Enhancement of Catalytic Activity and Durability of Pt Nanoparticle through Strong Chemical Interaction with Electrically Conductive Support of Magnéli Phase Titanium Oxide
Abstract
1. Introduction
2. Experimental
2.1. Preparation of MPTO
2.2. Preparation of Pt/MPTO and Pt/C
3. Characterizations
3.1. Physicochemical Characterizations
3.2. Electrochemical Characterizations
3.3. Model Systems and Computational Detail
4. Results and Discussion
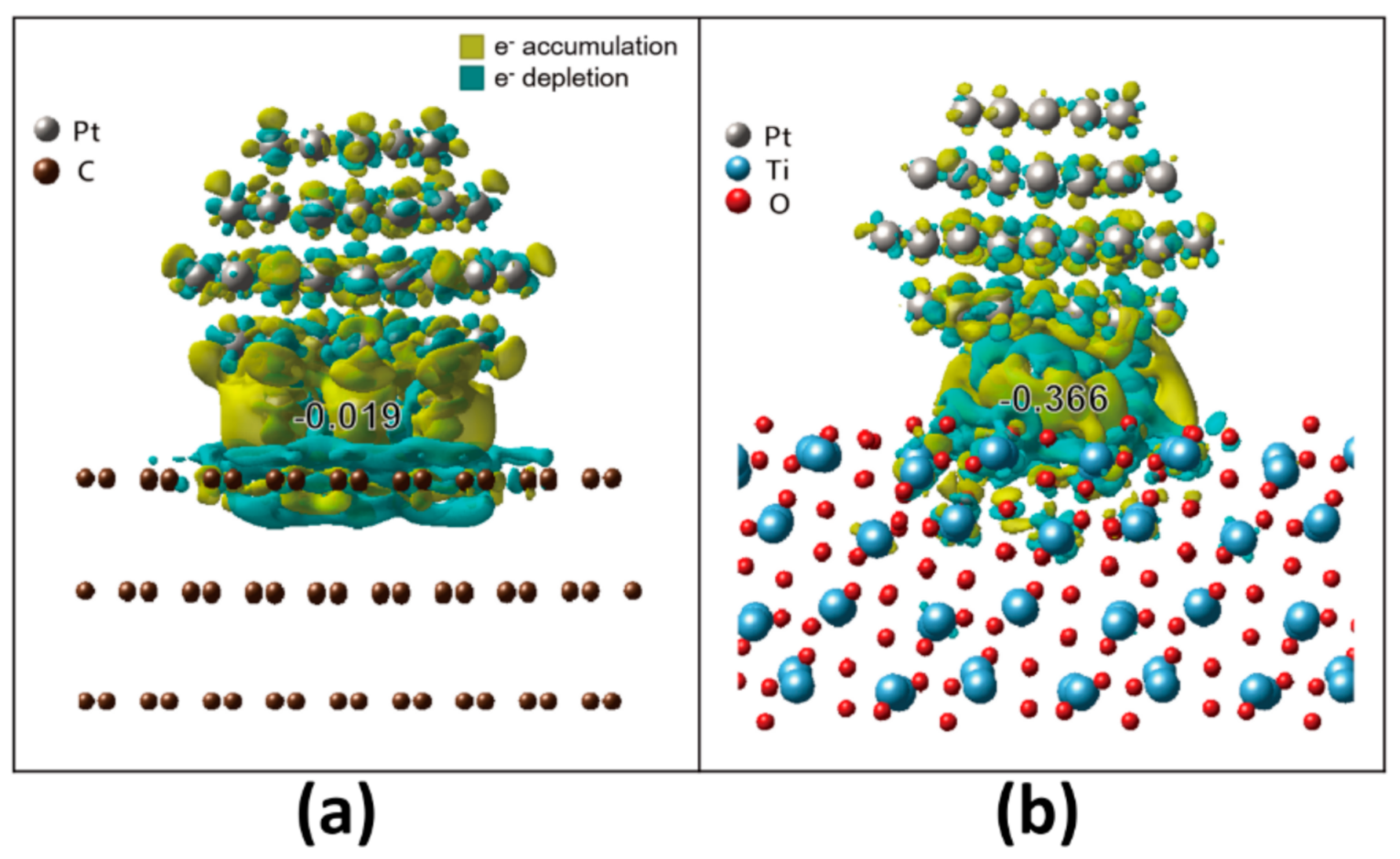
4.1. Interaction of Pt NP and Supports
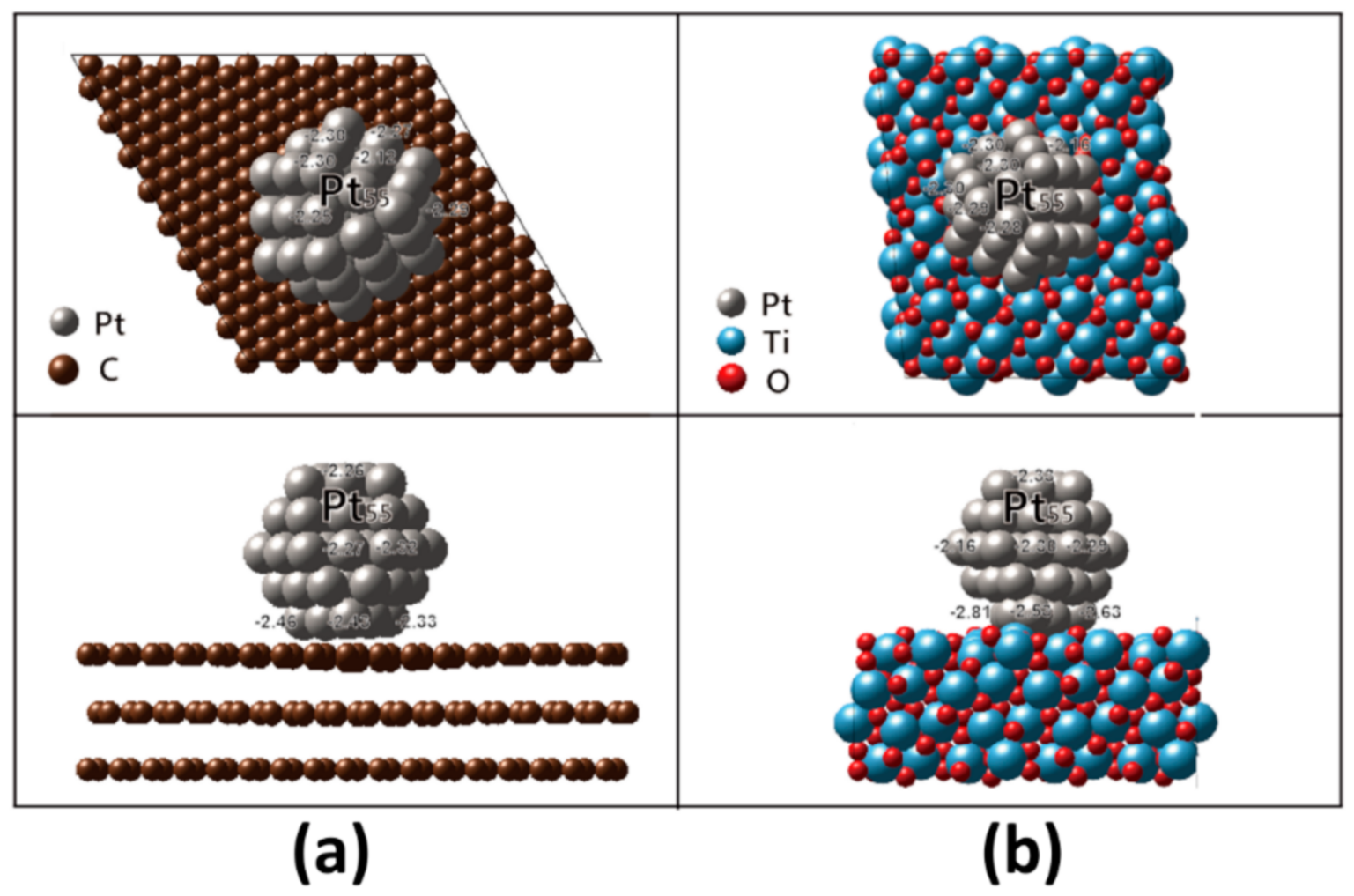
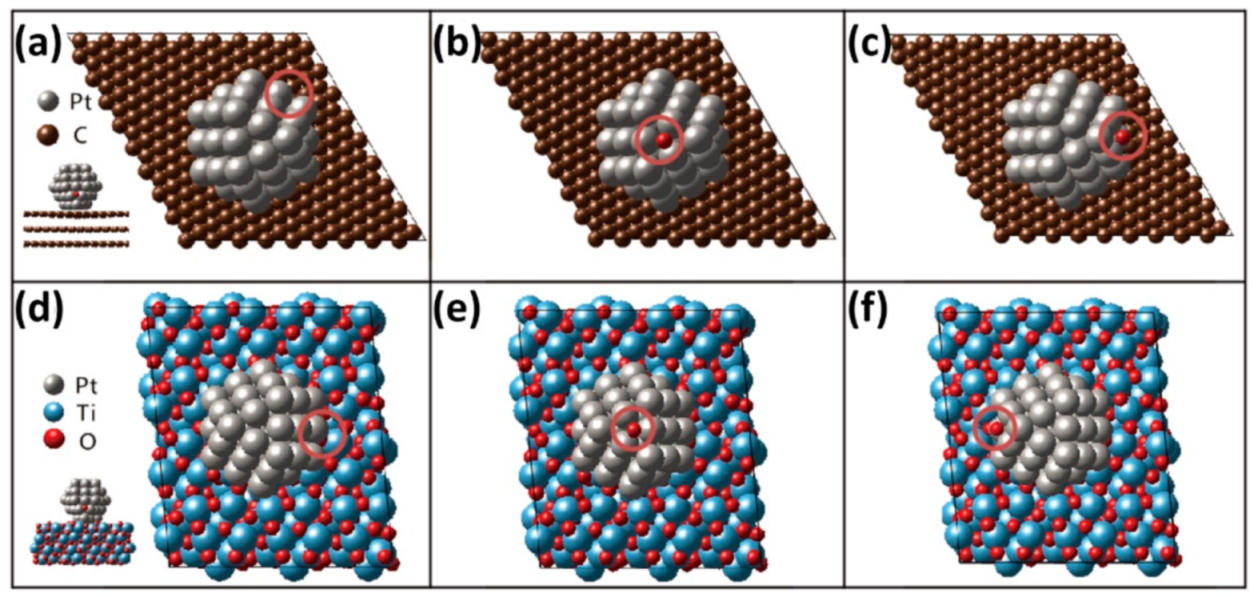
4.2. Ab-Initio Investigation of Durability of Pt NP on Carbon and MPTO
4.3. Catalytic Properties of MPTO Supported Pt NPs
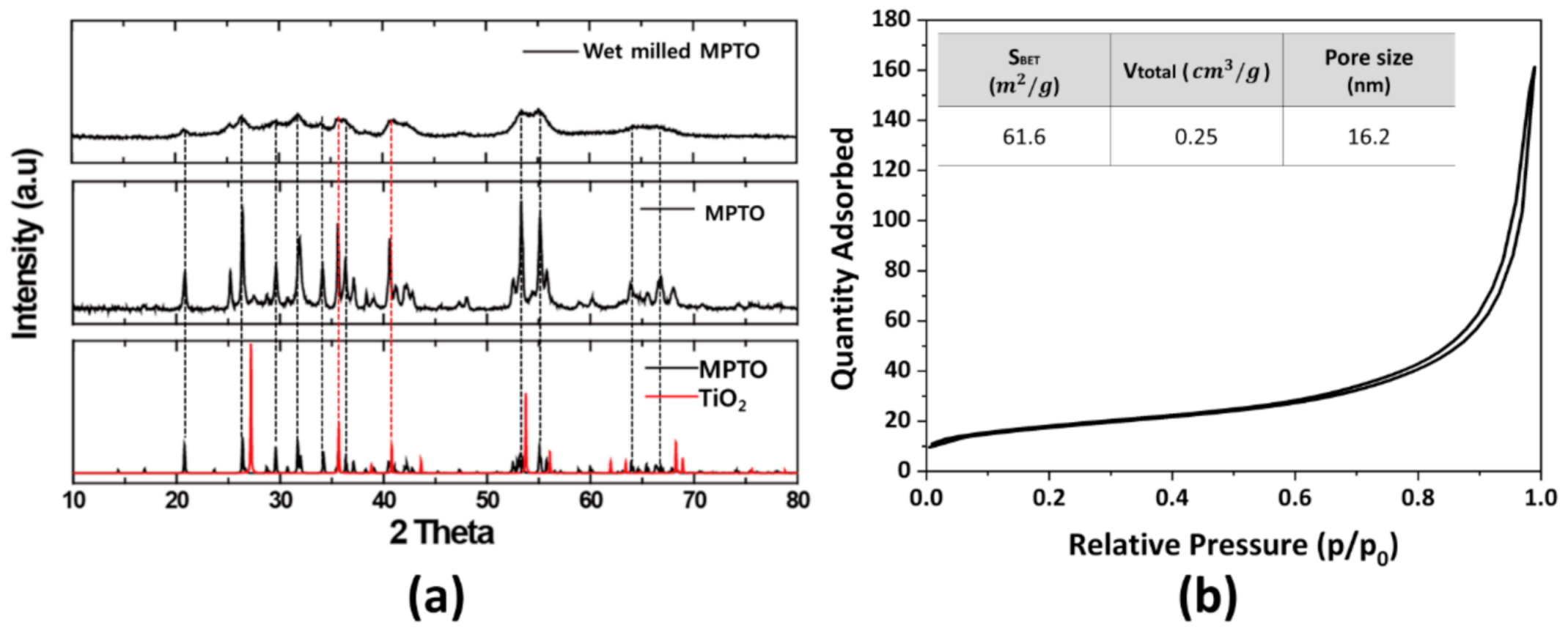
4.4. Characterization of MPTO and Pt/MPTO
4.5. Electrochemical Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabis, A.; Rodriguez, P.; Schmidt, T.J. Electrocatalysis for polymer electrolyte fuel cells: Recent achievements and future challenges. ACS Catal. 2012, 2, 864–890. [Google Scholar] [CrossRef]
- Carter, D.; Ryan, M.; Wing, J. The Fuel Cell Industry Review 2013. Platin. Met. Rev. 2013, 57, 310. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Sun, H.; Xiang, D.; Jiang, Q.; Lu, Z.; He, H.; Huang, H. Rh-decorated three-dimensional graphene aerogel networks as highly-efficient electrocatalysts for direct methanol fuel cells. Front. Energy Res. 2020, 8, 8. [Google Scholar] [CrossRef]
- Zheng, H.B.; An, L.; Zheng, Y.; Qu, C.; Fang, Y.; Liu, Q.; Dang, D. Tuning the catalytic activity of Ir@Pt nanoparticles through controlling Ir core size on cathode performance for PEM fuel cell application. Front. Chem. 2018, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.M.; Yu, Y.; Mirkin, M.V. Dissolution of Pt at moderately negative potentials during oxygen reduction in water and organic media. Langmuir 2013, 29, 1346–1350. [Google Scholar] [CrossRef]
- Brouzgou, A.; Seretis, A.; Song, S.; Shen, P.K.; Tsiakaras, P. CO tolerance and durability study of PtMe(Me = Ir or Pd) electrocatalysts for H2-PEMFC application. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Lim, S.H.; Poh, C.K.; Tang, Z.; Xia, Z.; Luo, Z.; Shen, P.K.; Chua, D.; Feng, Y.P.; Shen, Z.; et al. A highly order-structured membrane electrode assembly with vertically aligned carbon nanotubes for ultra-low Pt loading PEM fuel cells. Adv. Energy Mater. 2011, 1, 1205–1214. [Google Scholar] [CrossRef]
