Evaluation of Fe-Mg Binary Oxide for As (III) Adsorption—Synthesis, Characterization and Kinetic Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Nano-Adsorbent Synthesis
3. Results
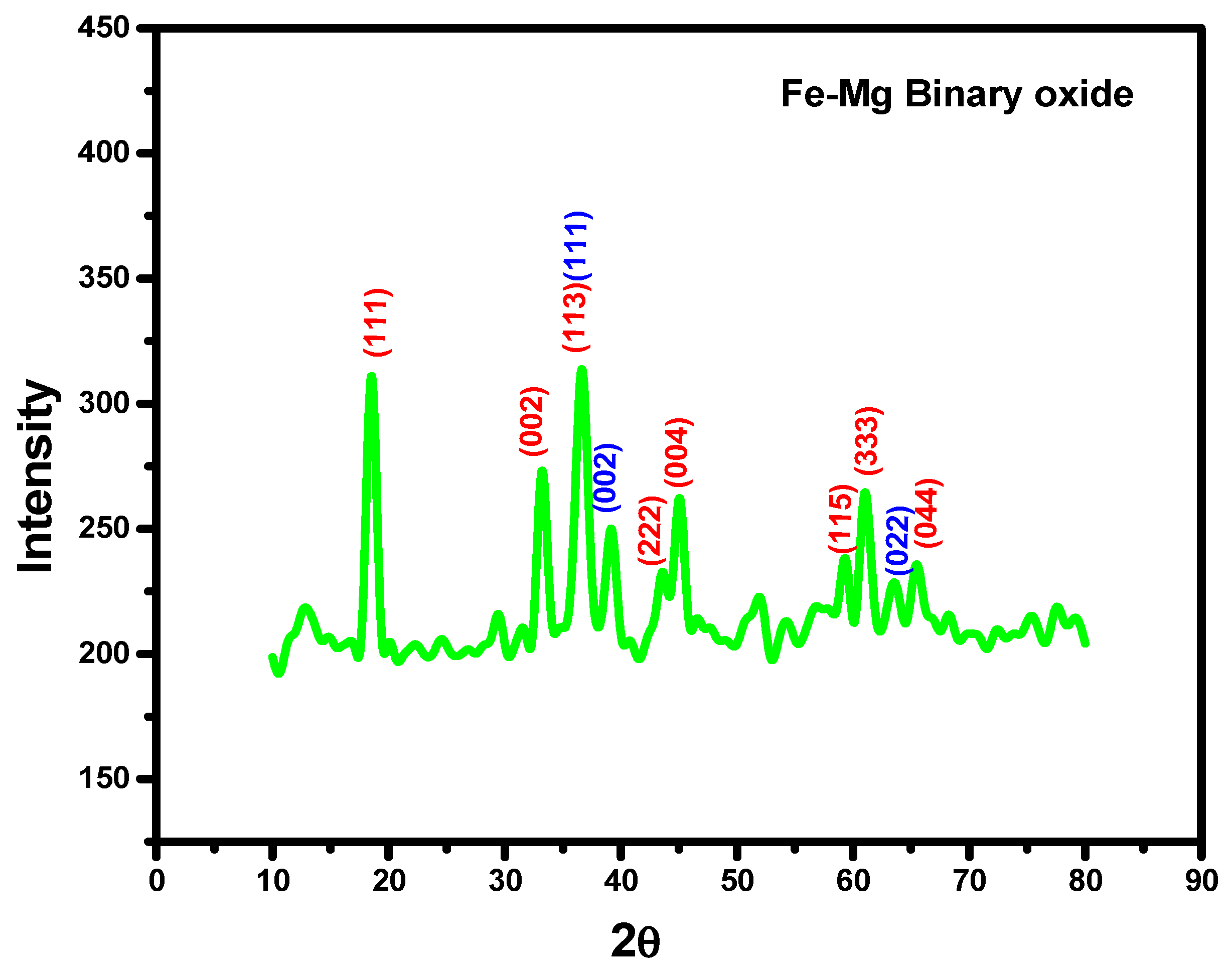
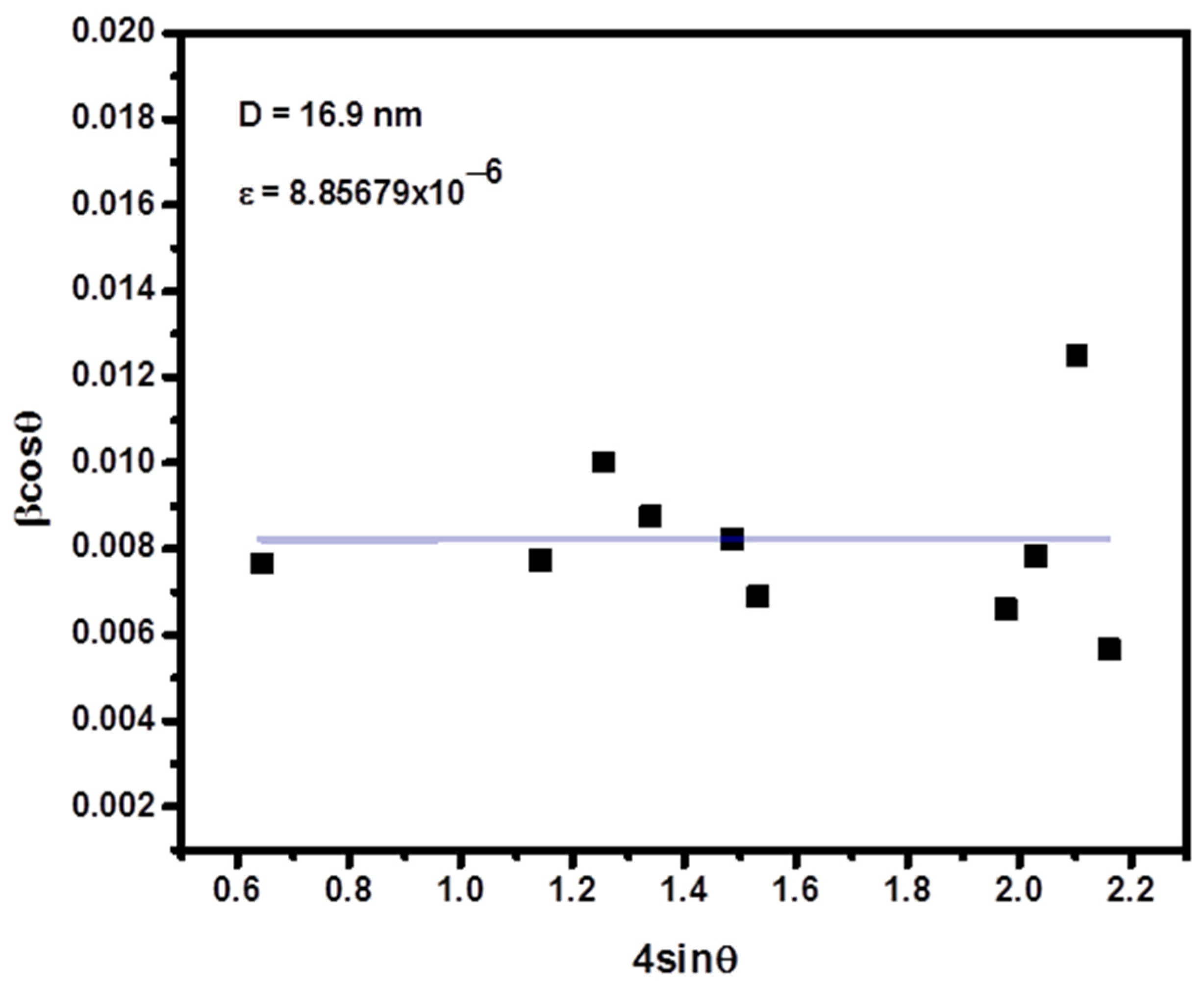
3.1. Characterization by Structural Properties: (XRD)
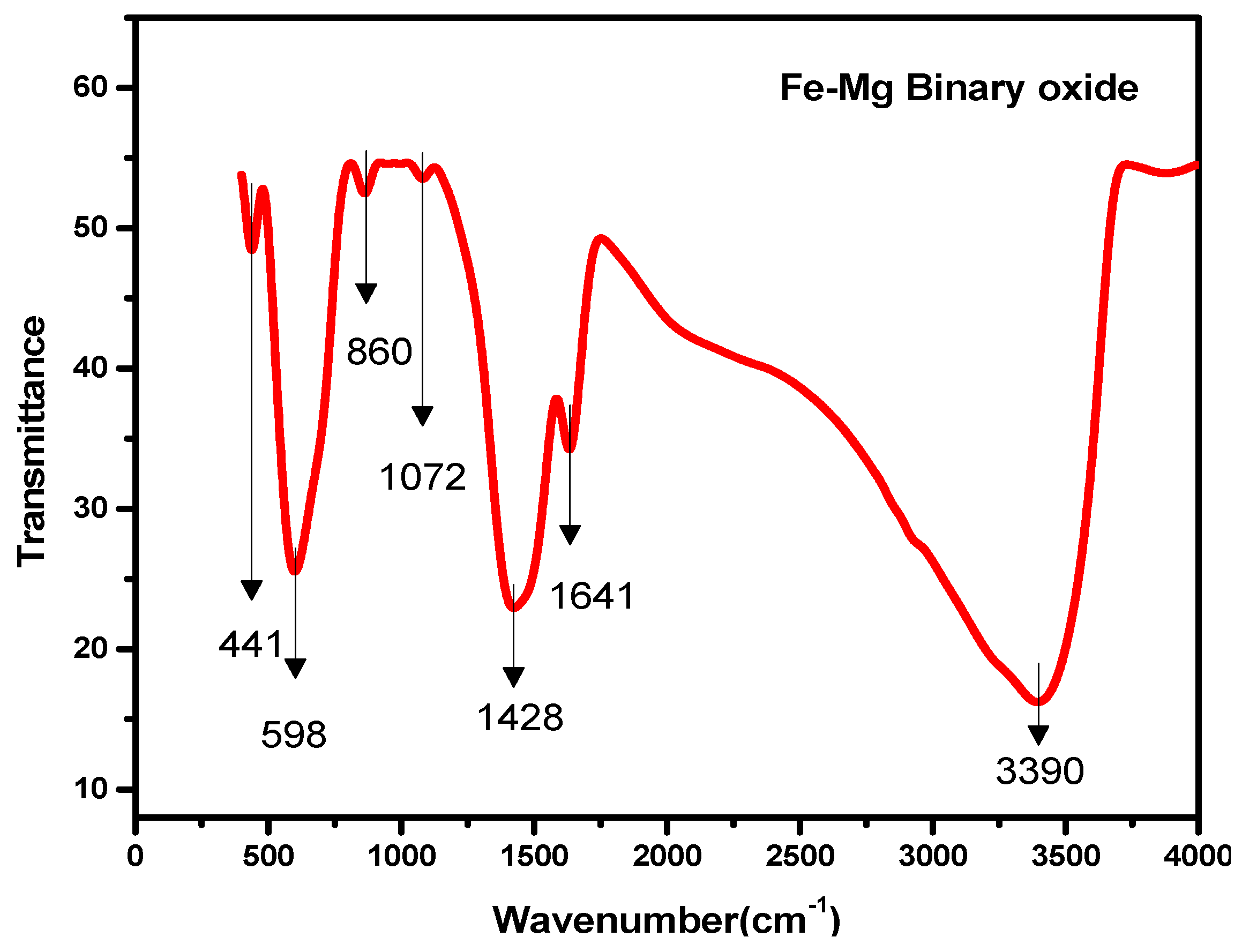
3.2. FTIR Studies
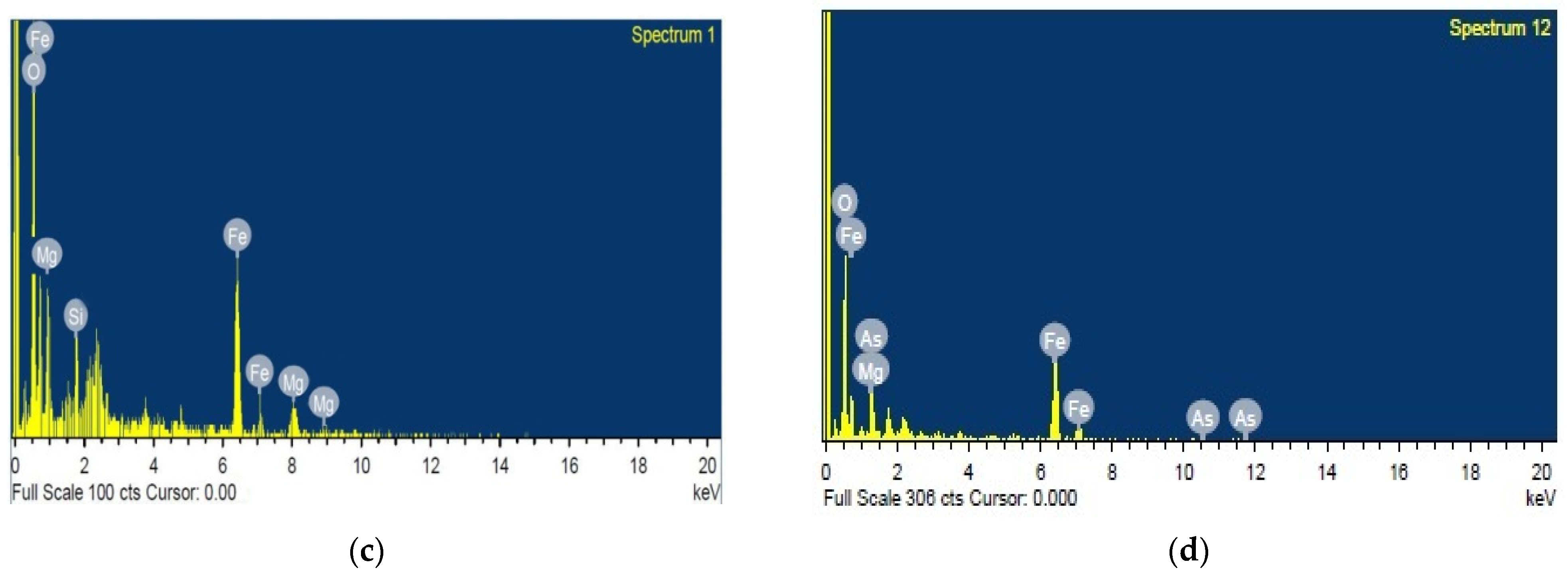
3.3. SEM with EDAX
3.4. Adsorption Experiments
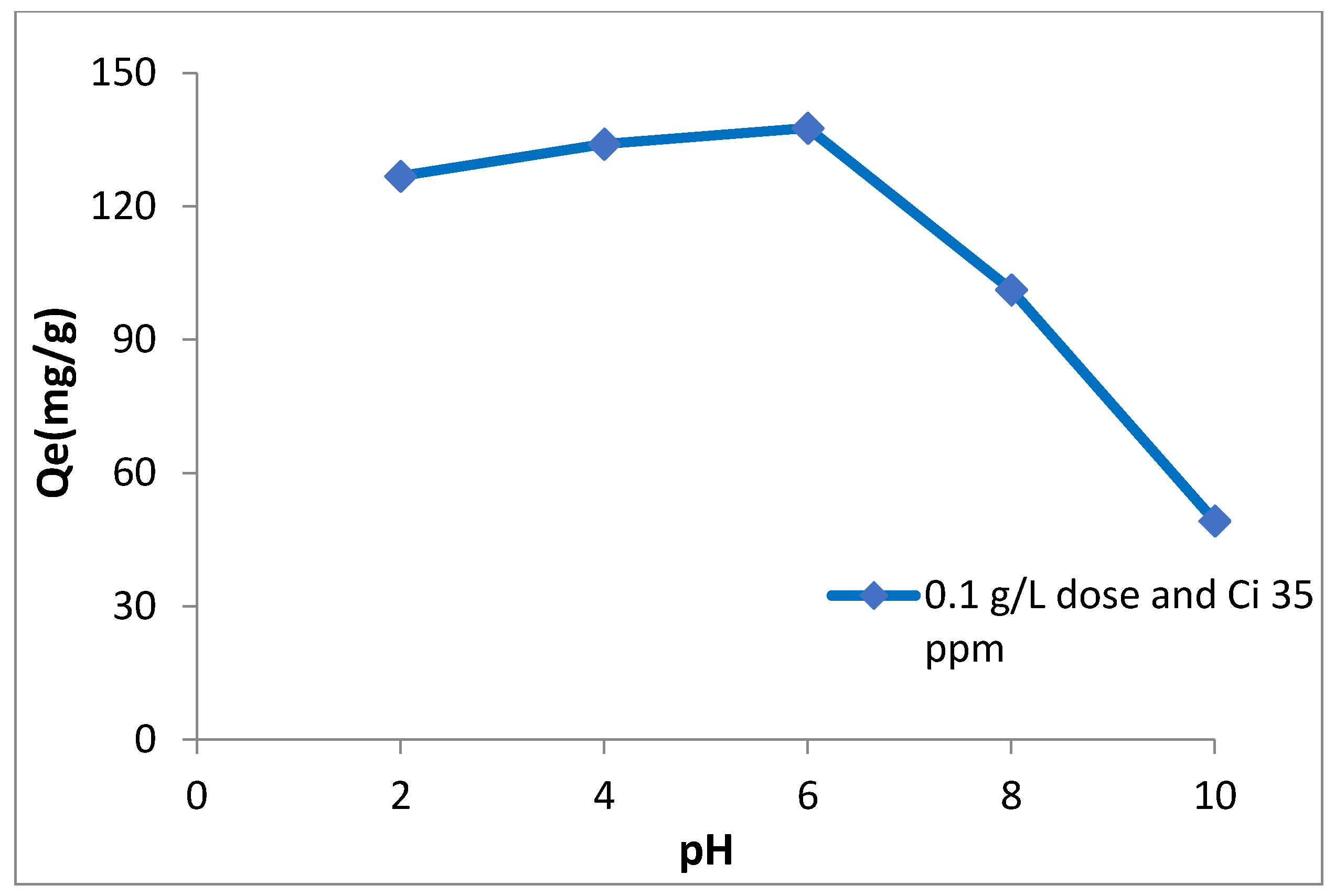
3.5. Effect of pH and Proposed Adsorption Mechanism
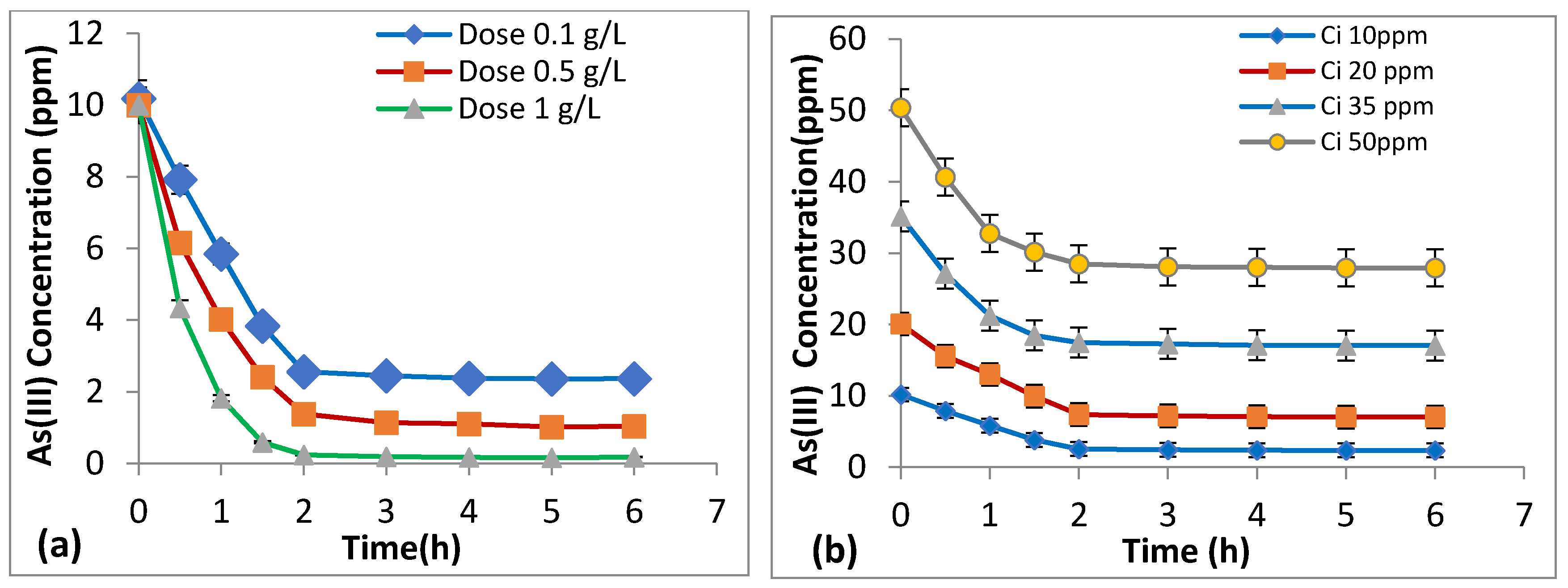
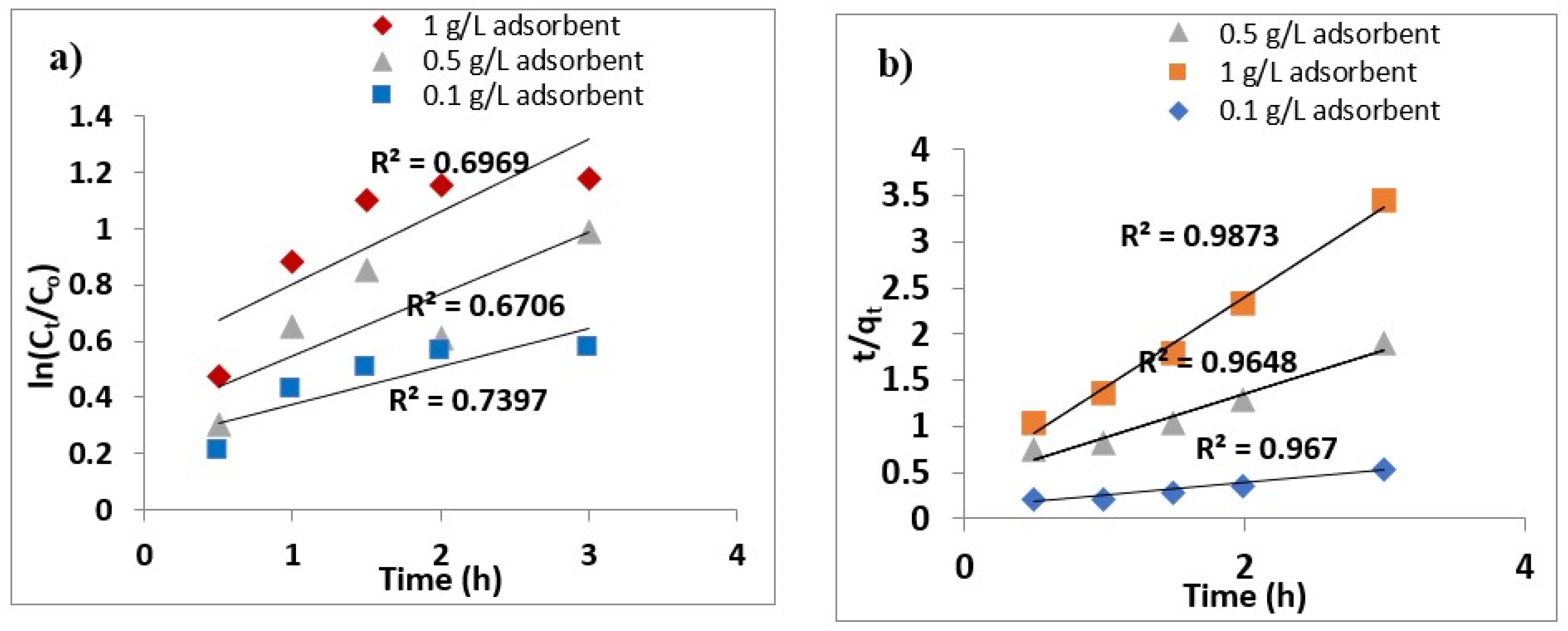
3.6. Effect of Dosage and Initial As (III) Concentration on Kinetics
