Graphene Nanosheet-Wrapped Mesoporous La0.8Ce0.2Fe0.5Mn0.5O3 Perovskite Oxide Composite for Improved Oxygen Reaction Electro-Kinetics and Li-O2 Battery Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Porous La1−xCexFe0.5Mn0.5O3 Perovskite Oxides
2.3. Preparation of Porous LCFM(8255)-gly/GNS Composite
2.4. Materials Characterization
2.5. Preparation of Electrode and Electrochemical Measurements
3. Results and Discussion
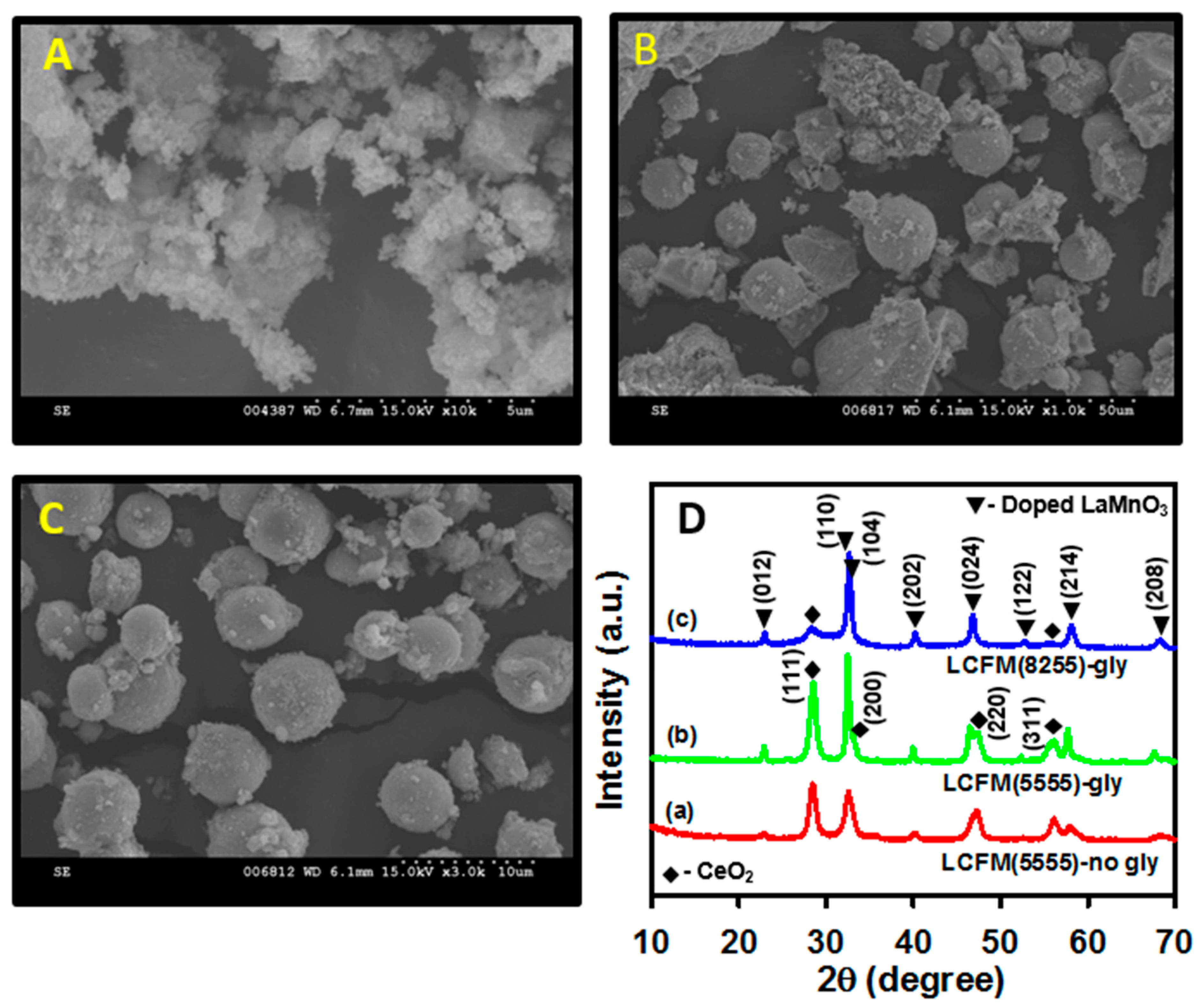
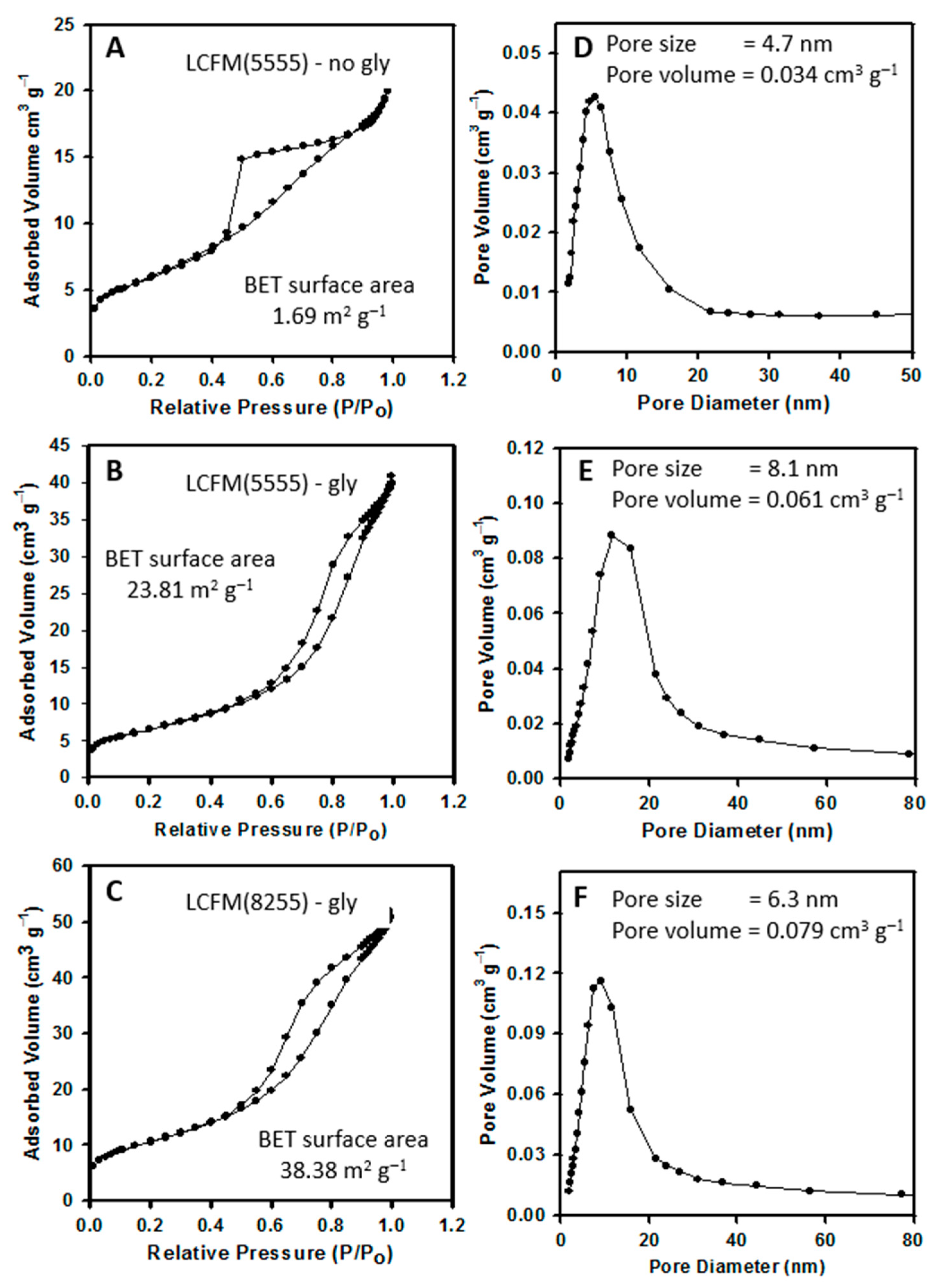
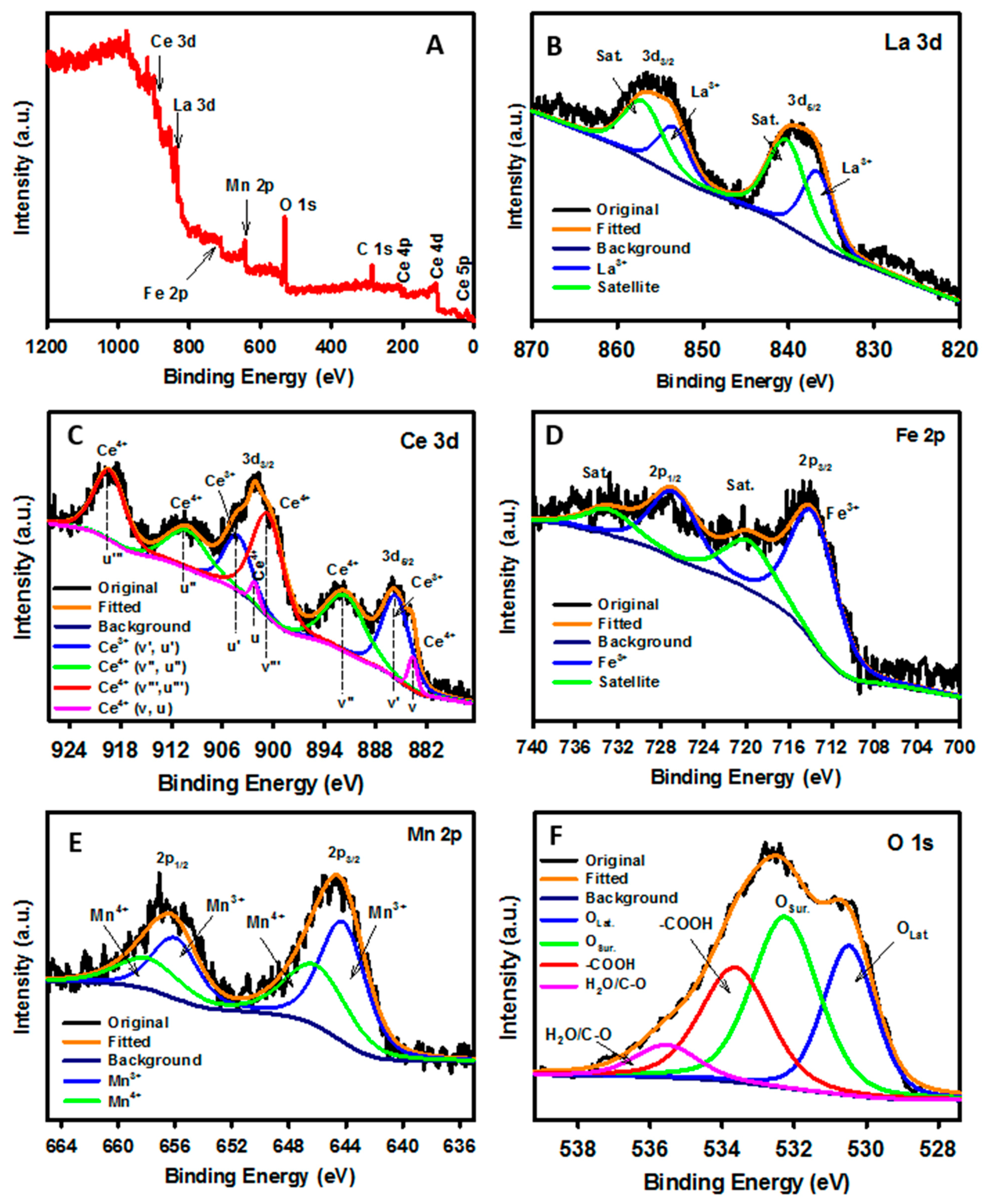
3.1. Surface and Structural Characterization of Perovskite Samples
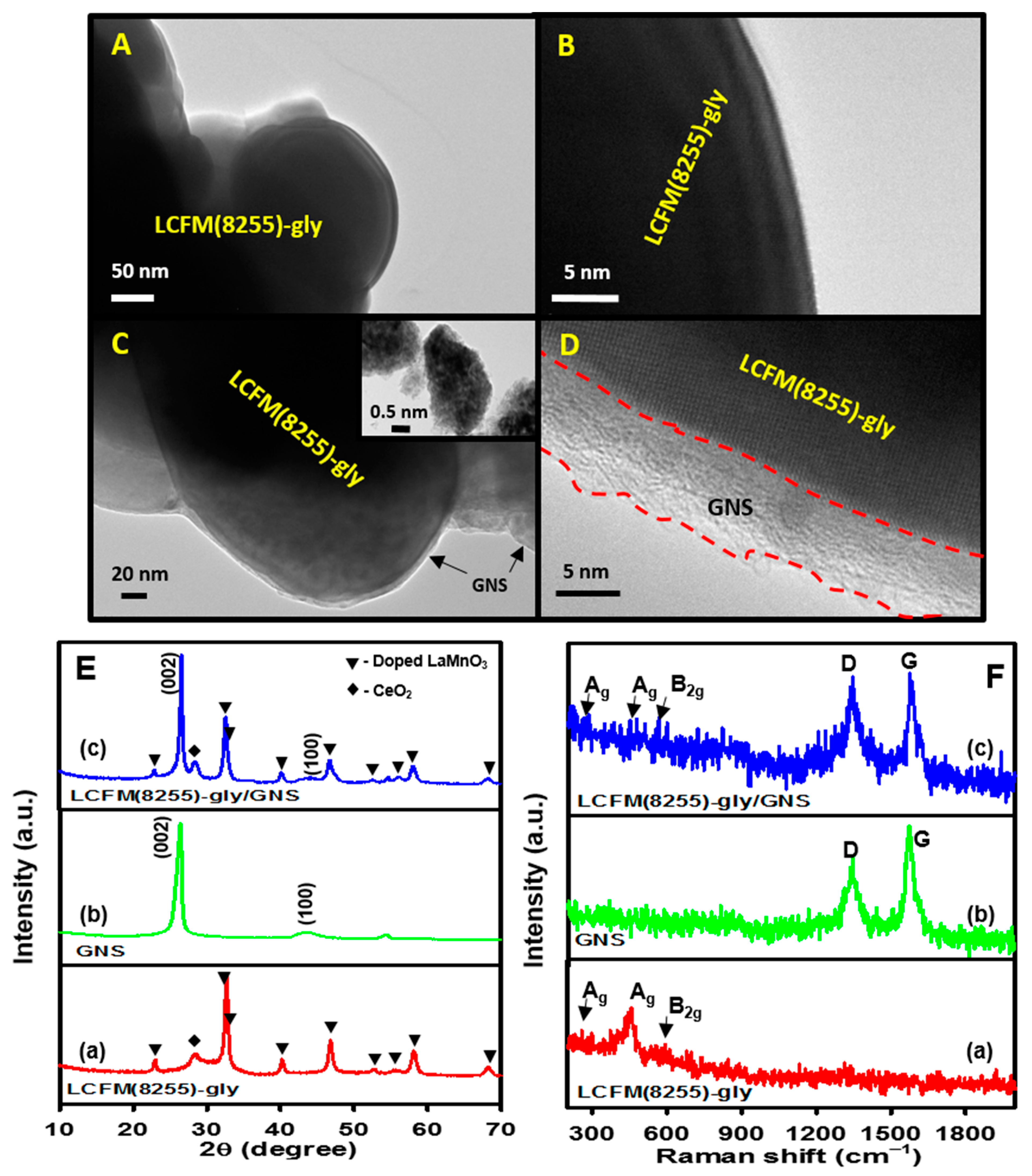
3.2. Surface and Structural Characterization of LCFM(8255)-gly/GNS Composite Catalyst
3.3. Electrochemical Performances
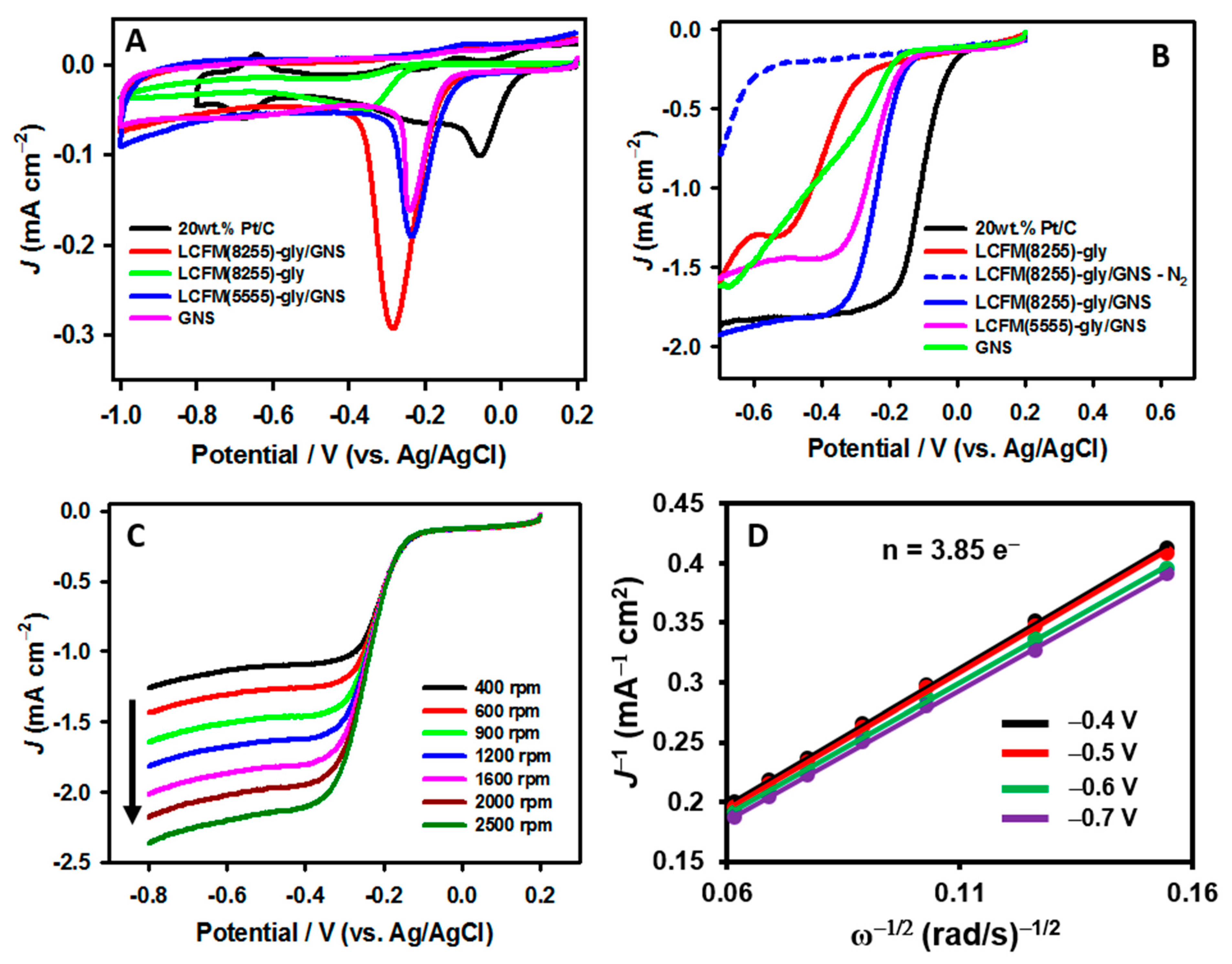
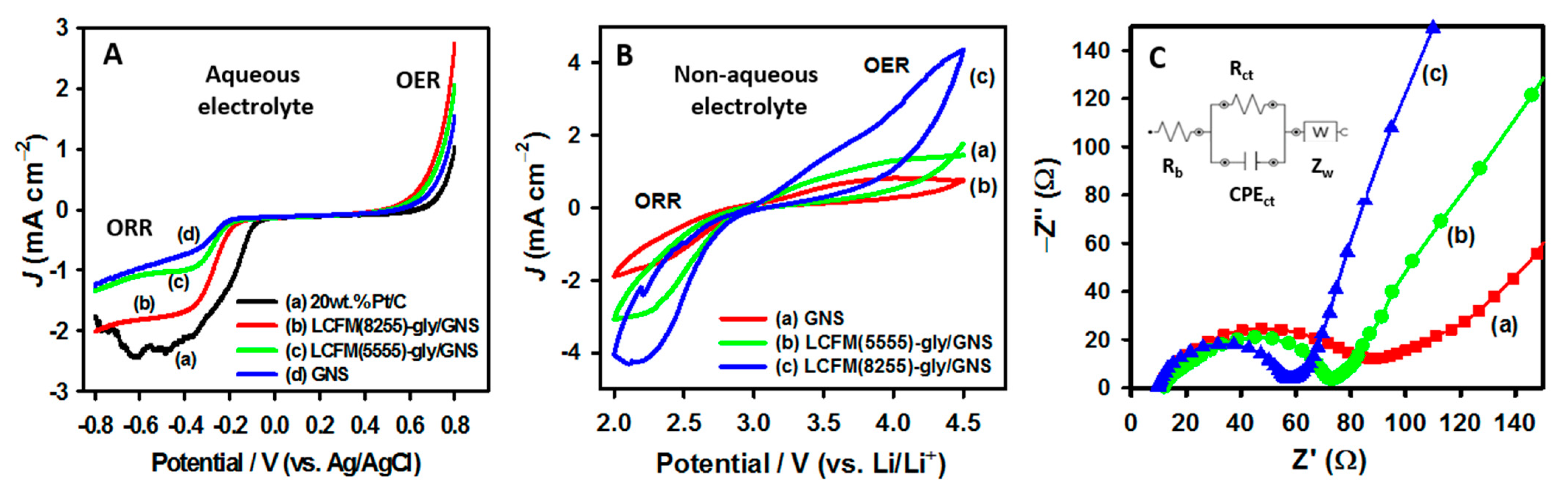
3.3.1. Oxygen Electrocatalysis
3.3.2. Bifunctional ORR/OER Activity
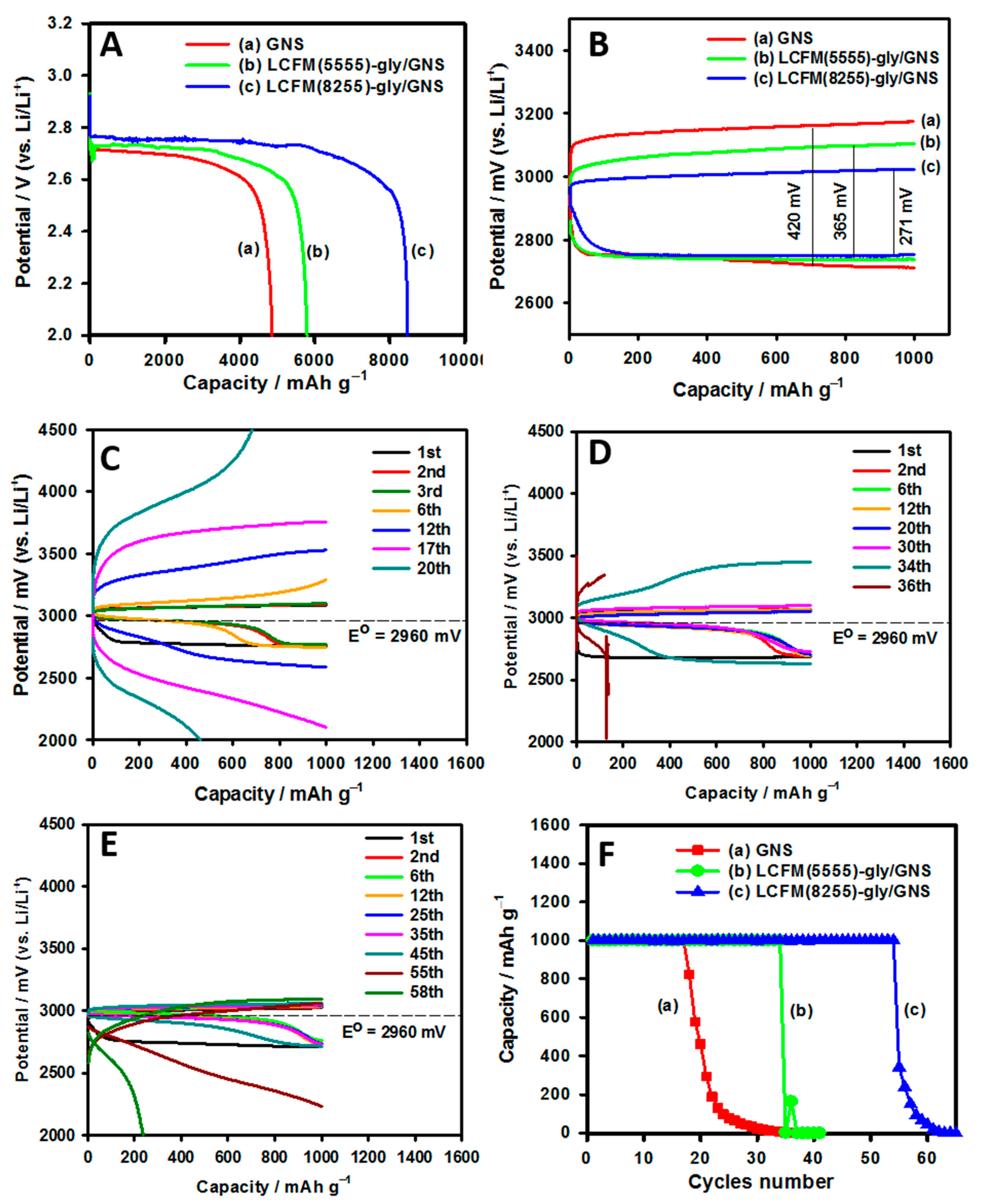
3.3.3. Li-O2 Battery Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Gong, H.; Wang, T.; Guo, H.; Song, L.; Xia, W.; Gao, B.; Jiang, Z.; Feng, L.; He, J. Cobalt-Doped Perovskite-Type Oxide LaMnO3 as Bifunctional Oxygen Catalysts for Hybrid Lithium–Oxygen Batteries. Chem. Asian