Influence of Colloidal Au on the Growth of ZnO Nanostructures
Abstract
1. Introduction
2. Experimental Section
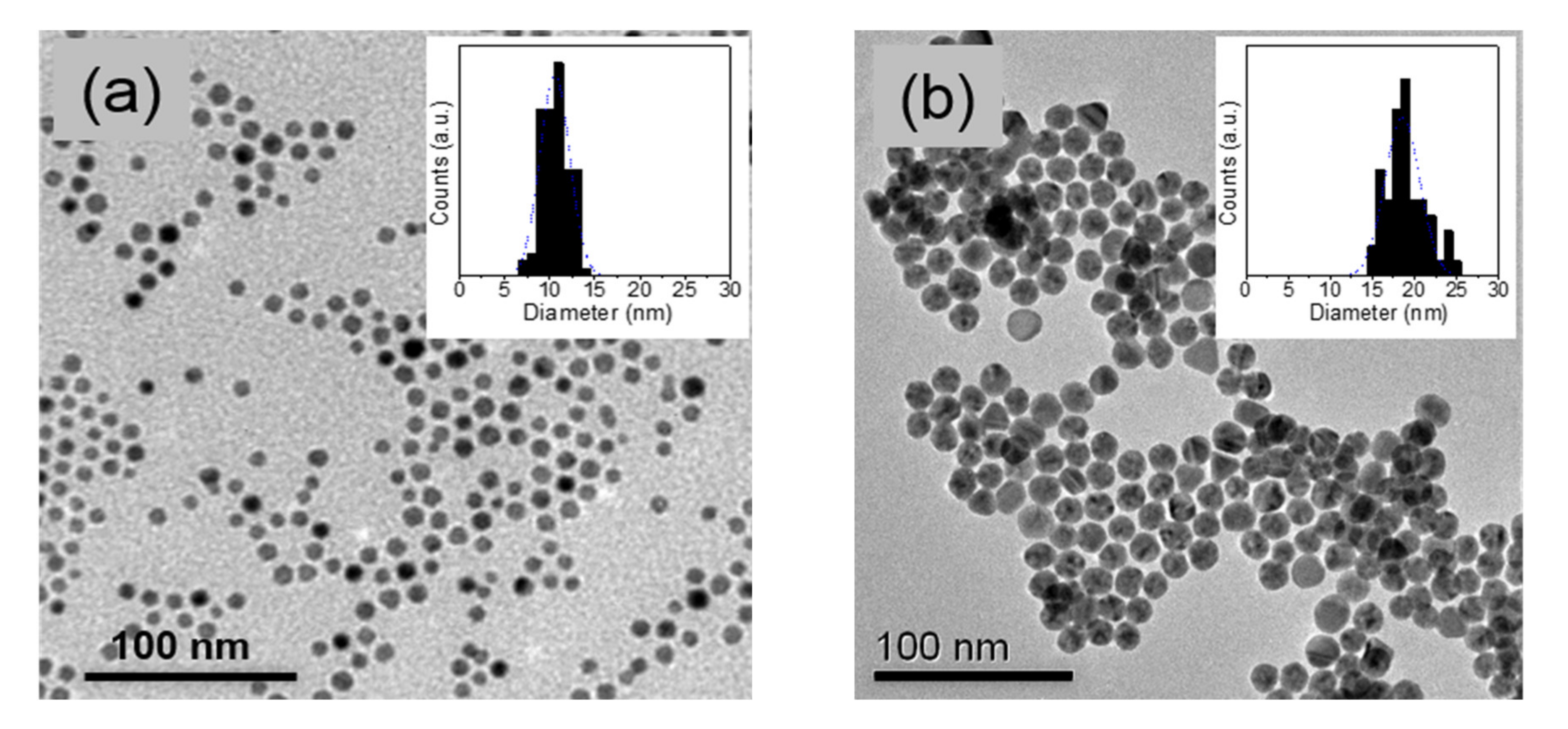
2.1. Au NP Synthesis and Deposition
2.2. ZnO Nanostructures Growth and Characterization
3. Results and Discussion
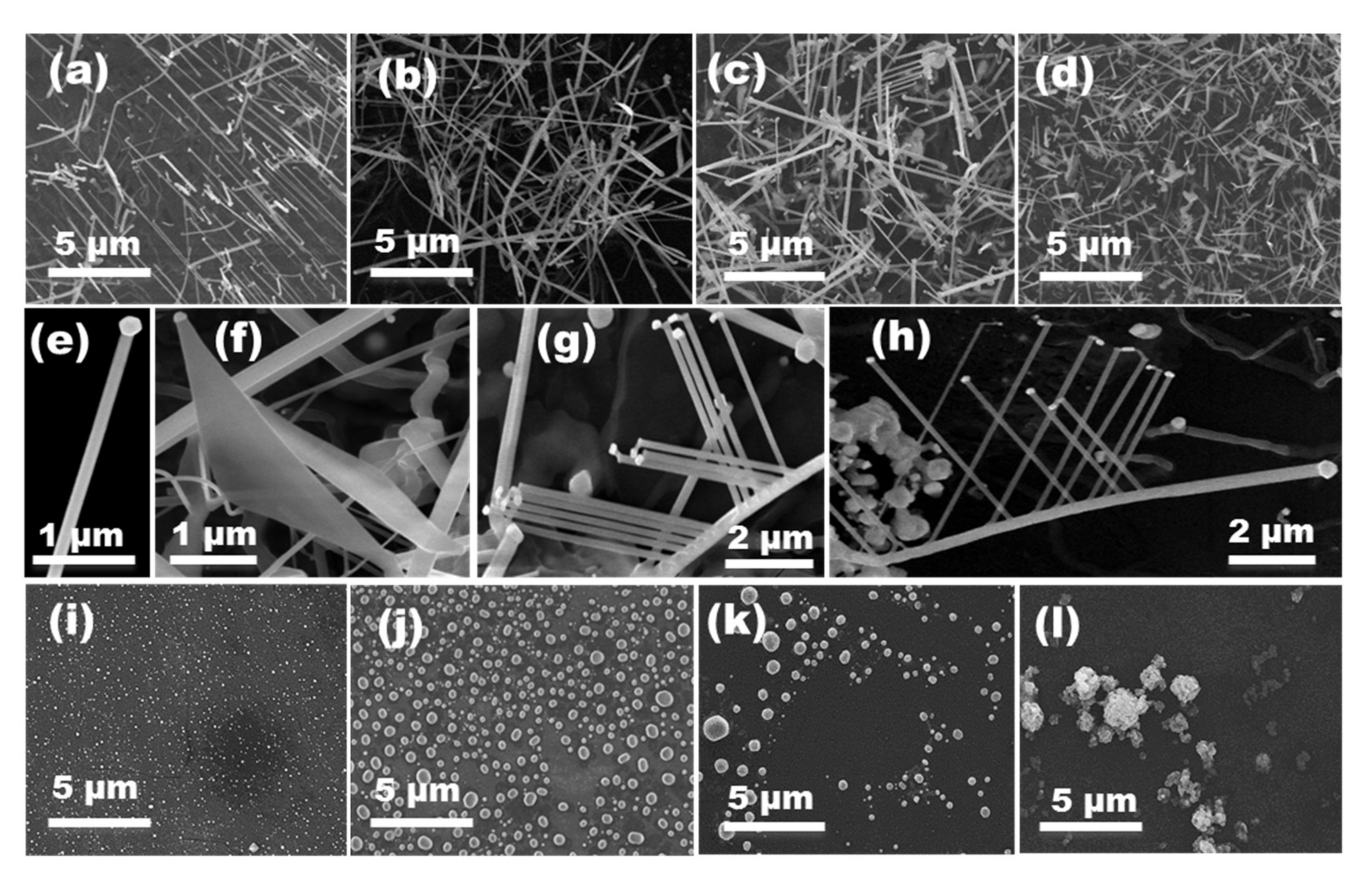
3.1. Effect of Growth Parameters
3.2. Solvent and Concentration Effect
3.3. Au Annealing Effect
3.4. Substrate Effect
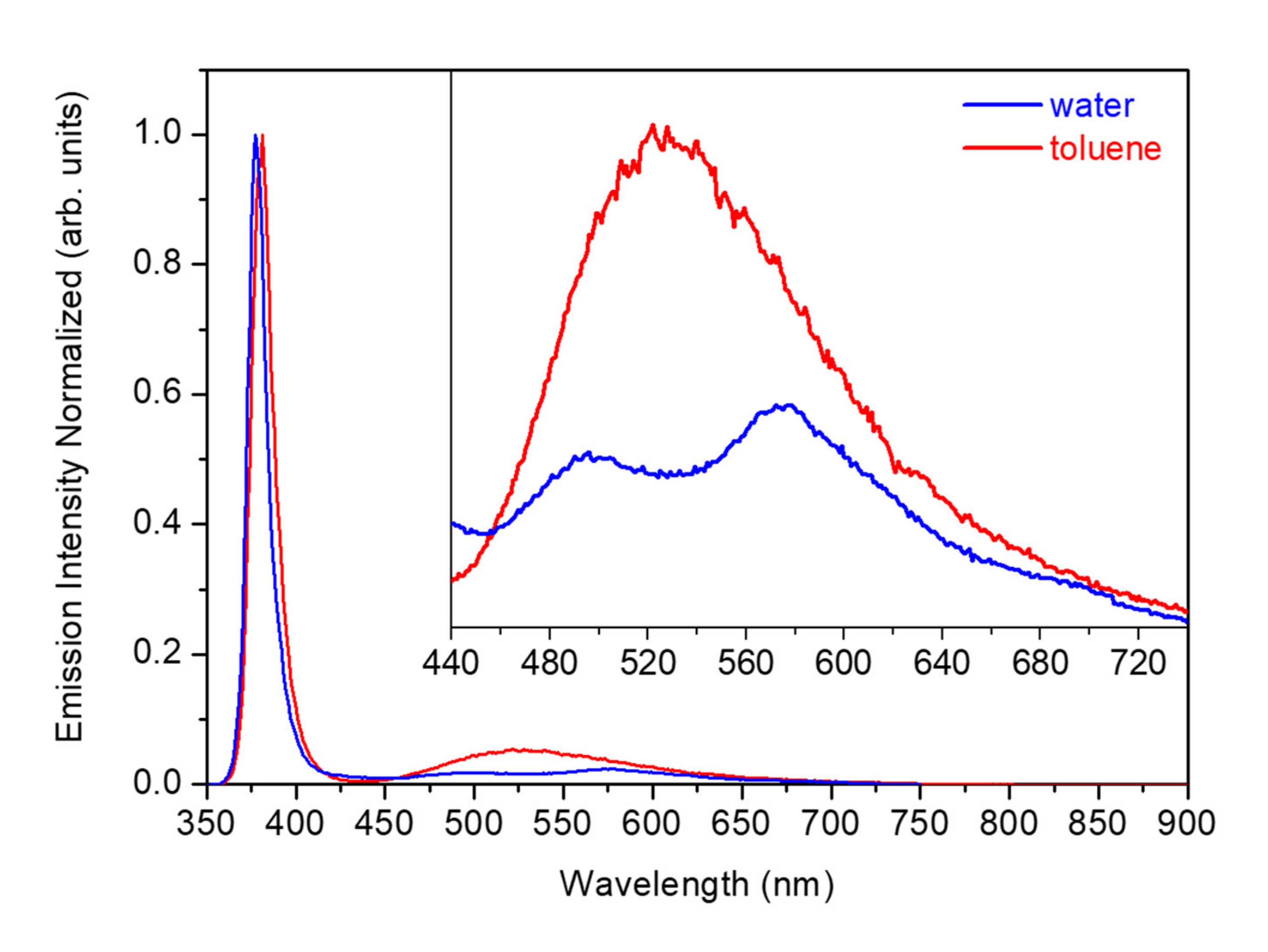
3.5. Optical and Structural Characterization
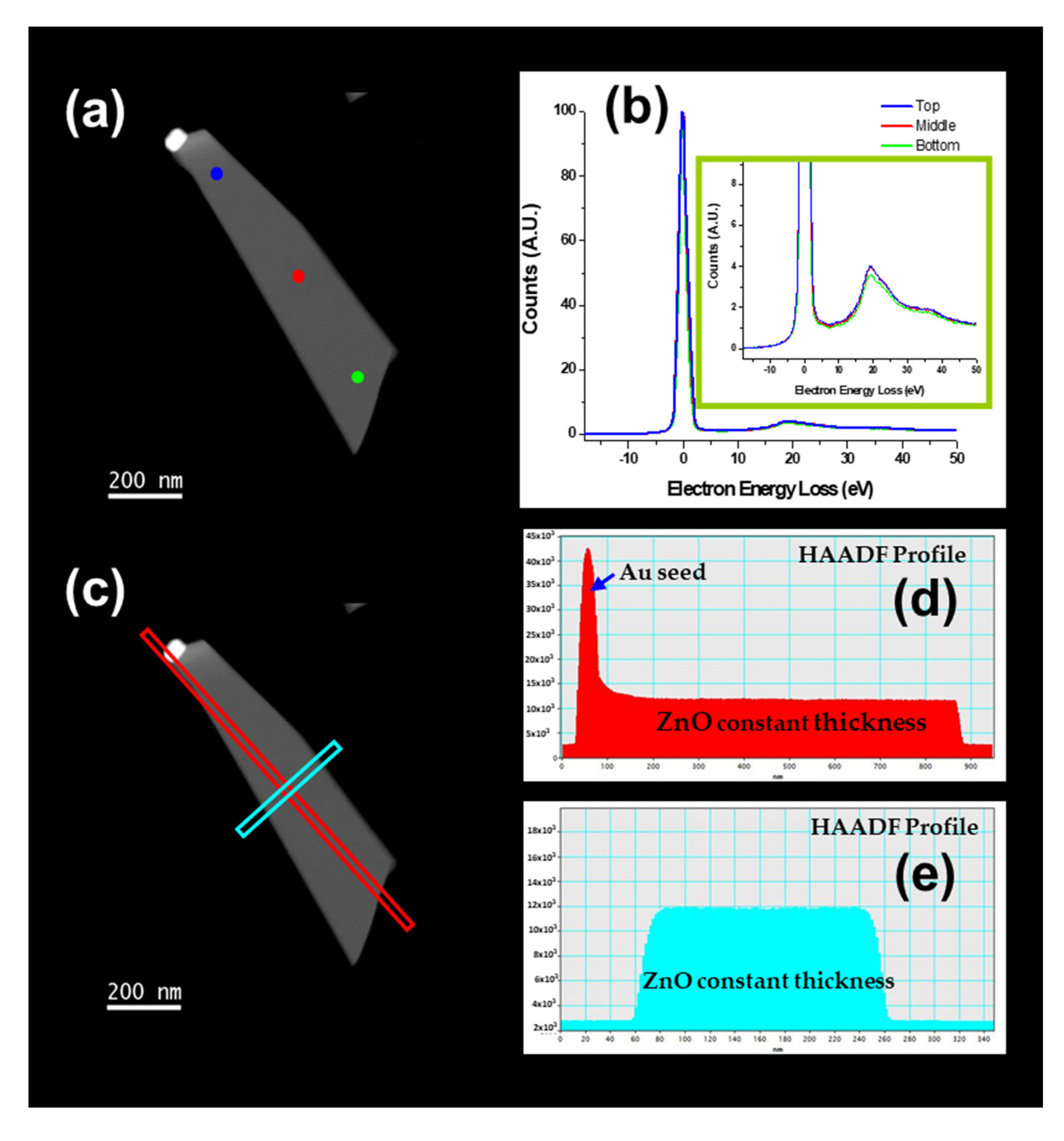
3.6. Nanostructures Growth Mechanisms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Reschikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter. 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Das, S.K.; Güell, F.; Gray, C.; Das, P.K.; Grunwald, R.; McGlynn, E. ZnO nanorods for efficient third harmonic UV Generation. Opt. Mat. Exp. 2014, 4, 701–709. [Google Scholar] [CrossRef]
- Gao, T.; Wang, T.H. Synthesis and properties of multipod-shaped ZnO nanorods for gas-sensor applications. Appl. Phys. A Mater. Sci. Process. 2005, 80, 1451–1454. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire Dye-Sensitized Solar Cells. J. Phys. Chem. C 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Güell, F.; Martínez-Alanis, P.R.; Khachadorian, S.; Zamani, R.R.; Franke, A.; Hoffmann, A.; Wagner, M.R.; Santana, G. Spatially controlled growth of highly crystalline ZnO nanowires by an inkjet-printing catalyst-free method. Mat. Res. Exp. 2016, 3, 025010. [Google Scholar] [CrossRef][Green Version]
