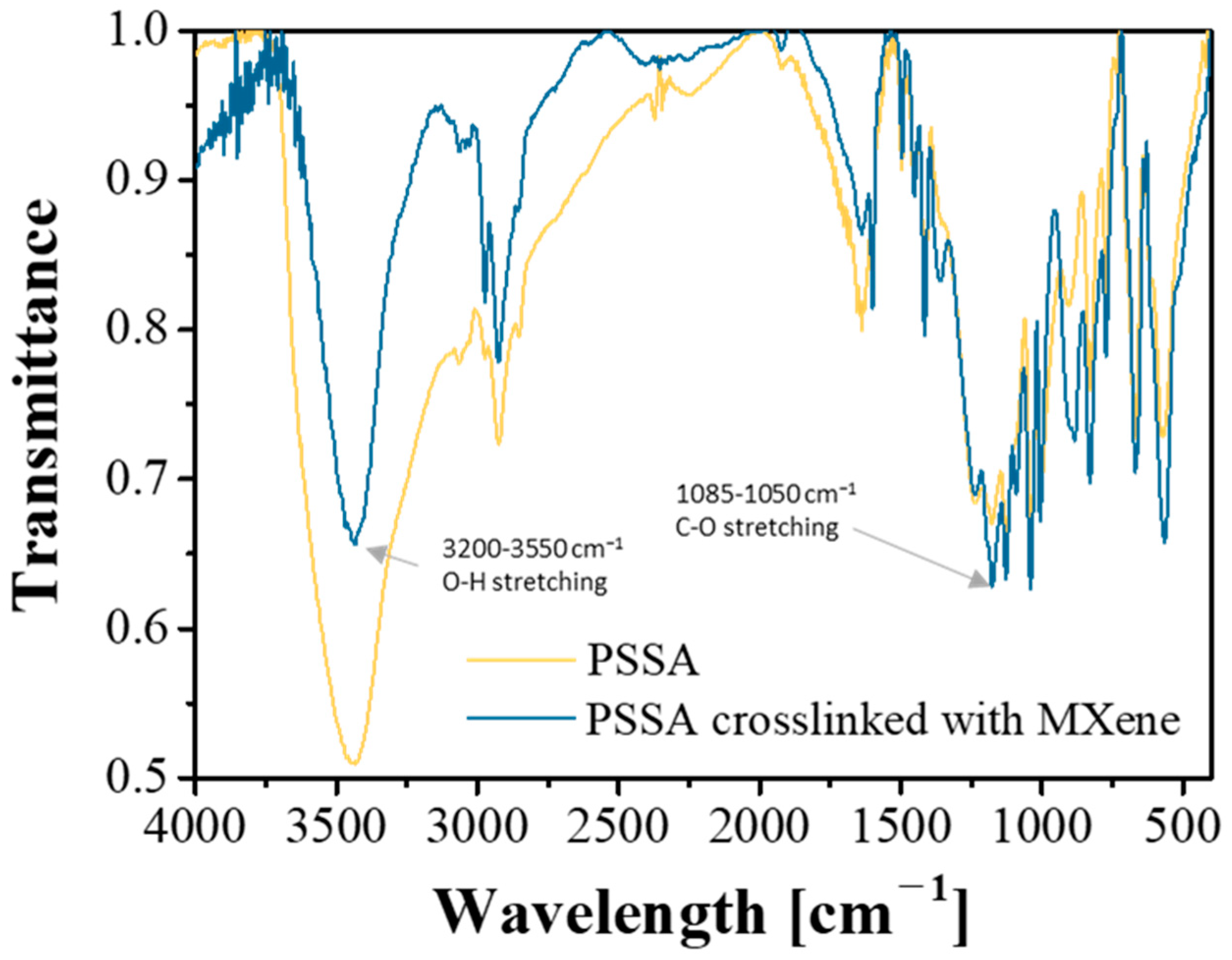
3.1. Synthesis and Characterization of PSSA Crosslinked with MXene
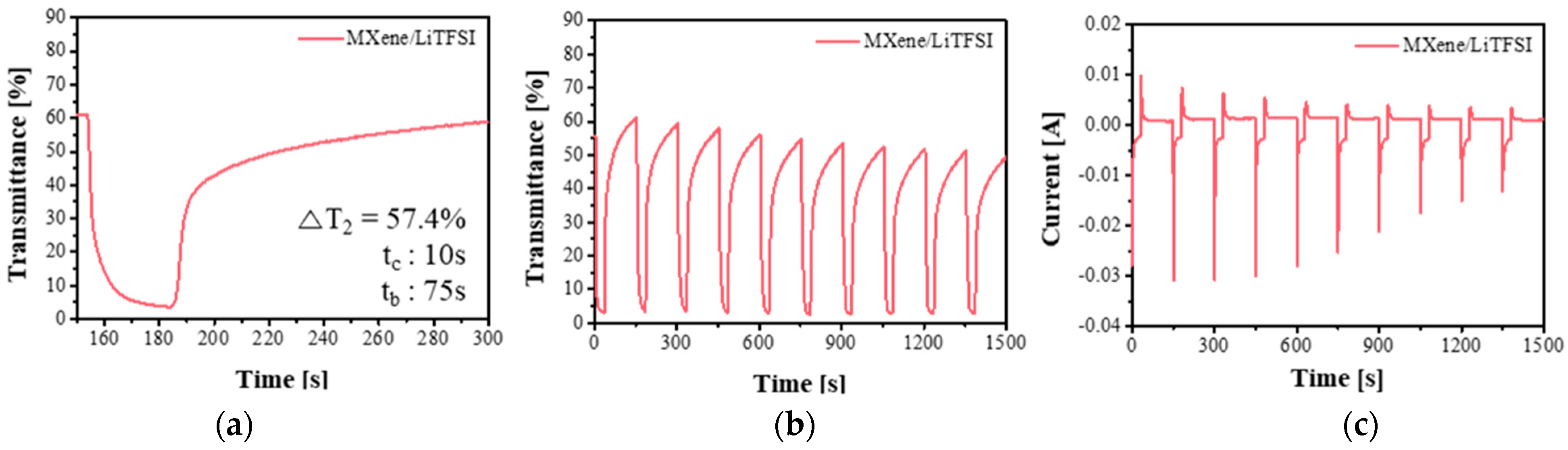
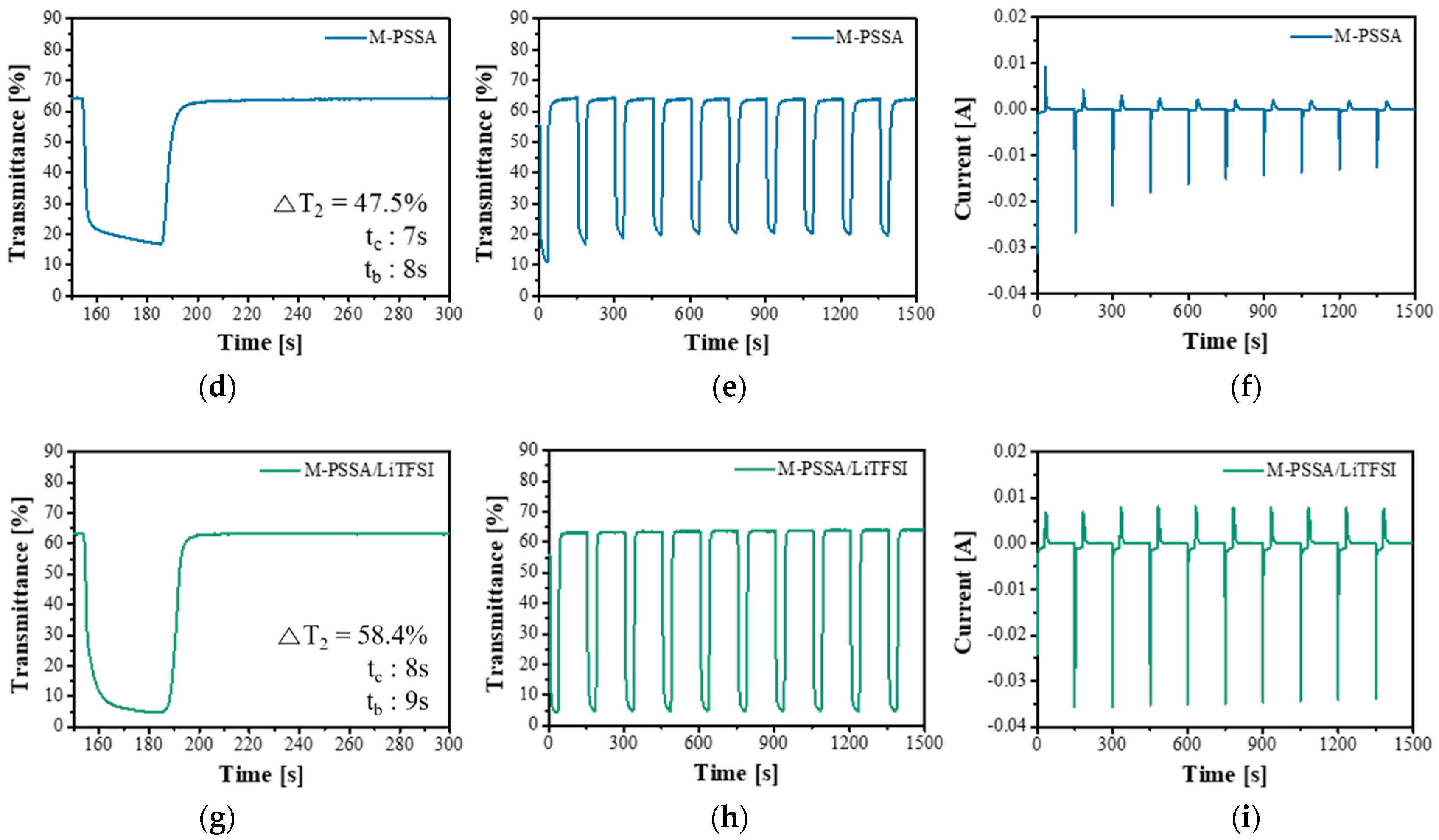
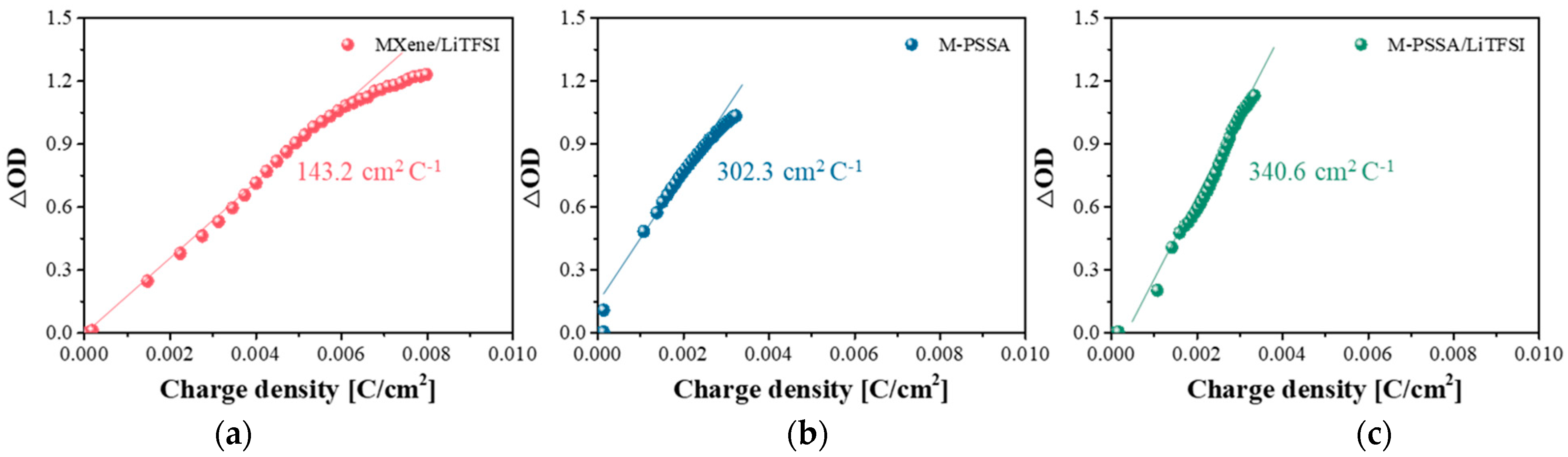
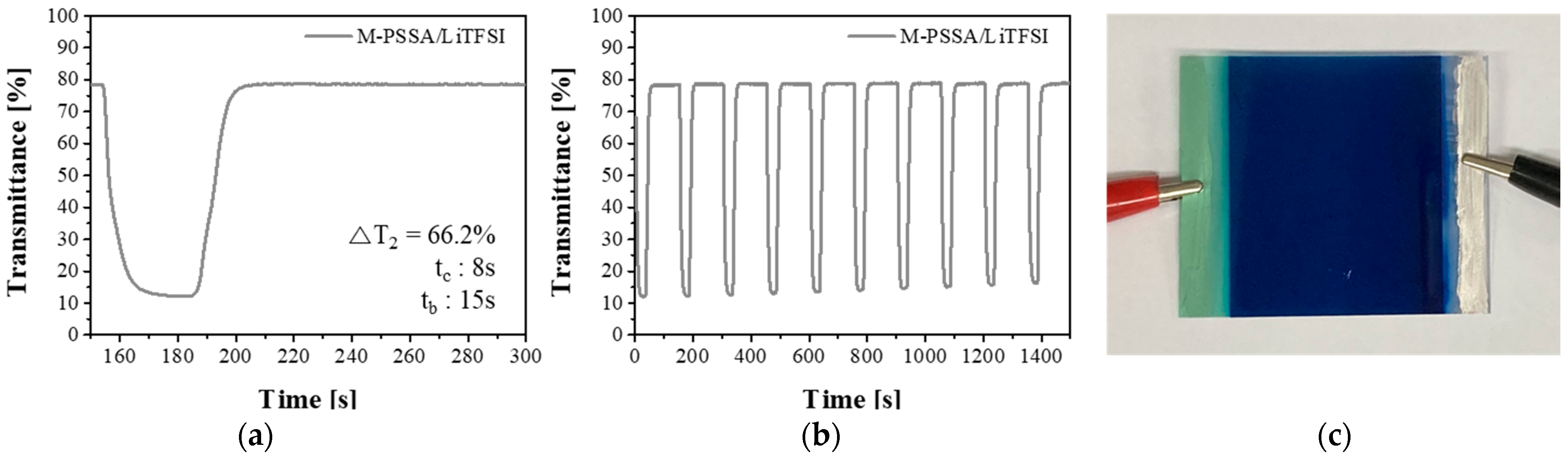
3.2. Comparison of Electrochromic Performances in Lithium, Acid and Dual-Cation Electrolytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panthi, D.; Hedayat, N.; Du, Y. Densification behavior of yttria-stabilized zirconia powders for solid oxide fuel cell electrolytes. J. Adv. Ceram. 2018, 7, 325–335. [Google Scholar] [CrossRef]
- Bi, L.; Shafi, S.P.; Da’as, E.H.; Traversa, E. Tailoring the cathode–electrolyte interface with nanoparticles for boosting the solid oxide fuel cell performance of chemically stable proton-conducting electrolytes. Small 2018, 14, 1801231. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ding, J.; Li, J.; Li, Y.; Zong, Y.; Zhang, P.; Wang, Z.; Liu, X. N, S and Transition-Metal Co-Doped Graphene Nanocomposites as High-Performance Catalyst for Glucose Oxidation in a Direct Glucose Alkaline Fuel Cell. Nanomaterials 2021, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Francis, C.F.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Benítez, A.; Morales, J.; Caballero, Á. Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries. Nanomaterials 2020, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, L.; Wu, H.; Cao, Y.; Zhang, J.; Xu, N.; Chen, S.; Decoppet, J.-D.; Zakeeruddin, S.M.; Grätzel, M. Stable and efficient organic dye-sensitized solar cell based on ionic liquid electrolyte. Joule 2018, 2, 2145–2153. [Google Scholar] [CrossRef]