J. 2018, 13, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, Y.; Li, S.; Sun, C. Porous Perovskite La0.6Sr0.4Co0.8Mn0.2O3 Nanofibers Loaded with RuO2 Nanosheets as an Efficient and Durable Bifunctional Catalyst for Rechargeable Li-O2 Batteries. ACS Catal. 2017, 7, 7737–7747. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Karuppiah, C.; Hsieh, Y.C.; Beshahwured, S.L.; Wu, X.W.; Wu, S.H.; Jose, R.; Lue, S.J.; Yang, C.C. Poly(vinyl alcohol)/Melamine Composite Containing LATP Nanocrystals as a High-Performing Nanofibrous Membrane Separator for High-Power, High-Voltage Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 8487–8499. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, W.; Zhang, Z.; Qu, Y.; Gan, Y.; Li, J. Fe/Fe3C decorated 3-D porous nitrogen-doped graphene as a cathode material for rechargeable Li-O2 batteries. Electrochim. Acta 2016, 191, 733–742. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Nan, B.; Jia, F.; Lu, Z.; Deng, H. In situ, facile synthesis of La0.8Sr0.2MnO3/nitrogen-doped graphene: A high-performance catalyst for rechargeable Li-O2 batteries. Ionics 2017, 23, 2241–2250. [Google Scholar] [CrossRef]
