One-Step Plasma Synthesis of Nitrogen-Doped Carbon Nanomesh
Abstract
1. Introduction
2. Materials and Methods
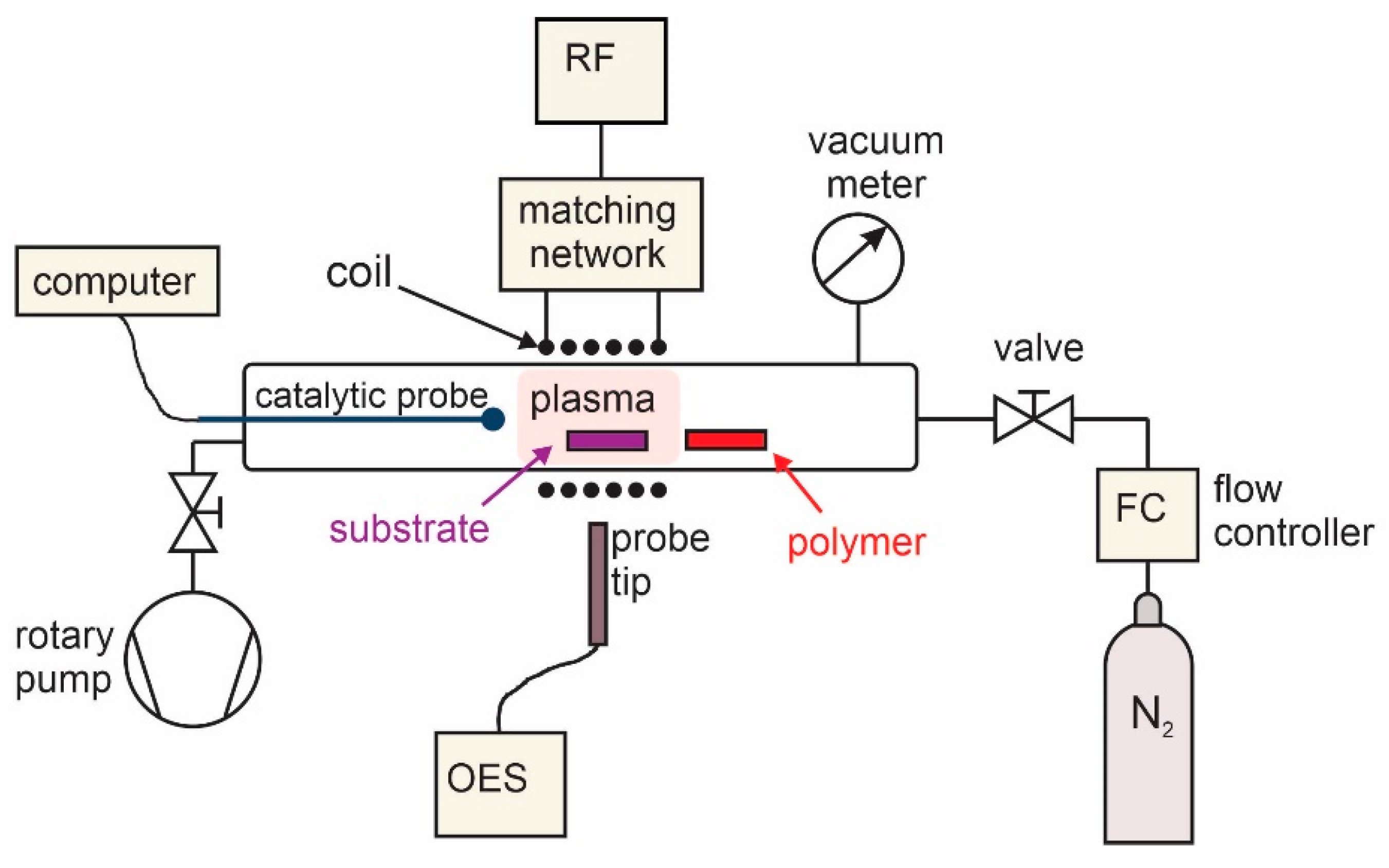
2.1. Plasma Synthesis
2.2. Characterization of the Samples
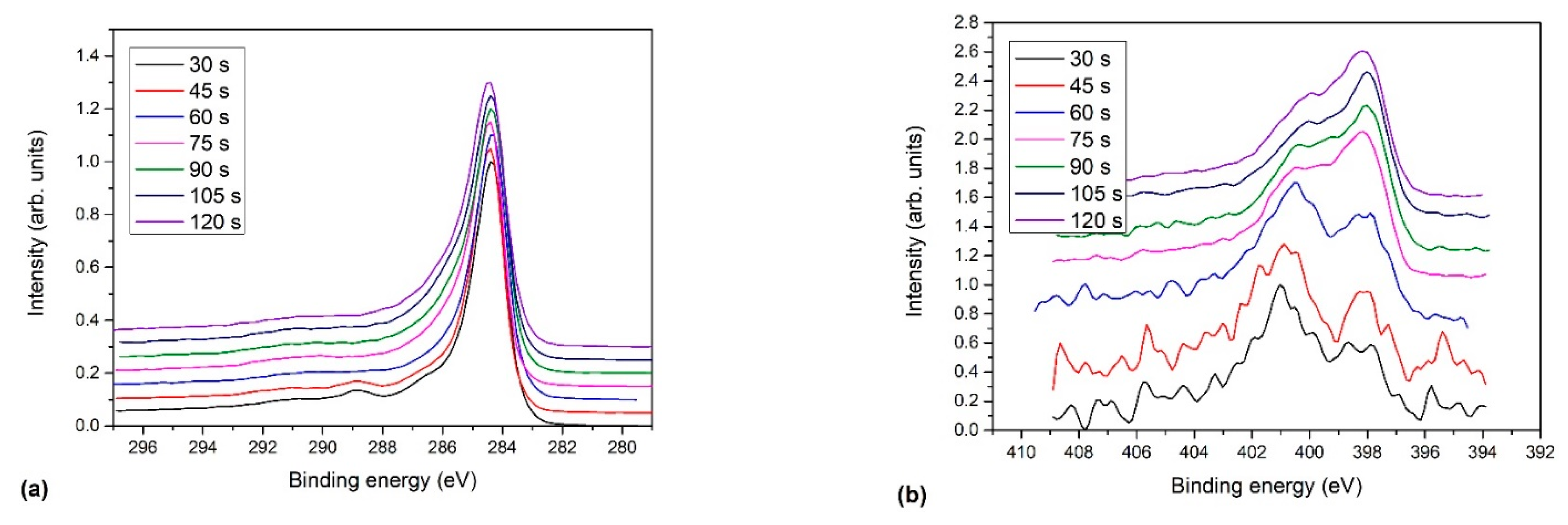
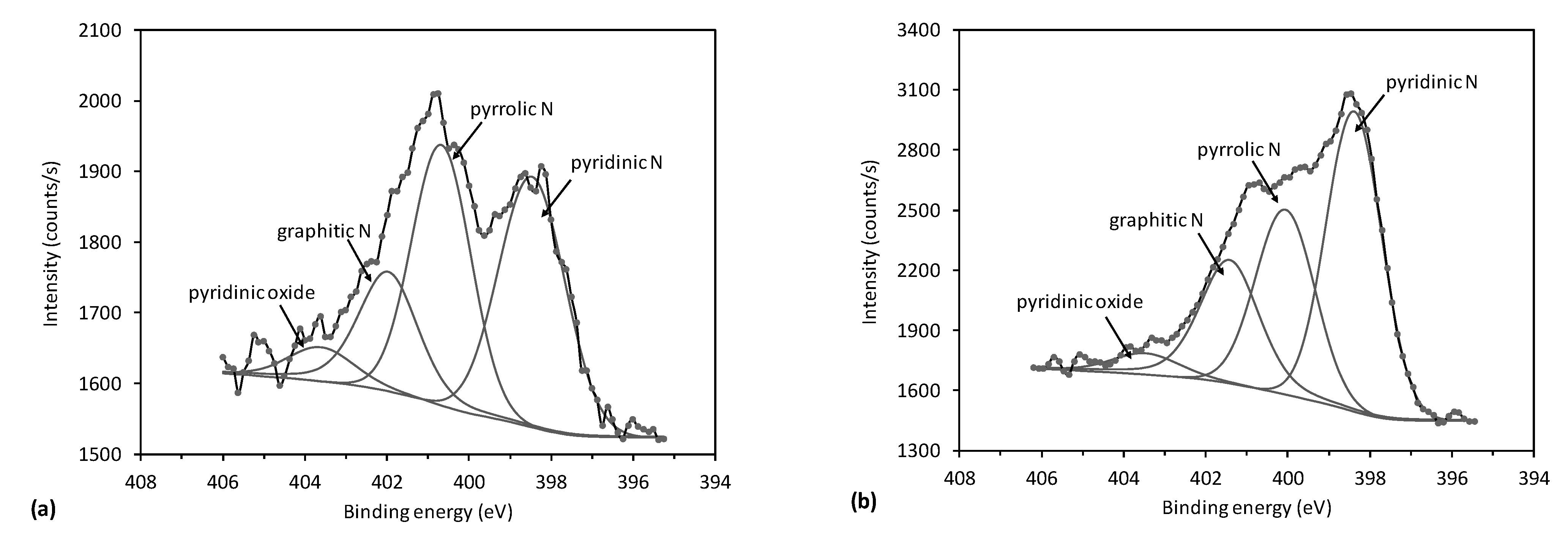
2.2.1. X-ray Photoelectron Spectroscopy (XPS)
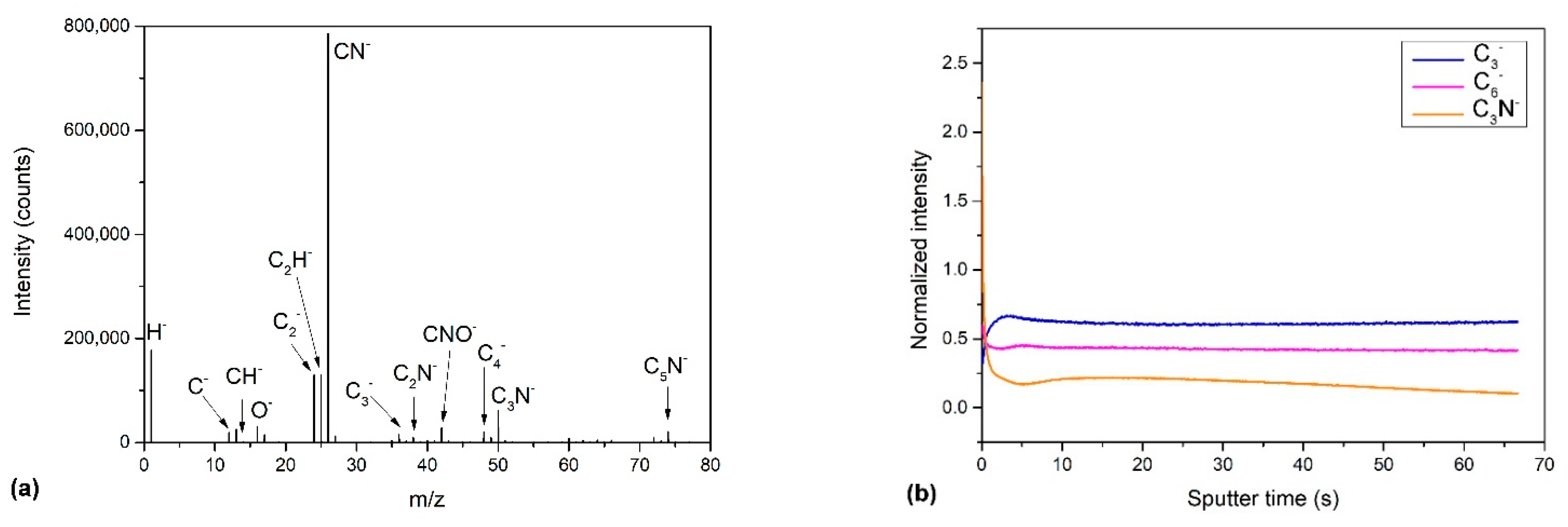