- Murata, S.; Imanishi, M.; Hasegawa, S.; Namba, R. Vertically aligned carbon nanotube electrodes for high current density operating proton exchange membrane fuel cells. J. Power Sources 2014, 253, 104–113. [Google Scholar] [CrossRef]
- Hwang, S.M.; Park, J.H.; Lim, S.; Jung, D.H.; Guim, H.; Yoon, Y.G.; Yim, S.D.; Kim, T.Y. Designing an ultrathin silica layer for highly durable carbon nanofibers as the carbon support in polymer electrolyte fuel cells. Nanoscale 2014, 6, 12111–12119. [Google Scholar] [CrossRef]
- Ghosh, A.; Basu, S.; Verma, A. Graphene and functionalized graphene supported platinum catalyst for PEMFC. Fuel Cells 2013, 13, 355–363. [Google Scholar] [CrossRef]
- Antolini, E. Graphene as a new carbon support for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2012, 123–124, 52–68. [Google Scholar] [CrossRef]
- Zhong, X.; Ye, S.; Tang, J.; Zhu, Y.; Wu, D.; Gu, M.; Pan, H.; Xu, B. Engineering Pt and Fe dual-metal single atoms anchored on nitrogen-doped carbon with high activity and durability towards oxygen reduction reaction for zinc-air battery. Appl. Catal. B Environ. 2021, 286, 119891. [Google Scholar] [CrossRef]
- Krishnan, P.; Advani, S.; Prasad, A.K. Magneli phase Ti n O2n—1 as corrosion-resistant PEM fuel cell catalyst support. J. Solid State Chem. 2012, 16, 2515–2521. [Google Scholar] [CrossRef]
- Yao, C.; Li, F.; Li, X.; Xia, D. Fiber-like nanostructured Ti4O7 used as durable fuel cell catalyst support in oxygen reduction catalysis. J. Mater. Chem. 2012, 22, 16560. [Google Scholar] [CrossRef]
- Kakinuma, K.; Chino, Y.; Senoo, Y.; Uchida, M.; Kamino, T.; Uchida, H.; Deki, S.; Watanabe, M. Characterization of Pt catalysts on Nb-doped and Sb-doped SnO2–δ support materials with aggregated structure by rotating disk electrode and fuel cell measurements. Electrochim. Acta. 2013, 110, 316–324. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, J.; Wang, Y.; Lin, Y. Novel catalyst support materials for PEMfuelcells: Current status and future prospects. J. Mater. Chem. 2009, 19, 46–59. [Google Scholar] [CrossRef]