- Wei, C.; Karuppiah, C.; Yang, C.; Shih, J.; Jessie, S. Journal of Physics and Chemistry of Solids Bifunctional perovskite electrocatalyst and PVDF/PET/PVDF separator integrated split test cell for high performance Li-O2 battery. J. Phys. Chem. Solids 2019, 133, 67–78. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Luo, S.; Ren, X.; Deng, L.; Mi, H.; Zhang, P.; Sun, L.; Gao, Y. 3D-ordered porous nitrogen and sulfur Co-Doped carbon supported PdCuW nanoparticles as efficient catalytic cathode materials for Li-O2 batteries. Electrochim. Acta 2018, 272, 33–43. [Google Scholar] [CrossRef]
- Wang, L.; Ara, M.; Wadumesthrige, K.; Salley, S.; Ng, K.Y.S. Graphene nanosheet supported bifunctional catalyst for high cycle life Li-air batteries. J. Power Sources 2013, 234, 8–15. [Google Scholar] [CrossRef]
- Cao, C.; Xie, J.; Zhang, S.; Pan, B.; Cao, G.; Zhao, X. Graphene-like δ-MnO2 decorated with ultrafine CeO2 as a highly efficient catalyst for long-life lithium-oxygen batteries. J. Mater. Chem. A 2017, 5, 6747–6755. [Google Scholar] [CrossRef]
- Kumar, S.; Munichandraiah, N. Nanoparticles of a Pt3Ni alloy on reduced graphene oxide (RGO) as an oxygen electrode catalyst in a rechargeable Li-O2 battery. Mater. Chem. Front. 2017, 1, 873–878. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Xie, J.; Huo, Y.; Yang, H.Y.; Zhu, T.; Cao, G.; Zhao, X.; Zhang, S. Direct growth of flower-like δ-MnO2 on three-dimensional graphene for high-performance rechargeable Li-O2 batteries. Adv. Energy Mater. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Jin, C.; Bie, S.; Yang, R.; Wu, J. MnOx decorated CeO2 nanorods as cathode catalyst for rechargeable lithium-air batteries. J. Mater. Chem. A 2015, 3, 13563–13567. [Google Scholar] [CrossRef]
- Li, J.; Shu, C.; Hu, A.; Ran, Z.; Li, M.; Zheng, R.; Long, J. Tuning oxygen non-stoichiometric surface via defect engineering to promote the catalysis activity of Co3O4 in Li-O2 batteries. Chem. Eng. J. 2020, 381. [Google Scholar] [CrossRef]
- Shui, J.; Lin, Y.; Connell, J.W.; Xu, J.; Fan, X.; Dai, L. Nitrogen-Doped Holey Graphene for High-Performance Rechargeable Li-O2 Batteries. ACS Energy Lett. 2016, 1, 260–265. [Google Scholar] [CrossRef]
- Karuppiah, C.; Rani, K.K.; Wang, S.F.; Devasenathipathy, R.; Yang, C.C. Dry particle coating preparation of highly conductive LaMnO3@C composite for the oxygen reduction reaction and hydrogen peroxide sensing. J. Taiwan Inst. Chem. Eng. 2018, 93, 94–102. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Islam, M.; Jeong, M.; Oh, I.; Nam, K.; Jung, H. Role of strontium as doping agent in LaMn0.5Ni0.5O3 for oxygen electro-catalysis. J. Ind. Eng. Chem. 2020, 85, 94–101. [Google Scholar] [CrossRef]