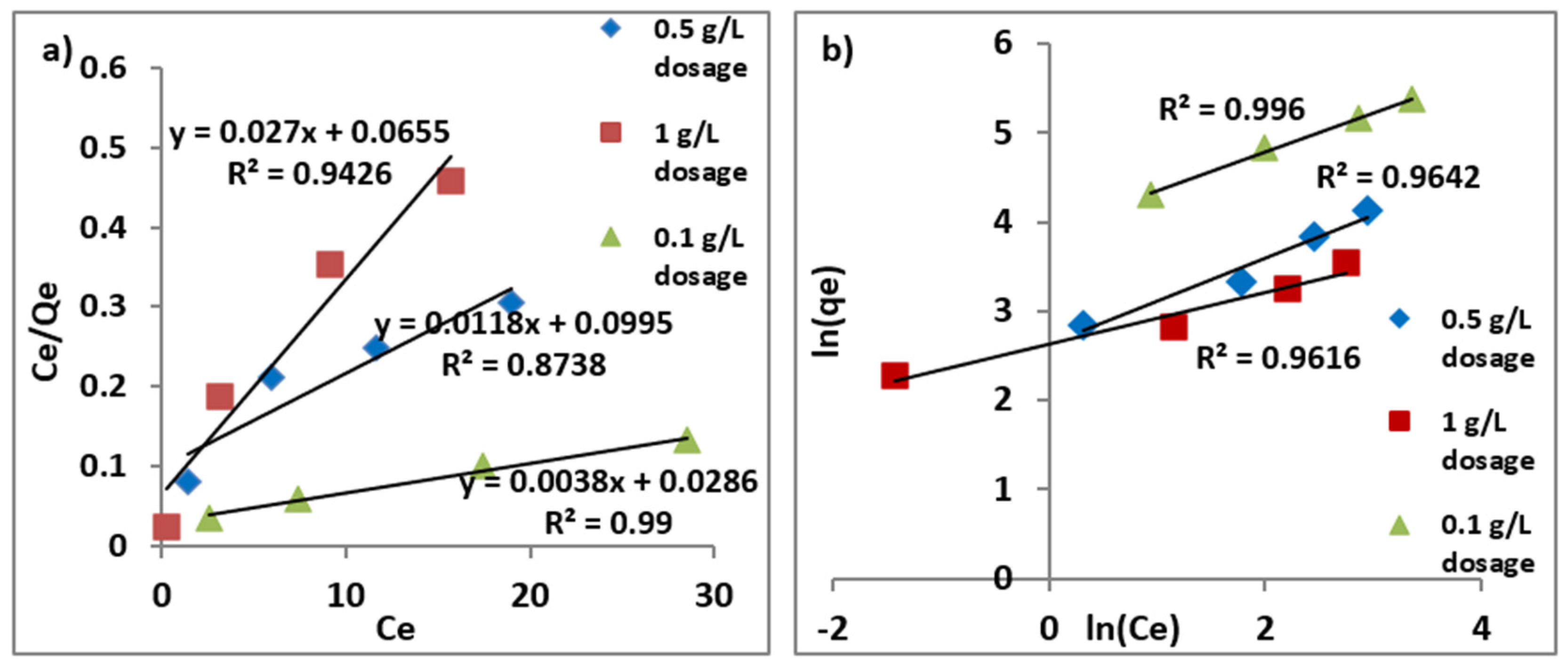
3.7. Kinetics and Isotherm Modeling
Langmuir and Freundlich Isotherm
- qe = adsorption capacity (the mass of adsorbate adsorbed over adsorbent at equilibrium), mg/g
- Ce = the equilibrium concentration of As, mg/L
- qmax = the maximum adsorption capacity, mg/g
- Kf = Freundlich constant
- n = Freundlich intensity factor
- b = Langmuir constant
3.8. Adsorption Kinetics
3.9. Effect of Co-Existing Ions
3.10. Comparison of Fe (III)-Mg (II) Binary Oxide with Other Adsorbents
3.11. Reusability of Synthesized Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Manning, B.A.; Hunt, M.L.; Amrhein, C.; Yarmoff, J.A. Arsenic (III) and arsenic (V) reactions with zerovalent iron corrosion products. Environ. Sci. Technol. 