- Yang, D.H.; Sui, X.L.; Zhao, L.; Huang, G.S.; Gu, D.M.; Wang, Z.B. Pt supported on carbon-coating antimony Tin oxide as anode catalyst for direct methanol fuel cell. Fuel Cells 2018, 18, 763–770. [Google Scholar] [CrossRef]
- Naik, K.M.; Higuchi, E.; Inoue, H. Two-dimensional oxygen-deficient TiO2 nanosheets-supported Pt nanoparticles as durable catalyst for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2020, 455, 227972. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Ebralidze, I.I.; Specchia, S.; Easton, E.B. A fuel cell catalyst support based on doped titanium suboxides with enhanced conductivity, durability and fuel cell performance. J. Mater. Chem. A 2018, 6, 14805–14815. [Google Scholar] [CrossRef]
- Jeon, Y.; Ji, Y.; Cho, Y.I.; Lee, C.; Park, D.H.; Shul, Y.G. Oxide-carbon nanofibrous composite support for a highly active and stable polymer electrolyte membrane fuel-cell catalyst. ACS Nano 2018, 12, 6819–6829. [Google Scholar] [CrossRef]
- Sullivan, M.T.; Alipour Moghadam Esfahani, R.; Easton, E.B. Conductive metal oxide-based fuel cell catalyst supports prepared by doping TiO2 with Si understanding the role of Si content. EXS Trans. 2020, 97, 659–670. [Google Scholar] [CrossRef]
- Wang, J.; Yin, G.; Shao, Y.; Zhang, S.; Wang, Z.; Gao, Y. Effect of carbon black support corrosion on the durability of Pt/C catalyst. J. Power Sources 2007, 171, 331–339. [Google Scholar] [CrossRef]
- Maass, S.; Finsterwalder, F.; Frank, G.; Hartmann, R.; Merten, C. Carbon support oxidation in PEM fuel cell cathodes. J. Power Sources 2008, 176, 444–451. [Google Scholar] [CrossRef]
- Tang, H.; Qi, Z.; Ramani, M.; Elter, J.F. PEM fuel cell cathode carbon corrosion due to the formation of air/fuel boundary at the anode. J. Power Sources 2006, 158, 1306–1312. [Google Scholar] [CrossRef]
- Hacker, V.; Baumgartner, W.; Wallnöfer, E.; Schaffer, T.; Besenhard, J.O. Characterization of carbon nanofiber-based fuel cell electrodes. EXS Trans. 2006, 3, 295. [Google Scholar] [CrossRef]
- Bartholomew, R.F.; Frankl, D.R. Electrical properties of some titanium oxides. Phys. Rev. B 1969, 187, 828–833. [Google Scholar] [CrossRef]