- Wang, C.C.; Cheng, Y.; Ianni, E.; Jiang, S.P.; Lin, B. A highly active and stable La0.5Sr0.5Ni0.4Fe0.6O3-δ perovskite electrocatalyst for oxygen evolution reaction in alkaline media. Electrochim. Acta 2017, 246, 997–1003. [Google Scholar] [CrossRef]
- Khellaf, N.; Kahoul, A.; Naamoune, F.; Alonso-Vante, N. Electrochemistry of Nanocrystalline La0.5Sr0.5MnO3 Perovskite for the Oxygen Reduction Reaction in Alkaline Medium. Electrocatalysis 2017, 8, 450–458. [Google Scholar] [CrossRef]
- Yan, L.; Lin, Y.; Yu, X.; Xu, W.; Salas, T.; Smallidge, H.; Zhou, M.; Luo, H. La0.8Sr0.2MnO3-Based Perovskite Nanoparticles with the A-Site Deficiency as High Performance Bifunctional Oxygen Catalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2017, 9, 23820–23827. [Google Scholar] [CrossRef]
- Sunarso, J.; Torriero, A.A.J.; Zhou, W.; Howlett, P.C.; Forsyth, M. Oxygen reduction reaction activity of La-based perovskite oxides in alkaline medium: A thin-film rotating ring-disk electrode study. J. Phys. Chem. C 2012, 116, 26108. [Google Scholar] [CrossRef]