- Zulkifli, A.M.; Said, N.; Aziz, S.B.; Hisham, S.; Shah, S.; Abu, A.; Bakar, Z.; Tajuddin, H.A.; Sulaiman, L.; Brza, M.A. Electrochemical characteristics of phthaloyl chitosan based gel polymer electrolyte for dye sensitized solar cell application. Int. J. Electrochem. Sci 2020, 15, 7434–7447. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, H.; Kim, E.; Kim, J.; Seo, I. Development of a Self-Charging Lithium-Ion Battery Using Perovskite Solar Cells. Nanomaterials 2020, 10, 1705. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Zhang, T.; Cao, S.; Yao, Q.; Lin, H.; Ye, H.; Fisher, A.; Lee, J.Y. Dual-band electrochromic devices with a transparent conductive capacitive charge-balancing anode. ACS Appl. Mater. Interfaces 2019, 11, 48062–48070. [Google Scholar] [CrossRef]
- Macher, S.; Schott, M.; Sassi, M.; Facchinetti, I.; Ruffo, R.; Patriarca, G.; Beverina, L.; Posset, U.; Giffin, G.A.; Löbmann, P. New Roll-to-Roll Processable PEDOT-Based Polymer with Colorless Bleached State for Flexible Electrochromic Devices. Adv. Funct. Mater. 2020, 30, 1906254. [Google Scholar] [CrossRef]
- Mouratis, K.; Tudose, I.V.; Bouranta, A.; Pachiu, C.; Romanitan, C.; Tutunaru, O.; Couris, S.; Koudoumas, E.; Suchea, M. Annealing Effect on the Properties of Electrochromic V2O5 Thin Films Grown by Spray Deposition Technique. Nanomaterials 2020, 10, 2397. [Google Scholar] [CrossRef]
- Alesanco, Y.; Viñuales, A.; Rodriguez, J.; Tena-Zaera, R. All-in-one gel-based electrochromic devices: Strengths and recent developments. Materials 2018, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zheng, P.; Ma, R.; Xu, C.; Yang, G.; Wang, Q.; Wang, H. Multifunctional hydrogel enables extremely simplified electrochromic devices for smart windows and ionic writing boards. Mater. Horiz. 