- Chen, G.; Bare, S.R.; Mallouk, T.E. Development of supported bifunctional electrocatalysts for unitized regenerative fuel cells. J. Electrochem. Soc. 2002, 149, A1092. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Fujiwara, N.; Yamazaki, S.; Yasuda, K. Sub-stoichiometric titanium oxide-supported platinum electrocatalyst for polymer electrolyte fuel cells. Electrochem. Commun. 2005, 7, 183–188. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of corrosion-resistant Magnéli-phase Ti4O7-supported PEMFC catalysts at high potentials. J. Electrochem. Soc. 2008, 155, B321. [Google Scholar] [CrossRef]
- Dogan, D.C.; Hwang, S.M.; Jang, E.H.; Yim, S.D.; Sohn, Y.J.; Kim, S.H.; Yang, T.H.; Park, G.G. Highly platinum-loaded Magneli phase titanium oxides as a high voltage tolerant electrocatalyst for polymer electrolyte fuel cells. J. Nanosci. Nanotechnol. 2015, 15, 6988–6994. [Google Scholar] [CrossRef]
- Islam, J.; Kim, S.K.; Kim, K.H.; Lee, E.; Park, G.G. Enhanced durability of Pt/C catalyst by coating carbon black with silica for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 1133–1143. [Google Scholar] [CrossRef]
- Warschkow, O.; Wang, Y.; Subramanian, A.; Asta, M.; Marks, L.D. Structure and local-equilibrium thermodynamics of the c(2x2) reconstruction of rutile TiO2 (100). Phys. Rev. Lett. 2008, 100, 086102. [Google Scholar] [CrossRef]
- Wang, Y.; Warschkow, O.; Marks, L.D. Surface evolution of rutile TiO2 (100) in an oxidizing environment. Surf. Sci. 2007, 601, 63–67. [Google Scholar] [CrossRef]
- Yoshida, K.; Kawai, T.; Nambara, T.; Tanemura, S.; Saitoh, K.; Tanaka, N. Direct observation of oxygen atoms in rutile titanium dioxide by spherical aberration corrected high-resolution transmission electron microscopy. Nanotechnology 2006, 17, 3944–3950. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blochl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B Condens. Matter 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wilkinson, D.P.; Zhang, J. Noncarbon support materials for polymer electrolyte membrane fuel cell electrocatalysts. Chem. Rev. 2011, 111, 7625–7651. [Google Scholar] [CrossRef]