2002, 36, 5455–5461. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, G.; Chen, J.P. Adsorptive removal of arsenic from water by an iron–zirconium binary oxide adsorbent. J. Colloid Interface Sci. 2011, 358, 230–237. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrodes: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Ahmad, A.; Cornelissen, E.; van de Wetering, S.; van Dijk, T.; van Genuchten, C.; Bundschuh, J.; van der Wal, A.; Bhattacharya, P. Arsenite removal in groundwater treatment plants by sequential Permanganate―Ferric treatment. J. Water Process Eng. 2018, 26, 221–229. [Google Scholar] [CrossRef]
- Aredes, S.; Klein, B.; Pawlik, M. The removal of arsenic from water using natural iron oxide minerals. J. Clean. Prod. 2013, 60, 71–76. [Google Scholar] [CrossRef]
- Mateen, Q.S.; Khan, S.U.; Islam, D.T.; Khan, N.A.; Farooqi, I.H. Copper (II) removal in a column reactor using electrocoagulation: Parametric optimization by response surface methodology using central composite design. Water Environ. Res. 2020, 92, 1350–1362. [Google Scholar] [PubMed]
- Polowczyk, I.; Cyganowski, P.; Ulatowska, J.; Sawiński, W.; Bastrzyk, A. Synthetic iron oxides for adsorptive removal of arsenic. Water Air Soil Pollut. 2018, 229, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Dhingra, A.; Hussain, A.; Changani, F. Applications of nanotechnology in water and wastewater treatment: A review. Asian J. Water Environ. Pollut. 