- Errico, V.; Arrabito, G.; Plant, S.R.; Medaglia, P.G.; Palmer, R.E.; Falconi, C. Chromium inhibition and size-selected Au nanocluster catalysis for the solution growth of low-density ZnO nanowire. Sci. Rep. 2015, 5, 12336. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, C.H.; Hong, Y.J.; Yi, G.C. Controlled selective growth of ZnO nanorod and microrod arrays on Si substrates by a wet chemical method. Appl. Phys. Lett. 2006, 89, 163128. [Google Scholar] [CrossRef]
- Liu, F.; Cao, P.J.; Zhang, H.R.; Shen, C.M.; Wang, Z.; Li, J.Q.; Gao, H.J. Well-aligned zinc oxide nanorods and nanowires prepared without catalyst. J. Cryst. Grow. 2005, 274, 126–131. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic Growth of Zinc Oxide Nanowires by Vapor Transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Güell, F.; Martínez-Alanis, P.R.; Roso, S.; Salas-Pérez, C.I.; García-Sánchez, M.F.; Santana, G.; Monroy, B.M. Plasma versus thermal annealing for the Au-catalyst growth of ZnO nanocones and nanowires on Al-doped ZnO buffer layers. Mat. Res. Exp. 2016, 3, 065013. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.; Wang, D.; Tseng, W.-Y.; Chiou, W.-A.; Hong, J.; Lu, J.G. ZnO Nanowires Synthesized by Vapor Trapping CVD Method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Gudiksen, M.S.; Lieber, C.M. Diameter-Selective Synthesis of Semiconductor Nanowires. J. Am. Chem. Soc. 2000, 122, 8801–8802. [Google Scholar] [CrossRef]