- Feldberg, S.W.; Enke, C.G.; Bricker, C.E. Formation and dissolution of platinum oxide film: Mechanism and kinetics. J. Electrochem. Soc. 1963, 110, 826–834. [Google Scholar] [CrossRef]
- Seo, M.H.; Choi, S.M.; Lim, E.J.; Kwon, I.H.; Seo, J.K.; Noh, S.H.; Kim, W.B.; Han, B. Toward new fuel cell support materials: A theoretical and experimental study of nitrogen-doped graphene. ChemSusChem 2014, 7, 2609–2620. [Google Scholar] [CrossRef]
- Higgins, D.; Hoque, M.A.; Seo, M.H.; Wang, R.; Hassan, F.; Choi, J.Y.; Pritzker, M.; Yu, A.; Zhang, J.; Chen, Z. Development and simulation of sulfur-doped graphene supported platinum with exemplary stability and activity towards oxygen reduction. Adv. Funct. Mater. 2014, 24, 4325–4336. [Google Scholar] [CrossRef]
- Seo, M.H.; Park, H.W.; Lee, D.U.; Park, M.G.; Chen, Z. Design of highly active perovskite oxides for oxygen evolution reaction by combining experimental and ab Initio studies. ACS Catal. 2015, 5, 4337–4344. [Google Scholar] [CrossRef]
- Lundqvist, B.I.; Gunnarsson, O.; Hjelmberg, H. Theoretical description of molecure-metal interaction and surface reactions. Surf. Sci. 1979, 89, 196–225. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Lett. Nat. 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Theoretical Surface Science and Catalysis—Calculations and Concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar]
- Calle-Vallejo, F.; Díaz-Morales, O.A.; Kolb, M.J.; Koper, M.T.M. Why Is bulk thermochemistry a good descriptor for the electrocatalytic activity of rransition metal oxides? ACS Catal. 2015, 5, 869–873. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Martinez, J.I.; Garcia-Lastra, J.M.; Mogensen, M.; Rossmeisl, J. Trends in stability of perovskite oxides. Angew. Chem. Int. Ed. 2010, 49, 7699–7701. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Inoglu, N.G.; Su, H.Y.; Martínez, J.I.; Man, I.C.; Koper, M.T.M.; Kitchin, J.R.; Rossmeisl, J. Number of outer electrons as descriptor for adsorption processes on transition metals and their oxides. Chem. Sci. 2013, 4, 1245. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in Molecules; Wiley online Library: Hoboken, NJ, USA, 1990. [Google Scholar]