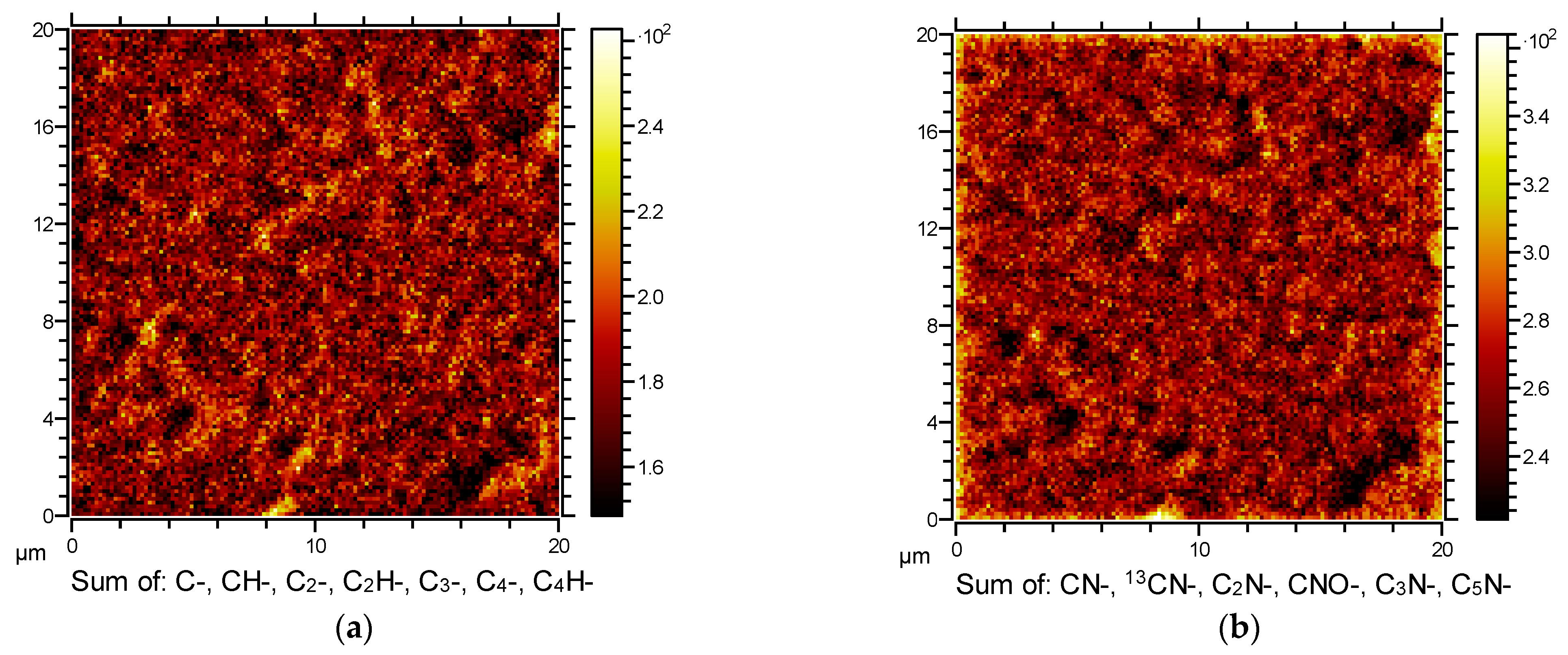
2.2.2. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
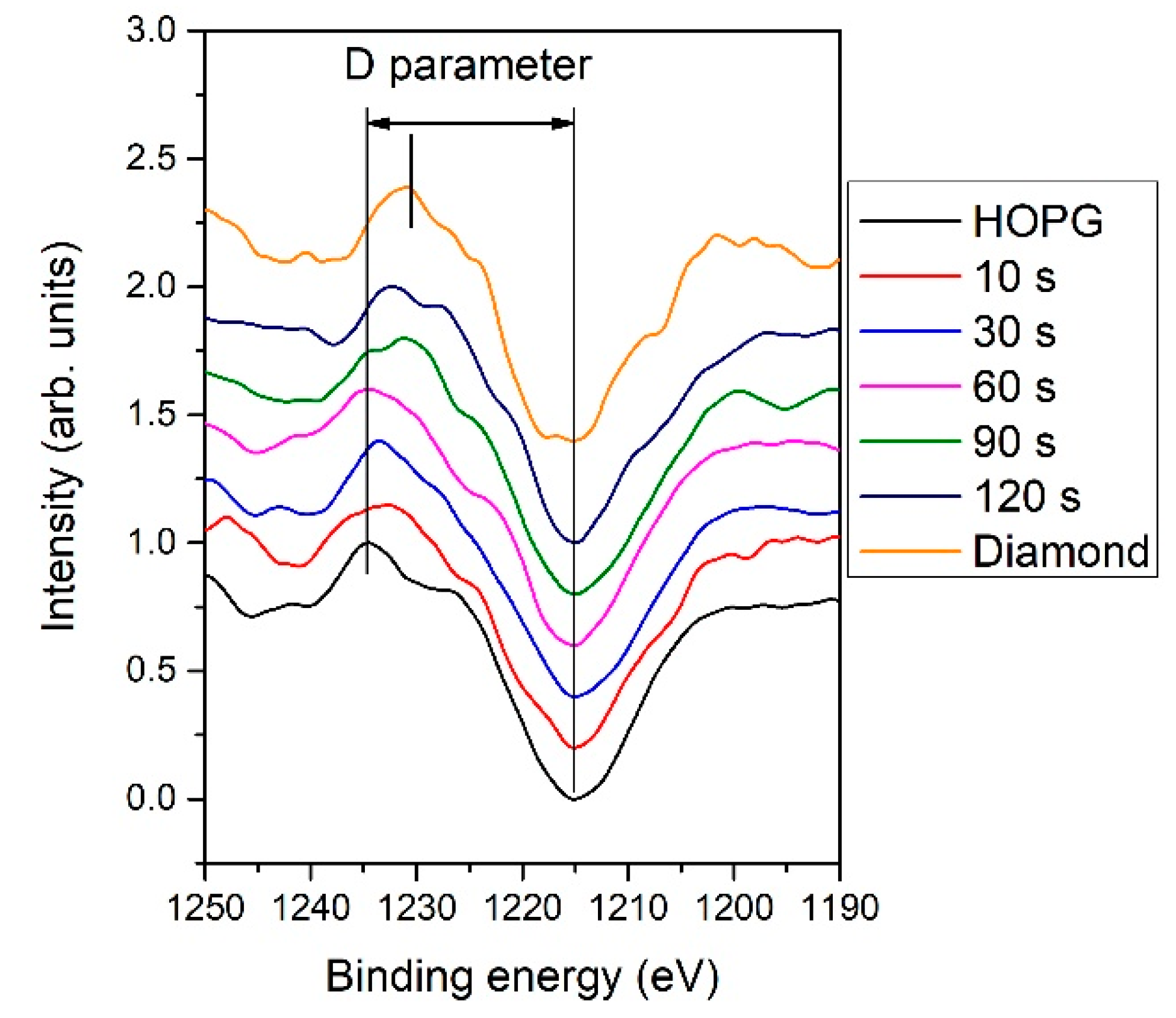
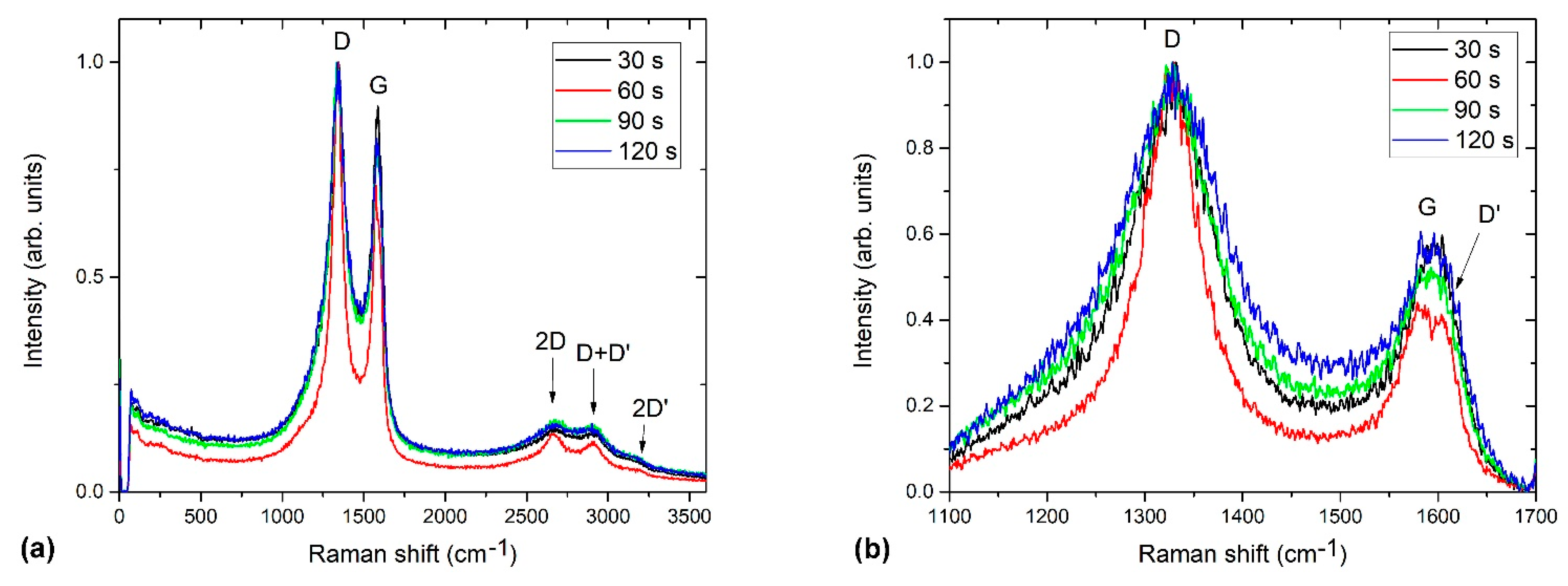
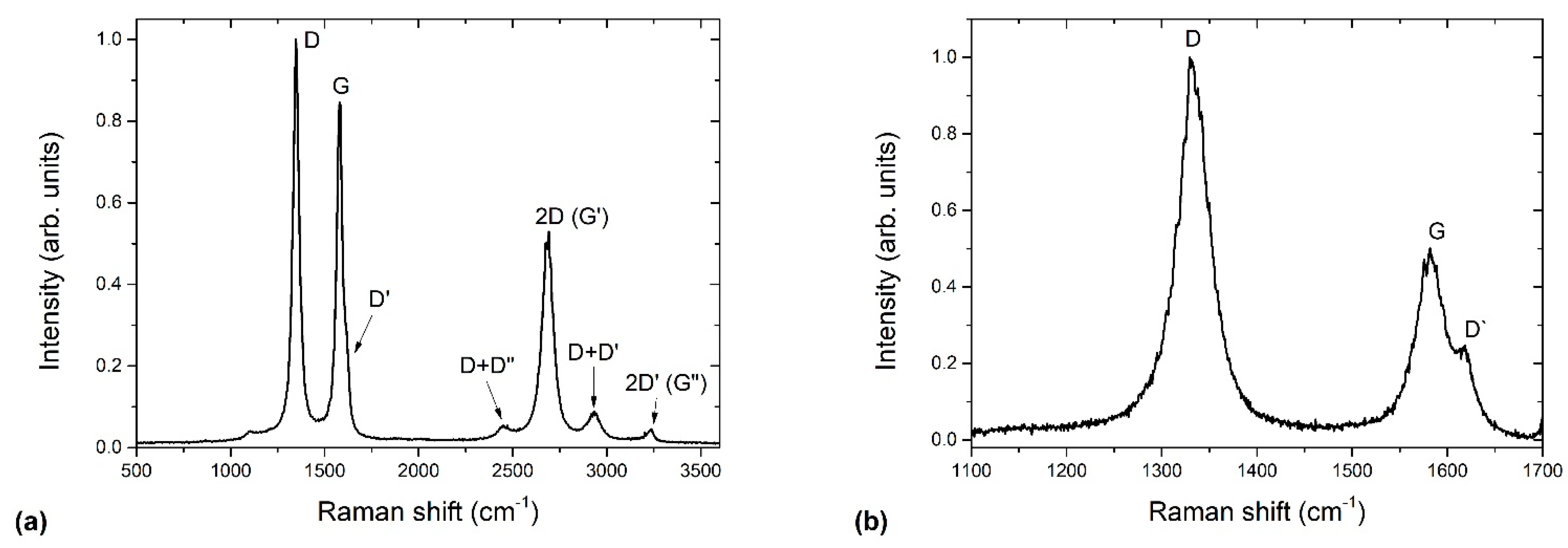
2.2.3. Raman Spectroscopy
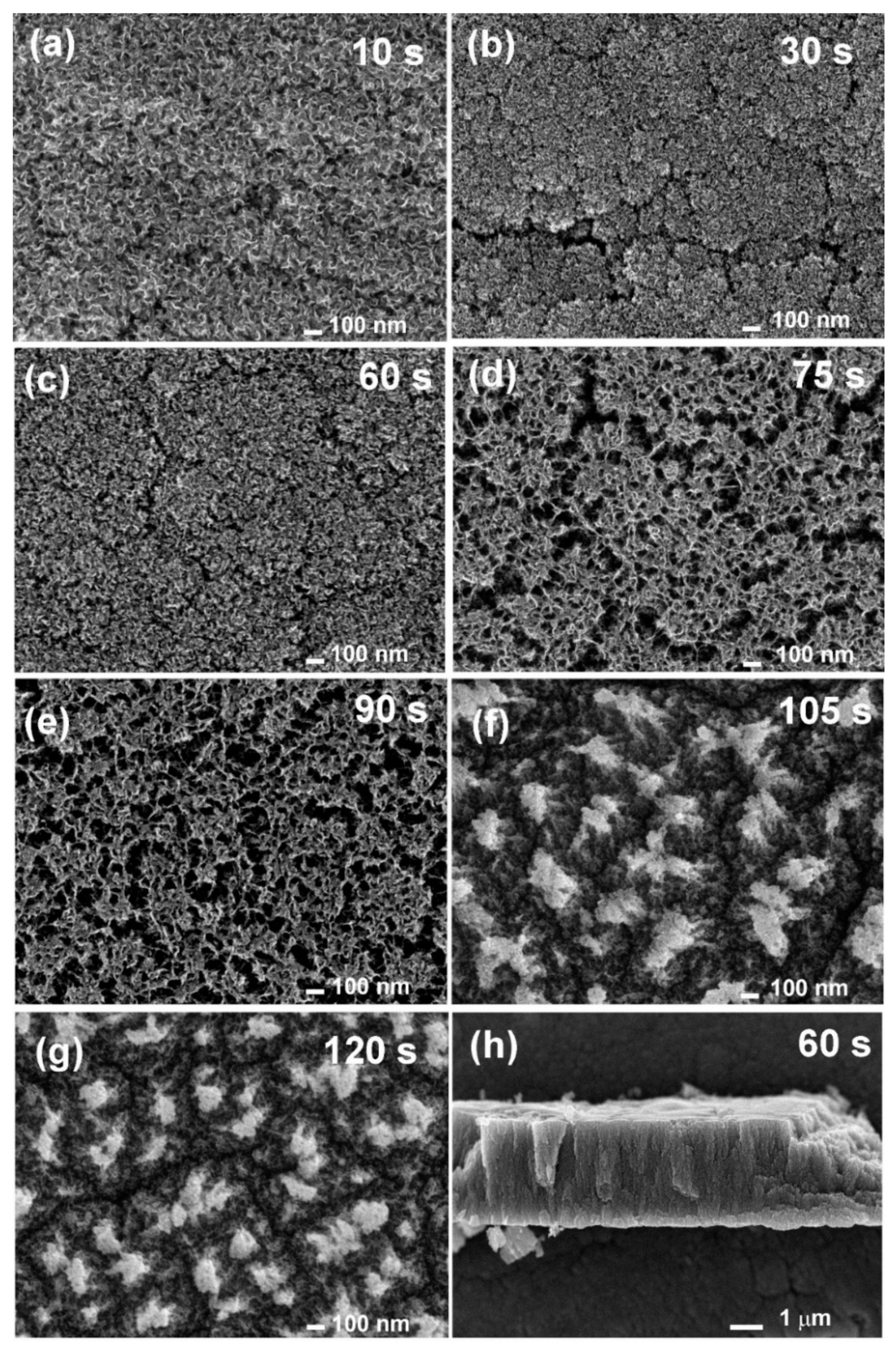
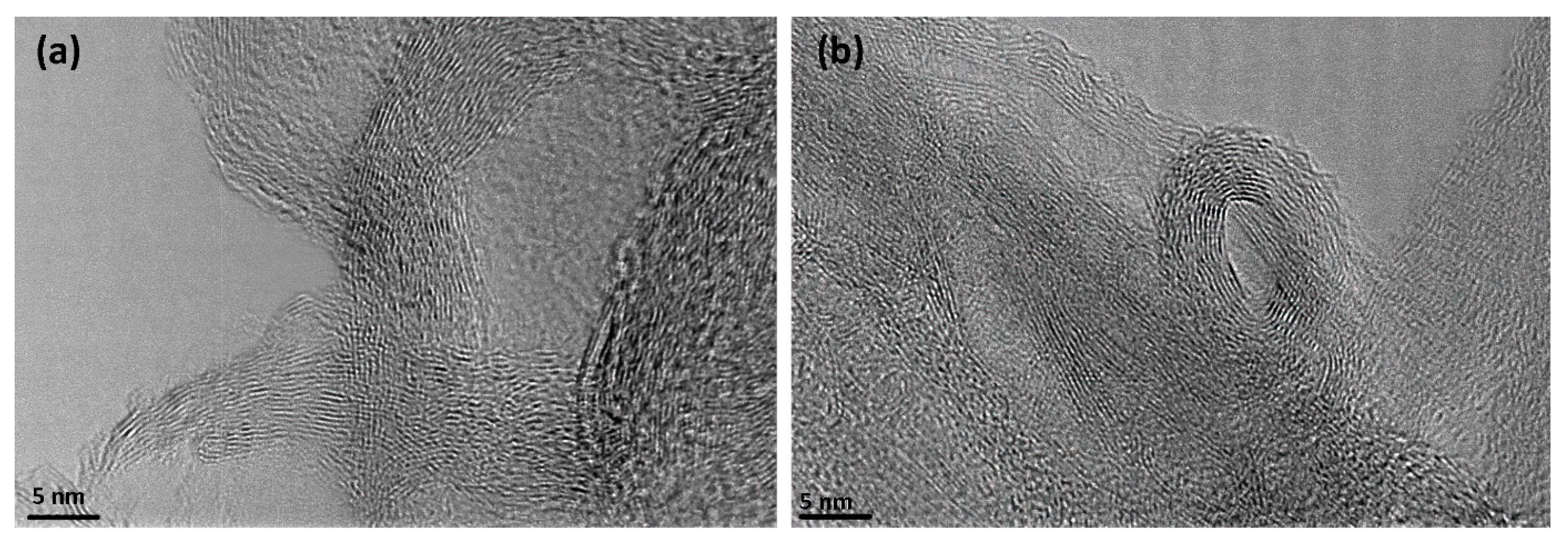