- Shin, H.S.; Sohn, J.I.; Kim, D.C.; Huck, W.T.S.; Welland, M.E.; Choi, H.C.; Kang, D.J. Density control of ZnO nanowires grown using Au-PMMA nanoparticles and their growth behavior. Nanotechnology 2009, 20, 085601. [Google Scholar] [CrossRef]
- Zhu, L.; Phillips, M.R.; Ton-That, C. Coalescence of ZnO nanowires grown from monodisperse Au nanoparticles. CrystEngComm 2015, 17, 4987–4991. [Google Scholar] [CrossRef]
- Jo, S.H.; Lao, J.Y.; Ren, Z.F.; Farrer, R.A.; Baldacchini, T.; Fourkas, J.T. Field-emission studies on thin films of zinc oxide nanowires. Appl. Phys. Lett. 2003, 83, 4821–4823. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Chen, T.; Yang, M.; Zhang, Z.; Wu, T.; Chen, H. Polymer-Encapsulated Gold-Nanoparticle Dimers: Facile Preparation and Catalytical Application in Guided Growth of Dimeric ZnO-Nanowires. Nano Lett. 2008, 8, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Jespersen, M.L.; Hutchison, J.E. Selective Growth of Vertical ZnO Nanowire Arrays Using Chemically Anchored Gold Nanoparticles. ACS Nano 2008, 2, 2001–2006. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Gutierrez-Wing, C.; Miki, M.; Yang, D.-Q.; Piyakis, K.N.; Sacher, E. Surface Diffusion and Coalescence of Mobile Metal Nanoparticles. J. Phys. Chem. B 2005, 109, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Cui, K.; Li, D.; Chen, D. Approach and Coalescence of Gold Nanoparticles Driven by Surface Thermodynamic Fluctuations and Atomic Interaction Forces. ACS Nano 2016, 10, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Jana, N.R.; Peng, X. Single-Phase and Gram-Scale Routes toward Nearly Monodisperse Au and Other Noble Metal Nanocrystals. J. Am. Chem. Soc. 2003, 125, 14280–14281. [Google Scholar] [CrossRef] [PubMed]