- Seo, J.K.; Khetan, A.; Seo, M.H.; Kim, H.; Han, B. First-principles thermodynamic study of the electrochemical stability of Pt nanoparticles in fuel cell applications. J. Power Sources 2013, 238, 137–143. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Toyoda, E.; Hatanaka, T.; Morimoto, Y. First principles calculations on site-dependent dissolution potentials of supported and unsupported Pt particles. J. Phys. Chem. C 2010, 114, 17557–17568. [Google Scholar] [CrossRef]
- Noh, S.H.; Seo, M.H.; Seo, J.K.; Fischer, P.; Han, B. First principles computational study on the electrochemical stability of Pt-Co nanocatalysts. Nanoscale 2013, 5, 8625–8633. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Han, B.; Persson, K.; Friesen, C.; He, T.; Sieradzki, K.; Ceder, G. Electrochemical stability of nanometer-scale Pt particles in acidic environments. J. Am. Chem. Soc. 2009, 132, 596–600. [Google Scholar] [CrossRef]
- Shao, M.; Liu, P.; Zhang, J.; Adzic, R. Origin of enhanced activity in palladium alloy electrocatalysts for oxygen reduction reaction. J. Phys. Chem. B 2007, 111, 6772–6775. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Demirci, U.B. Theoretical means for searching bimetallic alloys as anode electrocatalysts for direct liquid-feed fuel cells. J. Power Sources 2007, 173, 11–18. [Google Scholar] [CrossRef]
- Shao, M.; Liu, P.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Adzic, R.R. Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction. Langmuir 2006, 22, 10409–10415. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Alloy catalysts designed from first principles. Nat. Mater. 2004, 3, 810–815. [Google Scholar] [CrossRef]
- Shao, M.; Sasaki, K.; Marinkovic, N.; Zhang, L.; Adzic, R. Synthesis and characterization of platinum monolayer oxygen-reduction electrocatalysts with Co–Pd core–shell nanoparticle supports. Electrochem. Commun. 2007, 9, 2848–2853. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Lee, Y.; Kleis, J.; Rossmeisl, J.; Morgan, D. Ab initio energetics of LaBO3(001) (B=Mn, Fe, Co, and Ni) for solid oxide fuel cell cathodes. Phys. Rev. B 2009, 80, 224101. [Google Scholar] [CrossRef]