2018, 5, 1000–1007. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Tang, W.; Song, S.; Yao, J.; Wen, Z.; Lu, L.; Savilov, S.V.; Hu, N.; Molenda, J. Preparation of Nanocomposite Polymer Electrolyte via In Situ Synthesis of SiO2 Nanoparticles in PEO. Nanomaterials 2020, 10, 157. [Google Scholar] [CrossRef]
- He, J.; You, L.; Tran, D.T.; Mei, J. Low-temperature thermally annealed niobium oxide thin films as a minimally color changing ion storage layer in solution-processed polymer electrochromic devices. ACS Appl. Mater. Interfaces 2019, 11, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, S.; Hiller, M.; Wiemhöfer, H.-D. Preparation and electrochemical performance of polyphosphazene based salt-in-polymer electrolyte membranes for lithium ion batteries. J. Power Sources 2014, 253, 256–262. [Google Scholar] [CrossRef]
- Karatas, Y.; Banhatti, R.D.; Kaskhedikar, N.; Burjanadze, M.; Funke, K.; Wiemhöfer, H.-D. Synthesis and modeling of polysiloxane-based salt-in-polymer electrolytes with various additives. J. Phys. Chem. B 2009, 113, 15473–15484. [Google Scholar] [CrossRef]
- Xu, D.; Su, J.; Jin, J.; Sun, C.; Ruan, Y.; Chen, C.; Wen, Z. In situ generated fireproof gel polymer electrolyte with Li6. 4Ga0. 2La3Zr2O12 as initiator and ion-conductive filler. Adv. Energy Mater. 2019, 9, 1900611. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, Z.; Ganapathy, S.; Wang, C.; Haverkate, L.A.; Tułodziecki, M.; Unnikrishnan, S.; Wagemaker, M. Tandem interface and bulk Li-ion transport in a hybrid solid electrolyte with microsized active filler. ACS Energy Lett. 2019, 4, 2336–2342. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D.; Figueiredo, F.M. Carbon nanocomposite membrane electrolytes for direct methanol fuel cells—a concise review. Nanomaterials 2019, 9, 1292. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, Y.; Kota, S.; Huang, W.; Wang, S.; Qi, H.; Kim, S.; Tu, Y.; Barsoum, M.W.; Li, C.Y. 2D MXene-containing polymer electrolytes for all-solid-state lithium metal batteries. Nanoscale Adv. 2019, 1, 395–402. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.; Zhu, Q.; Shen, K.; Tang, W.; Xiang, Q.; Chen, W.; Liu, C.; Luo, J.; Yang, S. MXene-Based Mesoporous Nanosheets Toward Superior Lithium Ion Conductors. Adv. Energy Mater. 2020, 10, 1903534. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhang, X.; Li, Y.; Wang, J. Ti3C2T x Filler Effect on the Proton Conduction Property of Polymer Electrolyte Membrane. ACS Appl. Mater. Interfaces 2016, 8, 20352–20363. [Google Scholar] [CrossRef] [PubMed]
- Shimanoe, K.; Aoyama, K.; Shirai, M.; Endo, T.; Ito, T. Development of an Electrochromic Antiglare Mirror with Multi-layer Thin Films. JSAE Rev. 1997, 2, 192. [Google Scholar]
- Ohmi, S.; Hara, K.; Sagiike, M.; Sato, R. Fail-Safe Type Liquid Crystal Mirror for Automobiles. SAE Trans. 1987, 96, 900–902. [Google Scholar]
- Cossari, P.; Pugliese, M.; Gambino, S.; Cannavale, A.; Maiorano, V.; Gigli, G.; Mazzeo, M. Fully integrated electrochromic-OLED devices for highly transparent smart glasses. J. Mater. Chem. C 2018, 6, 7274–7284. [Google Scholar] [CrossRef]
- Ma, C.; Taya, M.; Xu, C. Smart sunglasses based on electrochromic polymers. Polym. Eng. Sci. 2008, 48, 2224–2228. [Google Scholar] [CrossRef]