- Güell, F.; Martínez-Alanis, P.R. Tailoring the Green, Yellow and Red defect emission bands in ZnO nanowires via the growth parameters. J. Lumin. 2019, 210, 128–134. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Zinc Oxide Nanostructures: Synthesis and Properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.H.; Baptista, D.L.; Teo, K.B.K.; Lacerda, R.G.; Jefferson, D.A.; Milne, W.I. Controllable growth of vertically aligned zinc oxide nanowires using vapour deposition. Nanotechnology 2006, 17, 4811–4818. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Riedo, E.; Wang, Z.L. Systematic Study on Experimental Conditions for Large-Scale Growth of Aligned ZnO Nanwires on Nitrides. J. Phys. Chem. B 2005, 109, 9869–9872. [Google Scholar] [CrossRef]
- Ye, C.; Fang, X.; Hao, Y.; Teng, X.; Zhang, L. Zinc Oxide Nanostructures: Morphology Derivation and Evolution. J. Phys. Chem. B 2005, 109, 19758–19765. [Google Scholar] [CrossRef] [PubMed]
- Givargivoz, E.I. Fundamental aspects of VLS growth. J. Cryst. Grow. 1975, 31, 20–30. [Google Scholar]
- Saunders, R.B.; Garry, S.; Byrne, D.; Henry, M.O.; McGlynn, E. Length versus Radius Relationship for ZnO Nanowires Grown via Vapor Phase Transport. Cryst. Grow. Des. 2012, 12, 5972–5979. [Google Scholar] [CrossRef]
- Datye, A.K.; Xu, Q.; Kharas, K.C.; McCarty, J.M. Particle size distributions in heterogeneous catalysts: What do they tell us about the sintering mechanism? Catal. Today 2006, 111, 59–67. [Google Scholar] [CrossRef]