- Pople, J.A.; HeadGordon, M.; Fox, D.J.; Raghavachari, K.; Curtiss, L.A. Gaussian-1 theory: A general procedure for prediction of molecular energies. J. Chem. Phys. 1989, 90, 5622–5629. [Google Scholar] [CrossRef]
- Han, B.C.; Miranda, C.R.; Ceder, G. Effect of particle size and surface structure on adsorption of O and OH on platinum nanoparticles: A first-principles study. Phys. Rev. B 2008, 77, 075410. [Google Scholar] [CrossRef]
- Sattiler, M.L.; Ross, P.N. The surface structure of Pt crystallites supported on carbon black. Ultramicroscopy 1986, 20, 21–28. [Google Scholar] [CrossRef]
- Kinoshita, K. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848. [Google Scholar] [CrossRef]
- Mukerjee, S.; McBreen, J. Effect of particle size on the electrocatalysis by carbon-supported Pt electrocatalysts: An in situ XAS investigation. J. Electroanal. Chem. 1998, 448, 163–171. [Google Scholar] [CrossRef]
- Ekuma, E.E.; Bagayoko, D. Ab-initio electronic and structural properties of rutile titanium dioxide. Jpn. J. Appl. Phys. 2011, 50, 101103. [Google Scholar] [CrossRef]
- Labat, F.; Baranek, P.; Domain, C.; Minot, C.; Adamo, C. Density functional theory analysis of the structural and electronic properties of TiO2 rutile and anatase polytypes: Performances of different exchange-correlation functionals. J. Chem. Phys. 2007, 126, 154703. [Google Scholar] [CrossRef]
- Zhang, J.; Su, N.; Hu, X.; Zhu, F.; Yu, Y.; Yang, H. Facile synthesis of Pt nanoparticles supported on anatase TiO2 nanotubes with good photo-electrocatalysis performance for methanol. RSC Adv. 2017, 7, 56194–56203. [Google Scholar] [CrossRef]
- Huang, K.; Sasaki, K.; Adzic, R.R.; Xing, Y. Increasing Pt oxygen reduction reaction activity and durability with a carbon-doped TiO2 nanocoating catalyst support. J. Mater. Chem. 2012, 22, 16824. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, P.N.; Markovic, N.M. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, J.; Lee, Y.H.; Kim, I.Y.; Park, M.H.; Yang, C.W.; Hwang, S.J.; Kwon, Y.U. One-pot synthesis of core–shell-like Pt3Co nanoparticle electrocatalyst with Pt-enriched surface for oxygen reduction reaction in fuel cells. Energy Environ. Sci. 2011, 4, 4947. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]