- Andersson, P.; Forchheimer, R.; Tehrani, P.; Berggren, M. Printable all-organic electrochromic active-matrix displays. Adv. Funct. Mater. 2007, 17, 3074–3082. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Chou, H.-H.; Nguyen, A.; Chortos, A.; To, J.W.; Lu, C.; Mei, J.; Kurosawa, T.; Bae, W.-G.; Tok, J.B.-H.; Bao, Z. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Koo, J.; Amoli, V.; Kim, S.Y.; Lee, C.; Kim, J.; Park, S.-M.; Kim, J.; Ahn, J.M.; Jung, K.J.; Kim, D.H. Low-power, deformable, dynamic multicolor electrochromic skin. Nano Energy 2020, 78, 105199. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Huang, Y.; Meng, W.; Gong, Q.; Li, G.; Zhi, C. An electrochromic supercapacitor and its hybrid derivatives: Quantifiably determining their electrical energy storage by an optical measurement. J. Mater. Chem. A 2015, 3, 21321–21327. [Google Scholar] [CrossRef]
- Yun, T.G.; Kim, D.; Kim, Y.H.; Park, M.; Hyun, S.; Han, S.M. Photoresponsive smart coloration electrochromic supercapacitor. Adv. Mater. 2017, 29, 1606728. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C.; Im, S.; Kim, J.H. Design of intrinsically stretchable and highly conductive polymers for fully stretchable electrochromic devices. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Lee, H.; Kim, Y.; Cho, H.; Lee, J.-G.; Kim, J.H. Improvement of PEDOT: PSS linearity via controlled addition process. RSC Adv. 2019, 9, 17318–17324. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Lee, H.; Park, C.; Im, S.; Kim, J.H. Synthesis of stretchable, environmentally stable, conducting polymer PEDOT using a modified acid template random copolymer. Macromol. Chem. Phys. 2020, 221, 1900465. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.J.; Park, C.; Choi, H.H.; Kim, J.-H. N-type organic thermoelectric materials based on polyaniline doped with the aprotic ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate. RSC Adv. 2016, 6, 37130–37135. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.-K.; Wang, H. Self-Crosslinked MXene (Ti3C2T x) Membranes with Good Antiswelling Property for Monovalent Metal Ion Exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.; Filho, G.R.; Ribeiro, S.J.; Messadeqq, Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Yun, T.Y.; Li, X.; Bae, J.; Kim, S.H.; Moon, H.C. Non-volatile, Li-doped ion gel electrolytes for flexible WO3-based electrochromic devices. Mater. Des. 2019, 162, 45–51. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, W.; Guan, Z.; Jin, B.; Xiao, D. A novel bis (dihydroxypropyl) viologen-based all-in-one electrochromic device with high cycling stability and coloration efficiency. Electrochim. Acta 2019, 298, 533–540. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Kim, Y.; Kim, J.M.; Kim, J.H. Dual-Cation Electrolytes Crosslinked with MXene for High-Performance Electrochromic Devices. Nanomaterials 2021, 11, 874. https://doi.org/10.3390/nano11040874
Bae S, Kim Y, Kim JM, Kim JH. Dual-Cation Electrolytes Crosslinked with MXene for High-Performance Electrochromic Devices. Nanomaterials. 2021; 11(4):874. https://doi.org/10.3390/nano11040874
Chicago/Turabian StyleBae, Soyoung, Youngno Kim, Jeong Min Kim, and Jung Hyun Kim. 2021. "Dual-Cation Electrolytes Crosslinked with MXene for High-Performance Electrochromic Devices" Nanomaterials 11, no. 4: 874. https://doi.org/10.3390/nano11040874
APA StyleBae, S., Kim, Y., Kim, J. M., & Kim, J. H. (2021). Dual-Cation Electrolytes Crosslinked with MXene for High-Performance Electrochromic Devices. Nanomaterials, 11(4), 874. https://doi.org/10.3390/nano11040874