- Hansen, T.W.; Delariva, A.T.; Challa, S.R.; Datye, A.K. Sintering of Catalytic Nanoparticles: Particle Migration or Ostwald Ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Deltour, P.; Barrat, J.L.; Jensen, P. Fast Diffusion of a Lennard-Jones Cluster on a Crystalline Surface. Phys. Rev. Lett. 1997, 78, 4597–4600. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Sacher, E. Ar+-induced surface defects on HOPG and their effect on the nucleation, coalescence and growth of evaporated copper. Surf. Sci. 2002, 516, 43–45. [Google Scholar] [CrossRef]
- Carrey, J.; Maurice, J.L.; Petroff, F.; Vaurès, A. Growth of Au Clusters on Amorphous Al2O3: Evidence of Cluster Mobility above a Critical Size. Phys. Rev. Lett. 2001, 86, 4600–4603. [Google Scholar] [CrossRef] [PubMed]
- Güell, F.; Martínez-Alanis, P.R.; Khachadorian, S.; Rubio-Garcia, J.; Franke, A.; Hoffmann, A.; Santana, G. Raman and photoluminescence properties of ZnO nanowires grown by a catalyst-free vapor-transport process using ZnO nanoparticle seeds. Phys. Stat. Sol. B 2016, 253, 883–888. [Google Scholar] [CrossRef]
- Reparaz, J.S.; Güell, F.; Wagner, M.R.; Callsen, G.; Kirste, R.; Claramunt, S.; Morante, J.R.; Hoffmann, A. Recombination dynamics in ZnO nanowires: Surfaces states versus mode quality factor. Appl. Phys. Lett. 2010, 97, 133116. [Google Scholar] [CrossRef]
- Güell, F.; Osso, J.O.; Goni, A.R.; Cornet, A.; Morante, J.R. Synthesis and optical spectroscopy of ZnO nanowires. Superlattices Microstruct. 2009, 45, 271–276. [Google Scholar] [CrossRef]