- Fu, Z.; Lin, X.; Huang, T.; Yu, A. Nano-sized La0.8Sr0.2MnO3 as oxygen reduction catalyst in nonaqueous Li/O2 batteries. J. Solid State Electrochem. 2012, 16, 1447–1452. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Y.; Jin, C.; Li, F.; Yang, R.; Chen, F. Microporous La0.8Sr0.2MnO3 perovskite nanorods as efficient electrocatalysts for lithium-air battery. J. Power Sources 2015, 293, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hang, Y.; Zhang, Y.; Wang, Z.; Yao, Y.; He, X.; Zhang, C.; Zhang, D. Strontium-doped perovskite oxide La1-xSrxMnO3 (x = 0, 0.2, 0.6) as a highly efficient electrocatalyst for nonaqueous Li-O2 batteries. Electrochim. Acta 2017, 232, 296–302. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Shi, Q.; Yang, Q.; Li, C.; Zhang, D.; Zhang, C. Perovskite oxides La0.4Sr0.6CoxMn1-xO3 (x = 0, 0.2, 0.4) as an effective electrocatalyst for lithium-air batteries. Green Energy Environ. 2018, 3, 78–85. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Z.; Yu, Y.; Yin, J.; Song, K.; Yang, B.; Yuan, L.; Hu, X. Copper/cobalt-doped LaMnO3 perovskite oxide as a bifunctional catalyst for rechargeable Li-O2 batteries. J. Alloys Compd. 2019, 801, 19–26. [Google Scholar] [CrossRef]