2.2.4. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
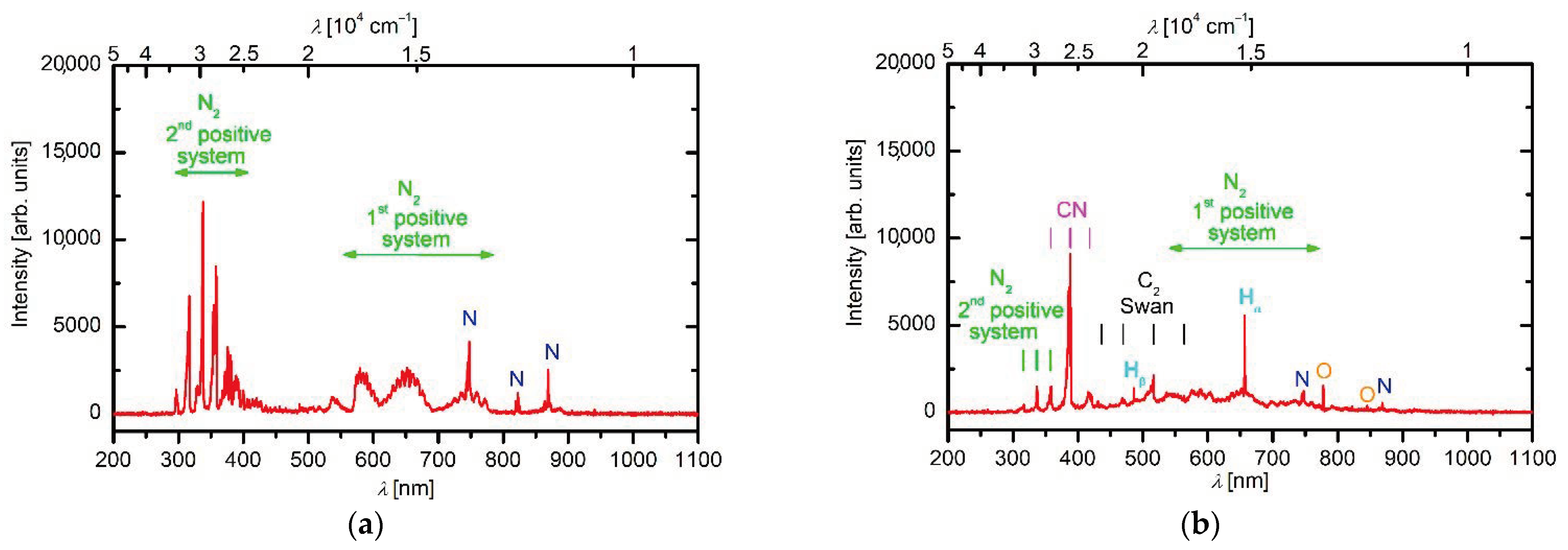
3. Results and Discussion
3.1. Synthesis Mechanism of N-Doped Carbon Nanomesh
3.2. Surface Characterization of N-Doped Carbon Nanomesh
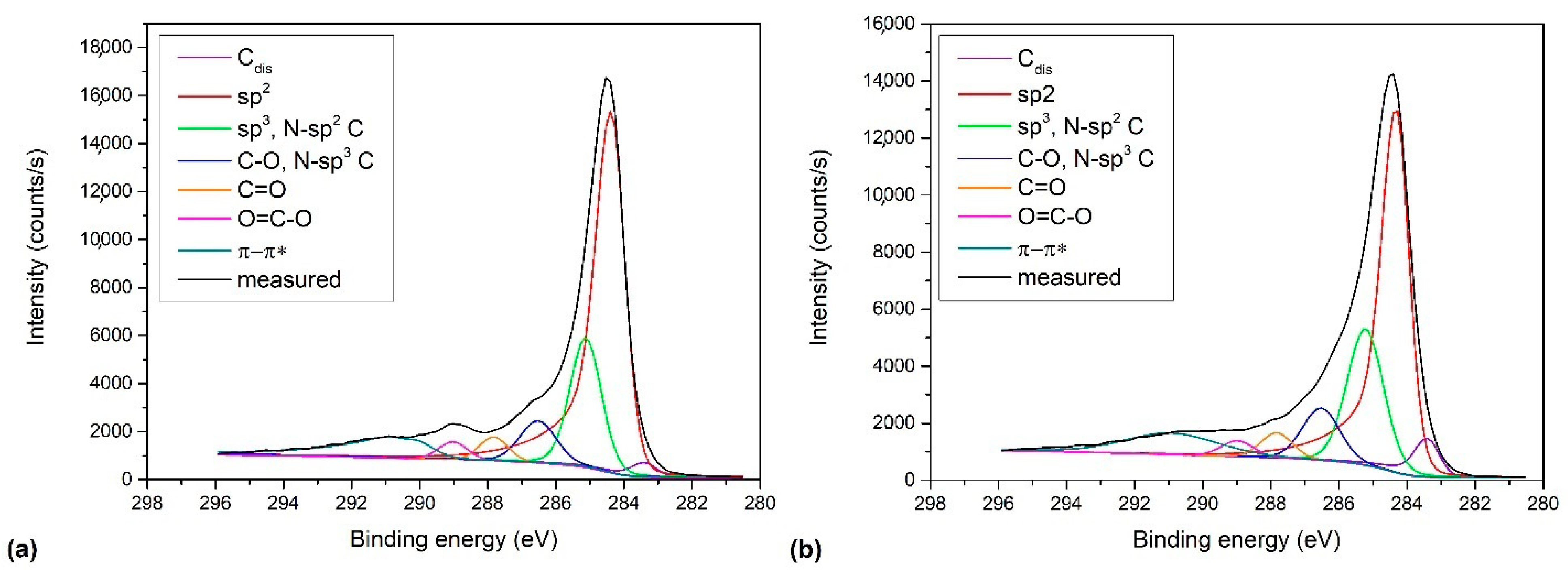
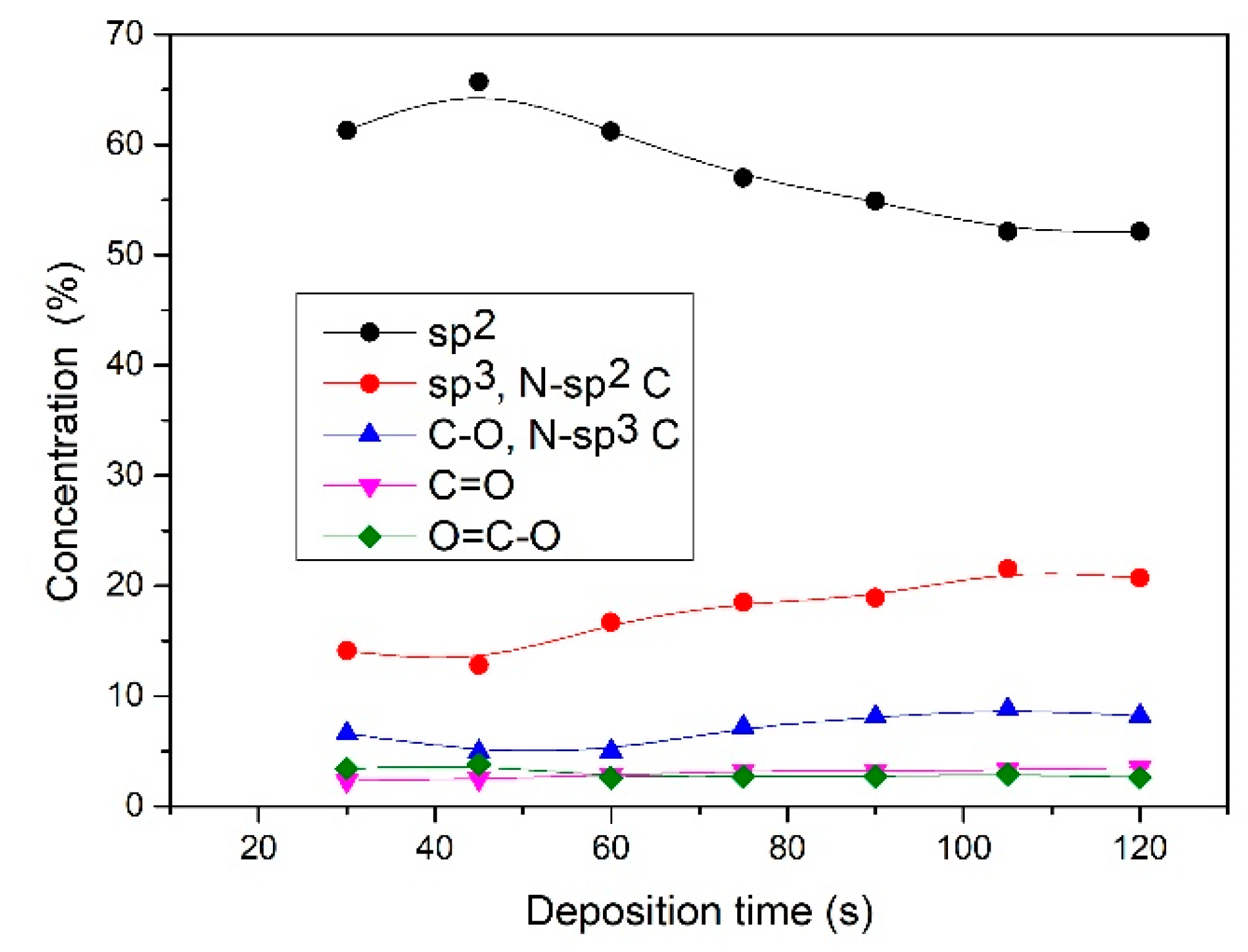
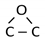 (peak 5), O=C-O (peak 6), and π-π* excitation. Two examples are shown in Figure 9. Figure 9a shows an example of the C1s peak for short deposition times, whereas Figure 9b for long deposition times. The results for all samples obtained from fitting as shown in Figure 9 are summarized in Figure 10. Figure 10 shows the evolution of functional groups with deposition time. Here we should also note that overlapping of the O=C-O peak at ~289 eV with the π-π* peak brings some uncertainties in determining the corresponding concentrations. In Figure 10, we can observe a decreasing concentration of sp2 carbon with deposition time, which is in agreement with the results of the D-parameter shown in Figure 8. We can also observe the increasing intensity of peaks related to sp3 and nitrogen content. The detailed analyses of the C1s peak, therefore, support the conclusion brought on the basis of the Auger CKLL spectra. Here, the overlapping of the oxygen and nitrogen-containing functional groups should be stressed again. For example, the functionality marked as “C-O and sp3 C-N” is likely to be almost free from oxygen at high deposition times.