- Fabbri, F.; Villani, M.; Catellani, A.; Calzolari, A.; Cicero, G.; Calestani, D.; Calestani, G.; Zappettini, A.; Dierre, B.; Sekiguchi, T.; et al. Zn vacancy induced green luminescence on non-polar surfaces in ZnO nanostructures. Sci. Rep. 2014, 4, 5158. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Güell, F.; Osso, J.O.; Goni, A.R.; Cornet, A.; Morante, J.R. Direct imaging of the visible emission bands from individual ZnO nanowires by near-field optical spectroscopy. Nanotechnology 2009, 20, 315701. [Google Scholar] [CrossRef]
- Allen, J.E.; Hemesath, E.R.; Perea, D.E.; Lensch-Falk, L.; Li, Z.Y.; Yin, F.; Gass, M.H.; Wang, P.; Bleloch, A.L.; Palmer, R.E.; et al. High-resolution detection of Au catalyst atoms in Si nanowires. Nat. Nanotechnol. 2009, 3, 168–173. [Google Scholar] [CrossRef]
- Méndez-Reyes, J.M.; Monroy, B.M.; Bizarro, M.; Güell, F.; Martínez, A.; Ramos, E. Gold as an intruder in ZnO nanowires. Phys. Chem. Chem. Phys. 2015, 17, 21525–21532. [Google Scholar] [CrossRef] [PubMed]
- Gruzintsev, A.N.; Volkov, V.T.; Khodos, I.I.; Nikiforova, T.V.; Koval’chuk, M.N. Luminescent Properties of ZnO Films Doped with Group-IB Acceptors. Russ. Microelectron. 2002, 31, 200–205. [Google Scholar] [CrossRef]
- Liao, H.; Wen, W.; Wong, G.K.L. Photoluminescence from Au nanoparticles embedded in Au:oxide composite films. J. Opt. Soc. Am. B 2006, 23, 2518–2521. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Grzelczak, M.; Altantzis, T.; Goris, B.; Pérez-Juste, J.; Bals, S.; Van Tendeloo, G.; Donaldson, S.H., Jr.; Chmelka, B.F. Hydrophobic Interactions Modulate Self-Assembly of Nanoparticles. ACS Nano 2012, 6, 11059–11065. [Google Scholar] [CrossRef]