- Han, X.; Hu, Y.; Yang, J.; Cheng, F.; Chen, J. Porous perovskite CaMnO3 as an electrocatalyst for rechargeable Li-O2 batteries. Chem. Commun. 2014, 50, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, C.; Thirumalraj, B.; Alagar, S.; Piraman, S.; Li, Y.J.J.; Yang, C.C. Solid-state ball-milling of Co3O4 nano/microspheres and carbon black endorsed LaMnO3 perovskite catalyst for bifunctional oxygen electrocatalysis. Catalysts 2021, 11, 76. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Shao, H.; Yang, F.; Wang, J.; Wang, Y.; Liu, J. Facile syntheses of cerium-based CeMO3 (M = Co, Ni, Cu) perovskite nanomaterials for high-performance supercapacitor electrodes. J. Mater. Sci. 2020, 55, 8421–8434. [Google Scholar] [CrossRef]
- Merten, S.; Shapoval, O.; Damaschke, B.; Samwer, K.; Moshnyaga, V. Magnetic-Field-Induced Suppression of Jahn-Teller Phonon Bands in (La0.6Pr0.4)0.7Ca0.3MnO3: The Mechanism of Colossal Magnetoresistance shown by Raman Spectroscopy. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppiah, C.; Wei, C.-N.; Karikalan, N.; Wu, Z.-H.; Thirumalraj, B.; Hsu, L.-F.; Alagar, S.; Piraman, S.; Hung, T.-F.; Li, Y.-J.J.; et al. Graphene Nanosheet-Wrapped Mesoporous La0.8Ce0.2Fe0.5Mn0.5O3 Perovskite Oxide Composite for Improved Oxygen Reaction Electro-Kinetics and Li-O2 Battery Application. Nanomaterials 2021, 11, 1025. https://doi.org/10.3390/nano11041025