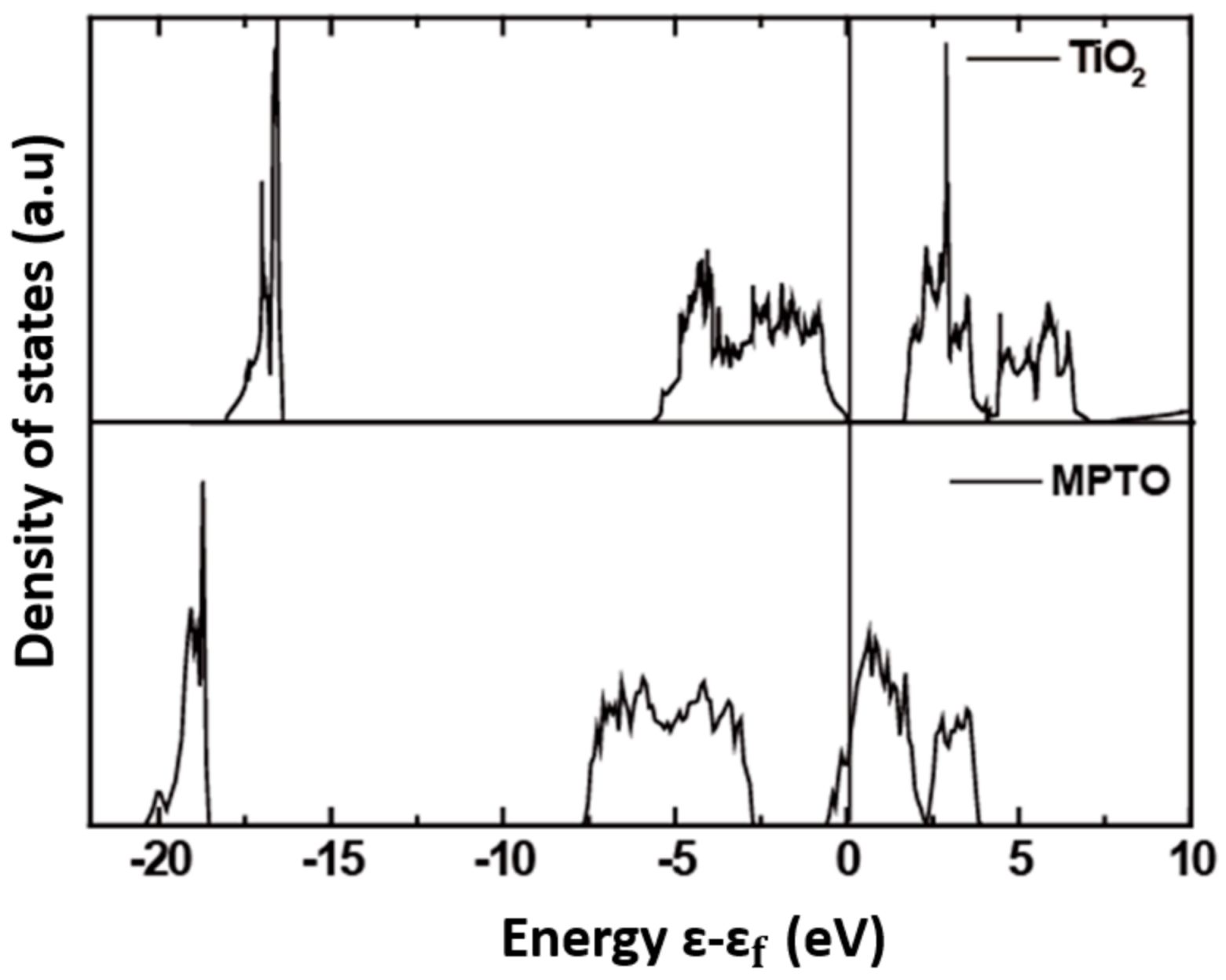
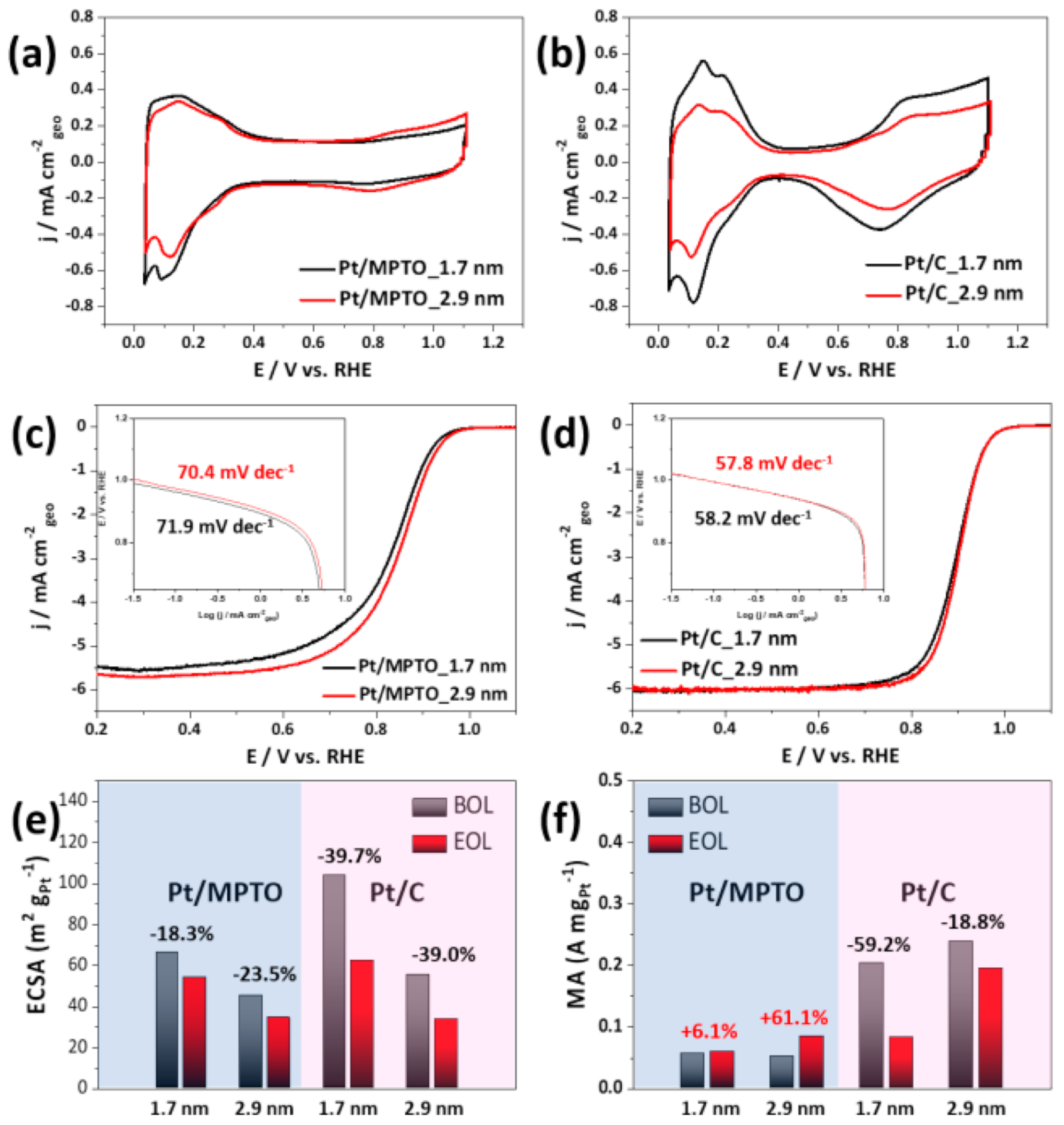
| Atomic Oxygen (eV/O) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pt (111) 1 | Pt55 NP | Pt55/Graphite | Pt55/MPTO | |||||
| Pt-FCC | Pt-FCC | top-FCC | side-FCC | bottom-FCC | top-FCC | side-FCC | bottom-FCC | |
| Eads, eV | −3.92 | −4.39 | −3.79 | −3.59 | −3.75 | −3.57 | −3.62 | −3.74 |
| ΔEO−ΔEOPt(111), eV | 0 | −0.47 | 0.12 | 0.32 | 0.16 | 0.34 | 0.30 | 0.17 |
| Ecoh, eV | 4.85 | 4.86 | 5.06 | |||||
| Udiss-Pt shell vs. SHE, eV | - | 0.62 | 0.63 | 0.76 | ||||
| Samples | Pt Loading (ICP-MS, wt%) | Particle Size (TEM, nm) | Particle Size (XRD, nm) |
|---|---|---|---|
| Pt/MPTO | 11.4 | 1.76 | - |
| Pt/MPTO | 8.6 | 2.91 | - |
| Pt/C | 19.2 | 1.70 | - |
| Pt/C | 20.1 | 2.90 | 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogan, D.C.; Choi, J.; Seo, M.H.; Lee, E.; Jung, N.; Yim, S.-D.; Yang, T.-H.; Park, G.-G. Enhancement of Catalytic Activity and Durability of Pt Nanoparticle through Strong Chemical Interaction with Electrically Conductive Support of Magnéli Phase Titanium Oxide. Nanomaterials 2021, 11, 829. https://doi.org/10.3390/nano11040829
Dogan DC, Choi J, Seo MH, Lee E, Jung N, Yim S-D, Yang T-H, Park G-G. Enhancement of Catalytic Activity and Durability of Pt Nanoparticle through Strong Chemical Interaction with Electrically Conductive Support of Magnéli Phase Titanium Oxide. Nanomaterials. 2021; 11(4):829. https://doi.org/10.3390/nano11040829
Chicago/Turabian StyleDogan, Didem C., Jiye Choi, Min Ho Seo, Eunjik Lee, Namgee Jung, Sung-Dae Yim, Tae-Hyun Yang, and Gu-Gon Park. 2021. "Enhancement of Catalytic Activity and Durability of Pt Nanoparticle through Strong Chemical Interaction with Electrically Conductive Support of Magnéli Phase Titanium Oxide" Nanomaterials 11, no. 4: 829. https://doi.org/10.3390/nano11040829
APA StyleDogan, D. C., Choi, J., Seo, M. H., Lee, E., Jung, N., Yim, S.-D., Yang, T.-H., & Park, G.-G. (2021). Enhancement of Catalytic Activity and Durability of Pt Nanoparticle through Strong Chemical Interaction with Electrically Conductive Support of Magnéli Phase Titanium Oxide. Nanomaterials, 11(4), 829. https://doi.org/10.3390/nano11040829