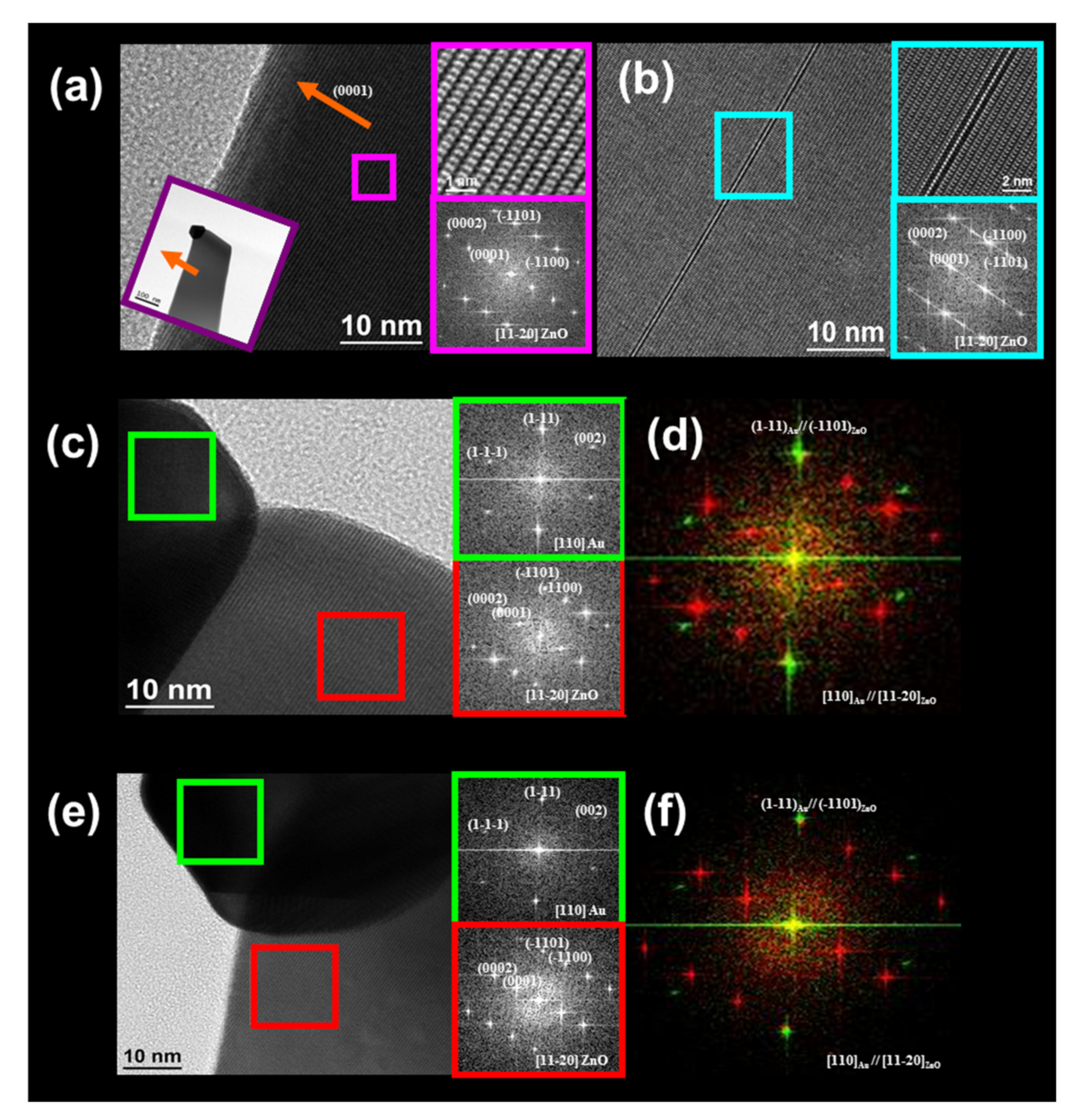

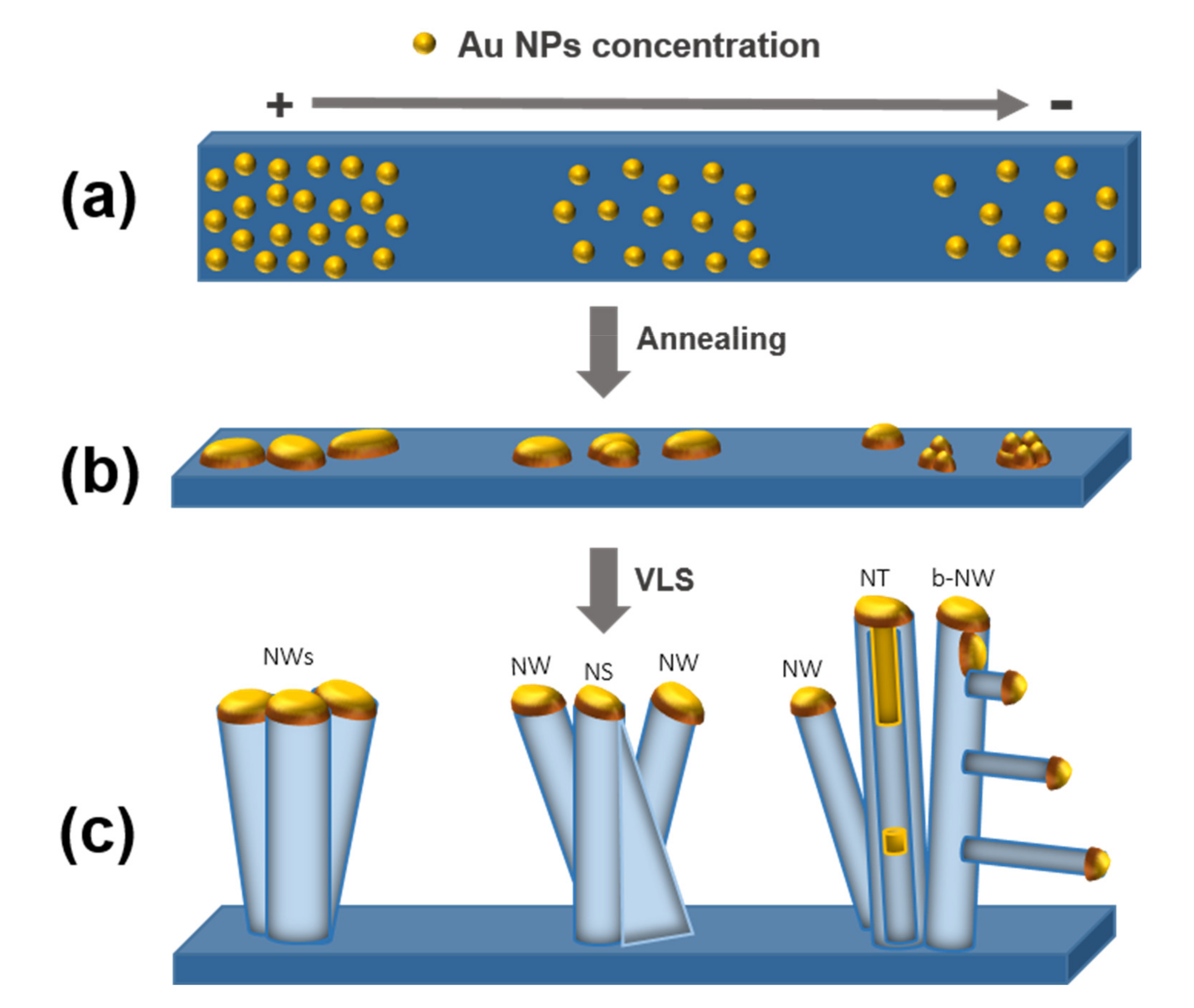
| Sample Number | Growth Parameter and Dimensions of the ZnO NWs | ||||
|---|---|---|---|---|---|
| Temperature (°C) | Pressure (Torr) | Ar Gas Flow (sccm) | Diameter (nm) | Length (µm) | |
| 1 | 700 | 760 | 400 | - | - |
| 2 | 800 | 760 | 400 | - | - |
| 3 | 850 | 760 | 400 | 70 ± 10 | 0.7 ± 0.1 |
| 4 | 900 | 760 | 400 | 100 ± 10 | 2.8 ± 0.2 |
| 5 | 900 | 76 | 400 | - | - |
| 6 | 900 | 200 | 400 | - | - |
| 7 | 900 | 380 | 400 | 100 ± 10 | 1.3 ± 0.1 |
| 8 | 900 | 600 | 400 | 120 ± 10 | 2.3 ± 0.2 |
| 9 | 900 | 760 | 50 | - | - |
| 10 | 900 | 760 | 100 | 120 ± 10 | 3.7 ± 0.2 |
| 11 | 900 | 760 | 200 | 140 ± 10 | 3.4 ± 0.2 |
| 12 | 900 | 760 | 600 | 100 ± 10 | 2.7 ± 0.2 |
| Sample Number | Colloidal Au NPs | ZnO NWs | |||
|---|---|---|---|---|---|
| Solvent | Concentration (NPs·cm−3) | Substrate | Diameter (nm) | Length (µm) | |
| 4 | water | 6.1·1016 | SiO2/Si | 100 ± 10 | 2.8 ± 0.2 |
| 13 | water | 6.1·1015 | SiO2/Si | 100 ± 10 | 2.8 ± 0.2 |
| 14 | water | 1.2·1015 | SiO2/Si | 100 ± 10 | 2.8 ± 0.2 |
| 15 | toluene | 6.1·1016 | SiO2/Si | 150 ± 10 | 2.0 ± 0.2 |
| 16 | toluene | 6.1·1015 | SiO2/Si | 120 ± 10 | 2.2 ± 0.2 |
| 17 | toluene | 1.2·1015 | SiO2/Si | 100 ± 10 | 2.5 ± 0.2 |
| 18 | hexane | 6.1·1016 | SiO2/Si | 160 ± 10 | 1.9 ± 0.2 |