Karuppiah C, Wei C-N, Karikalan N, Wu Z-H, Thirumalraj B, Hsu L-F, Alagar S, Piraman S, Hung T-F, Li Y-JJ, et al. Graphene Nanosheet-Wrapped Mesoporous La0.8Ce0.2Fe0.5Mn0.5O3 Perovskite Oxide Composite for Improved Oxygen Reaction Electro-Kinetics and Li-O2 Battery Application. Nanomaterials. 2021; 11(4):1025. https://doi.org/10.3390/nano11041025
Chicago/Turabian StyleKaruppiah, Chelladurai, Chao-Nan Wei, Natarajan Karikalan, Zong-Han Wu, Balamurugan Thirumalraj, Li-Fan Hsu, Srinivasan Alagar, Shakkthivel Piraman, Tai-Feng Hung, Ying-Jeng Jame Li, and et al. 2021. "Graphene Nanosheet-Wrapped Mesoporous La0.8Ce0.2Fe0.5Mn0.5O3 Perovskite Oxide Composite for Improved Oxygen Reaction Electro-Kinetics and Li-O2 Battery Application" Nanomaterials 11, no. 4: 1025. https://doi.org/10.3390/nano11041025
APA StyleKaruppiah, C., Wei, C.-N., Karikalan, N., Wu, Z.-H., Thirumalraj, B., Hsu, L.-F., Alagar, S., Piraman, S., Hung, T.-F., Li, Y.-J. J., & Yang, C.-C. (2021). Graphene Nanosheet-Wrapped Mesoporous La0.8Ce0.2Fe0.5Mn0.5O3 Perovskite Oxide Composite for Improved Oxygen Reaction Electro-Kinetics and Li-O2 Battery Application. Nanomaterials, 11(4), 1025. https://doi.org/10.3390/nano11041025