(peak 5), O=C-O (peak 6), and π-π* excitation. Two examples are shown in Figure 9. Figure 9a shows an example of the C1s peak for short deposition times, whereas Figure 9b for long deposition times. The results for all samples obtained from fitting as shown in Figure 9 are summarized in Figure 10. Figure 10 shows the evolution of functional groups with deposition time. Here we should also note that overlapping of the O=C-O peak at ~289 eV with the π-π* peak brings some uncertainties in determining the corresponding concentrations. In Figure 10, we can observe a decreasing concentration of sp2 carbon with deposition time, which is in agreement with the results of the D-parameter shown in Figure 8. We can also observe the increasing intensity of peaks related to sp3 and nitrogen content. The detailed analyses of the C1s peak, therefore, support the conclusion brought on the basis of the Auger CKLL spectra. Here, the overlapping of the oxygen and nitrogen-containing functional groups should be stressed again. For example, the functionality marked as “C-O and sp3 C-N” is likely to be almost free from oxygen at high deposition times.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joucken, F.; Henrard, L.; Lagoute, J. Electronic properties of chemically doped graphene. Phys. Rev. Mater. 2019, 3, 110301. [Google Scholar] [CrossRef]
- Tison, Y.; Lagoute, J.; Repain, V.; Chacon, C.; Girard, Y.; Rousset, S.; Joucken, F.; Sharma, D.; Henrard, L.; Amara, H.; et al. Electronic Interaction between Nitrogen Atoms in Doped Graphene. ACS Nano 2015, 9, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Joucken, F.; Tison, Y.; le Fèvre, P.; Tejeda, A.; Taleb-Ibrahimi, A.; Conrad, E.; Repain, V.; Chacon, C.; Bellec, A.; Girard, Y.; et al. Charge transfer and electronic doping in nitrogen-doped graphene. Sci. Rep. 2015, 5, 14564. [Google Scholar] [CrossRef]
- Joucken, F.; Tison, Y.; Lagoute, J.; Dumont, J.; Cabosart, D.; Zheng, B.; Repain, V.; Chacon, C.; Girard, Y.; Botello-Méndez, A.R.; et al. Localized state and charge transfer in nitrogen-doped graphene. Phys. Rev. B 2012, 85, 161408. [Google Scholar] [CrossRef]
- Hu, M.; Lv, Q.; Lv, R. Controllable synthesis of nitrogen-doped graphene oxide by tablet-sintering for efficient lithi-um/sodium-ion storage. ES Energ. Environ. 2019, 3, 45–54. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, X.; Cao, D. Biomass-derived nitrogen-doped porous carbons (NPC) and NPC/ polyaniline composites as high performance supercapacitor materials. Eng. Sci. 2018, 1, 55–63. [Google Scholar] [CrossRef]
- Lv, Y.; Zhu, L.; Xu, H.; Yang, L.; Liu, Z.; Cheng, D.; Cao, X.; Yun, J.; Cao, D. Core/shell Template-derived Co, N-doped Carbon Bifunctional Electrocatalysts for Rechargeable Zn-air Battery. Eng. Sci. 2019, 7, 26–37. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of Oxygen Reduction Reaction on Nitrogen-Doped Graphene for Fuel Cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Deng, H.; Zhang, C.; Su, J.-W.; Lin, J. Nitrogen Doped Coal with High Electrocatalytic Activity for Oxygen Reduction Reaction. Eng. Sci. 2019, 8, 39–45. [Google Scholar] [CrossRef]
- Yu, H.; Yang, L.; Cheng, D.; Cao, D. Zeolitic-imidazolate framework (ZIF)@ZnCo-ZIF core-shell template derived Co, N-doped carbon catalysts for oxygen reduction reaction. Eng. Sci. 2018, 3, 54–61. [Google Scholar] [CrossRef][Green Version]
- Evlashin, S.A.; Maksimov, Y.M.; Dyakonov, P.V.; Pilevsky, A.A.; Maslakov, K.I.; Mankelevich, Y.A.; Voronina, E.N.; Vavilov, S.V.; Pavlov, A.A.; Zenova, E.V.; et al. N-Doped Carbon NanoWalls for Power Sources. Sci. Rep. 2019, 9, 6716. [Google Scholar] [CrossRef] [PubMed]
- Bundaleska, N.; Henriques, J.; Abrashev, M.; Rego, A.M.B.D.; Ferraria, A.M.; Almeida, A.; Dias, F.M.; Valcheva, E.; Arnaudov, B.; Upadhyay, K.K.; et al. Large-scale synthesis of free-standing N-doped graphene using microwave plasma. Sci. Rep. 2018, 8, 12595. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Hong, J.-Y.; Han, A.-R.; Lee, J.S.; Lee, J.S.; Oh, J.H.; Jang, J. Flexible FET-Type VEGF Aptasensor Based on Nitrogen-Doped Graphene Converted from Conducting Polymer. ACS Nano 2012, 6, 1486–1493. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C.-P. 3D Nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy 2013, 2, 241–248. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef]
- Zhang, L.; Qing, X.; Chen, Z.; Wang, J.; Yang, G.; Qian, Y.; Liu, D.; Chen, C.; Wang, L.; Lei, W. All pseudocapacitive nitrogen-doped reduced graphene oxide and polyaniline nanowire network for high-performance flexible on-chip en-ergy storage. ACS Appl. Energy Mater. 2020, 3, 6845–6852. [Google Scholar] [CrossRef]
- Kumar, N.A.; Nolan, H.; McEvoy, N.; Rezvani, E.; Doyle, R.L.; Lyons, M.E.G.; Duesberg, G.S. Plasma-assisted simul-taneous reduction and nitrogen doping of graphene oxide nanosheets. J. Mater. Chem. A 2013, 1, 4431–4435. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. A review of strategies for the synthesis of N-doped graphene-like ma-terials. Nanomaterials 2020, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-H.; Shiue, S.-T. Effects of acetylene/ammonia mixtures on the properties of carbon films prepared by thermal chemical vapor deposition. Surf. Coat. Technol. 2013, 215, 161–169. [Google Scholar] [CrossRef]
- Prioli, R.; Zanette, S.I.; Caride, A.O.; Franceschini, D.F. Atomic force microscopy of amorphous hydrogenated carbon–nitrogen films deposited by radio-frequency-plasma decomposition of methane–ammonia gas mixtures. J. Vacuum Sci. Technol. A 1996, 14, 2351–2355. [Google Scholar] [CrossRef]
- Fu, Y.B.; Chen, Q.; Xu, W.C. Effects of ammonia on the growth of carbon nanotubesby plasma synthesis. Beijing Ligong Daxue Xuebao/Trans. Beijing Inst. Technol. 2009, 29, 146–148. [Google Scholar]
- Bell, M.S.; Teo, K.B.K.; Lacerda, R.G.; Milne, W.I.; Hash, D.B.; Meyyappan, M. Carbon nanotubes by plasma-enhanced chemical vapor deposition. Pure Appl. Chem. 2006, 78, 1117–1125. [Google Scholar] [CrossRef]
- Guo, Z.; Yi, Y.; Wang, L.; Yan, J.; Guo, H. Pt/TS-1 Catalyst Promoted C–N Coupling Reaction in CH4–NH3 Plasma for HCN Synthesis at Low Temperature. ACS Catal. 2018, 8, 10219–10224. [Google Scholar] [CrossRef]
- Denysenko, I.B.; von Wahl, E.; Mikikian, M.; Berndt, J.; Ivko, S.; Kersten, H.; Kovacevic, E.; Azarenkov, N. Plasma properties as function of time in Ar/C2H2 dust-forming plasma. J. Phys. D Appl. Phys. 2019, 53, 135203. [Google Scholar] [CrossRef]
- Holland, B.; Hay, J. The thermal degradation of PET and analogous polyesters measured by thermal analysis–Fourier transform infrared spectroscopy. Polymer 2002, 43, 1835–1847. [Google Scholar] [CrossRef]
- Sirse, N.; Harvey, C.; Gaman, C.; Ellingboe, A.R. Investigation of plasma uniformity, rotational and vibrational tem-perature in a 162 MHz multi-electrode capacitive discharge. J. Phys. D Appl. Phys. 2020, 53, 335203. [Google Scholar] [CrossRef]