| 19 | hexane | 6.1·1015 | SiO2/Si | 140 ± 10 | 1.8 ± 0.2 |
| 20 | hexane | 1.2·1015 | SiO2/Si | 100 ± 10 | 2.2 ± 0.2 |
| 21 | water | 6.1·1016 | Al2O3 | 70 ± 10 | 0.8 ± 0.2 |
| 22 | water | 6.1·1015 | Al2O3 | 70 ± 10 | 1.0 ± 0.2 |
| 23 | water | 1.2·1015 | Al2O3 | 70 ± 10 | 1.4 ± 0.2 |
| 24 | toluene | 6.1·1016 | Al2O3 | 70 ± 10 | 1.4 ± 0.2 |
| 25 | toluene | 6.1·1015 | Al2O3 | 70 ± 10 | 1.6 ± 0.2 |
| 26 | toluene | 1.2·1015 | Al2O3 | 70 ± 10 | 2.2 ± 0.2 |
| 27 | hexane | 6.1·1016 | Al2O3 | 70 ± 10 | 1.3 ± 0.2 |
| 28 | hexane | 6.1·1015 | Al2O3 | 70 ± 10 | 1.5 ± 0.2 |
| 29 | hexane | 1.2·1015 | Al2O3 | 70 ± 10 | 2.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güell, F.; Cabot, A.; Claramunt, S.; Moghaddam, A.O.; Martínez-Alanis, P.R. Influence of Colloidal Au on the Growth of ZnO Nanostructures. Nanomaterials 2021, 11, 870. https://doi.org/10.3390/nano11040870
Güell F, Cabot A, Claramunt S, Moghaddam AO, Martínez-Alanis PR. Influence of Colloidal Au on the Growth of ZnO Nanostructures. Nanomaterials. 2021; 11(4):870. https://doi.org/10.3390/nano11040870
Chicago/Turabian StyleGüell, Frank, Andreu Cabot, Sergi Claramunt, Ahmad Ostovari Moghaddam, and Paulina R. Martínez-Alanis. 2021. "Influence of Colloidal Au on the Growth of ZnO Nanostructures" Nanomaterials 11, no. 4: 870. https://doi.org/10.3390/nano11040870
APA StyleGüell, F., Cabot, A., Claramunt, S., Moghaddam, A. O., & Martínez-Alanis, P. R. (2021). Influence of Colloidal Au on the Growth of ZnO Nanostructures. Nanomaterials, 11(4), 870. https://doi.org/10.3390/nano11040870