- Fantz, U.; Briefi, S.; Rauner, D.; Wünderlich, D. Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas. Plasma Sources Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]
- Jamroz, P.; Zyrnicki, W. Optical emission spectroscopy study for nitrogen–acetylene–argon and nitrogen–acetylene–helium 100 kHz and dc discharges. Vacuum 2010, 84, 940–946. [Google Scholar] [CrossRef]
- Borillo, G.C.; Tadano, Y.S.; Godoi, A.F.L.; Pauliquevis, T.; Sarmiento, H.; Rempel, D.; Yamamoto, C.I.; Marchi, M.R.; Potgieter-Vermaak, S.; Godoi, R.H. Polycyclic Aromatic Hydrocarbons (PAHs) and nitrated analogs associated to particulate matter emission from a Euro V-SCR engine fuelled with diesel/biodiesel blends. Sci. Total. Environ. 2018, 644, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, H.X.; Zhang, H.; Sasaoka, H.; Nishimura, K. Characterization and surface modification of carbon nanowalls. J. Mater. Chem. 2010, 20, 5070–5073. [Google Scholar] [CrossRef]
- Sakulsermsuk, S.; Singjai, P.; Chaiwong, C. Influence of plasma process on the nitrogen configuration in graphene. Diam. Relat. Mater. 2016, 70, 211–218. [Google Scholar] [CrossRef]
- McClure, J.P.; Thornton, J.D.; Jiang, R.Z.; Chu, D.; Cuomo, J.J.; Fedkiw, P.S. Oxygen reduction on metal-free nitro-gen-doped carbon nanowall electrodes. J. Electrochem. Soc. 2012, 159, F733–F742. [Google Scholar] [CrossRef]
- Dias, A.; Bundaleski, N.; Tatarova, E.; Dias, F.M.; Abrashev, M.; Cvelbar, U.; Teodoro, O.M.N.D.; Henriques, J. Pro-duction of N-graphene by microwave N2-Ar plasma. J. Phys. D Appl. Phys. 2016, 49, 055307. [Google Scholar] [CrossRef]
- Singh, G.; Sutar, D.S.; Botcha, V.D.; Narayanam, P.K.; Talwar, S.S.; Srinivasa, R.S.; Major, S.S. Study of simultaneous reduction and nitrogen doping of graphene oxide Langmuir–Blodgett monolayer sheets by ammonia plasma treatment. Nanotechnology 2013, 24, 355704. [Google Scholar] [CrossRef]
- Boas, C.R.S.V.; Focassio, B.; Marinho, E.; Larrude, D.G.; Salvadori, M.C.; Leão, C.R.; dos Santos, D.J. Characterization of nitrogen doped graphene bilayers synthesized by fast, low temperature microwave plasma-enhanced chemical vapour deposition. Sci. Rep. 2019, 9, 13715. [Google Scholar] [CrossRef]
- Turnbull, L.; Liggat, J.; Macdonald, W. Thermal degradation chemistry of poly(ethylene naphthalate)—A study by thermal volatilisation analysis. Polym. Degrad. Stab. 2013, 98, 2244–2258. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Schiros, T.; Nordlund, D.; Pálová, L.; Prezzi, D.; Zhao, L.; Kim, K.S.; Wurstbauer, U.; Gutiérrez, C.; Delongchamp, D.; Jaye, C.; et al. Connecting Dopant Bond Type with Electronic Structure in N-Doped Graphene. Nano Lett. 2012, 12, 4025–4031. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196. [Google Scholar] [CrossRef]
- Mezzi, A.; Kaciulis, S. Surface investigation of carbon films: From diamond to graphite. Surf. Interface Anal. 2010, 42, 1082–1084. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Calvani, P.; Trucchi, D.M. Electron spectroscopy of the main allotropes of carbon. Surf. Interface Anal. 2014, 46, 966–969. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated gra-phene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Blume, R.; Rosenthal, D.; Tessonnier, J.-P.; Li, H.; Knop-Gericke, A.; Schlögl, R. Characterizing Graphitic Carbon with X-ray Photoelectron Spectroscopy: A Step-by-Step Approach. ChemCatChem 2015, 7, 2871–2881. [Google Scholar] [CrossRef]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with het-eroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef]
- Leiro, J.; Heinonen, M.; Laiho, T.; Batirev, I. Core-level XPS spectra of fullerene, highly oriented pyrolitic graphite, and glassy carbon. J. Electron. Spectrosc. Relat. Phenom. 2003, 128, 205–213. [Google Scholar] [CrossRef]
- Maddi, C.; Bourquard, F.; Barnier, V.; Avila, J.; Asensio, M.-C.; Tite, T.; Donnet, C.; Garrelie, F. Nano-Architecture of nitrogen-doped graphene films synthesized from a solid CN source. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Yamada, Y.; Koinuma, M.; Sato, S. Origins of sp3C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials. Anal. Chem. 2016, 88, 6110–6114. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kondo, H.; Ishikawa, K.; Sekine, M.; Hiramatsu, M.; Hori, M. Effects of nitrogen plasma post-treatment on electrical conduction of carbon nanowalls. Jpn. J. Appl. Phys. 2014, 53, 40307. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, C.E.; Lyu, S.C.; Lee, T.J.; Lee, C.J. Structural study of nitrogen-doping effects in bamboo-shaped mul-tiwalled carbon nanotubes. Appl. Phys. Lett. 2004, 84, 2877–2879. [Google Scholar] [CrossRef]
- Yanilmaz, A.; Tomak, A.; Akbali, B.; Bacaksiz, C.; Ozceri, E.; Ari, O.; Senger, R.T.; Selamet, Y.; Zareie, H.M. Nitrogen doping for facile and effective modification of graphene surfaces. RSC Adv. 2017, 7, 28383–28392. [Google Scholar] [CrossRef]
- Tartz, M.; Neumann, H. Sputter Yields of Carbon Materials under Xenon Ion Incidence. Plasma Process. Polym. 2007, 4, S633–S636. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; Macdonald, B.J.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls: Synthesis and Emerging Applications; Springer: Wien, Austria, 2010. [Google Scholar] [CrossRef]
- Manojkumar, P.A.; Krishna, N.G.; Mangamma, G.; Albert, S.K. Understanding the structural and chemical changes in vertical graphene nanowalls upon plasma nitrogen ion implantation. Phys. Chem. Chem. Phys. 2019, 21, 10773–10783. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, M.; Stavale, F.; Ferreira, E.M.; Vilani, C.; Moutinho, M.; Capaz, R.B.; Achete, C.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]

| Time (s) | C (at.%) | N (at.%) | O (at.%) | N/C (%) | O/C (%) |
|---|---|---|---|---|---|
| 10 | 91.4 | 0.8 | 7.9 | 0.8 | 8.6 |
| 20 | 90.6 | 2.2 | 7.2 | 2.4 | 7.9 |
| 30 | 90.2 | 1.3 | 8.5 | 1.5 | 9.4 |
| 45 | 91.6 | 1.4 | 7.1 | 1.5 | 7.7 |
| 60 | 93.8 | 3.0 | 3.2 | 3.2 | 3.4 |
| 75 | 89.9 | 8.3 | 1.8 | 9.3 | 2.0 |
| 90 | 88.2 | 10.2 | 1.6 | 11.6 | 1.8 |
| 105 | 85.6 | 12.7 | 1.7 | 14.8 | 2.0 |
| 120 | 85.7 | 12.5 | 1.7 | 14.6 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A.; Zaplotnik, R.; Primc, G.; Pirker, L.; Mozetič, M. One-Step Plasma Synthesis of Nitrogen-Doped Carbon Nanomesh. Nanomaterials 2021, 11, 837. https://doi.org/10.3390/nano11040837
Vesel A, Zaplotnik R, Primc G, Pirker L, Mozetič M. One-Step Plasma Synthesis of Nitrogen-Doped Carbon Nanomesh. Nanomaterials. 2021; 11(4):837. https://doi.org/10.3390/nano11040837
Chicago/Turabian StyleVesel, Alenka, Rok Zaplotnik, Gregor Primc, Luka Pirker, and Miran Mozetič. 2021. "One-Step Plasma Synthesis of Nitrogen-Doped Carbon Nanomesh" Nanomaterials 11, no. 4: 837. https://doi.org/10.3390/nano11040837
APA StyleVesel, A., Zaplotnik, R., Primc, G., Pirker, L., & Mozetič, M. (2021). One-Step Plasma Synthesis of Nitrogen-Doped Carbon Nanomesh. Nanomaterials, 11(4), 837. https://doi.org/10.3390/nano11040837