2019, 16, 81–86. [Google Scholar] [CrossRef]
- Zaidi, R.; Khan, S.U.; Azam, A.; Farooqi, I.H. A study on effective adsorption of lead from an aqueous solution using Copper Oxide nanoparticles. IOP Conf. Series: Mat. Sci. Eng. 2021, 1058, 012074. [Google Scholar] [CrossRef]
- Kalfa, O.M.; Yalçınkaya, Ö.; Türker, A.R. Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to preconcentration and separation of cadmium. J. Hazard. Mater. 2009, 166, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Self-assembled mesoporousƔ-Al2O3 spherical nanoparticles and their efficiency for the removal of arsenic from water. J. Hazard. Mater. 2012, 201, 170–177. [Google Scholar] [CrossRef]
- Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. Ind. Eng. Chem. Res. 2012, 18, 1418–1427. [Google Scholar] [CrossRef]
- La, D.D.; Patwari, J.M.; Jones, L.A.; Antolasic, F.; Bhosale, S.V. Fabrication of a GNP/Fe–Mg binary oxide composite for effective removal of arsenic from aqueous solution. ACS Omega 2017, 2, 218–226. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Ayub, S. Studies on application of Fe based binary oxide nanoparticles for treatment of lead (Pb2+) contaminated water- A batch study. Mater. Today Proc. 2017, 4, 9650–9655. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, G.; Wu, Q.; Wang, D. Facile fabrication of nanostructured cerium-manganese binary oxide for enhanced arsenite removal from water. Chem. Eng. J. 2018, 334, 1518–1526. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.A.; Isakovski, M.K.; Tubić, A.; Šolić, M.; Kordić, B.; Agbaba, J. Synthesis, characterization and application of magnetic nanoparticles modified with Fe-Mn binary oxide for enhanced removal of As (III) and As (V). Environ. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- He, K.Q.; Yuan, C.G.; Jiang, Y.H.; Duan, X.L.; Li, Y.; Shi, M.D. Synergistic effects of Fe–Mn binary oxide for gaseous arsenic removal in flue gas. Ecotoxicol. Environ. Saf. 2021, 207, 111491. [Google Scholar] [CrossRef]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Li, G.T. Removal mechanism of As (III) by a Novel Fe–Mn binary oxide adsorbent: Oxidation and sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhao, D. Immobilization of As (III) in soil and groundwater using a new class of polysaccharide stabilized Fe–Mn oxide nanoparticles. J. Hazard. Mater. 2012, 211, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.; Ghosh, U.C. Nano-structured iron (III)–cerium (IV) mixed oxide: Synthesis, characterization and arsenic sorption kinetics in the presence of co-existing ions aiming to apply for high arsenic groundwater treatment. Appl. Surf. Sci. 2013, 283, 471–481. [Google Scholar] [CrossRef]
- Khan, S.U.; Zaidi, R.; Hassan, S.Z.; Farooqi, I.H.; Azam, A. Application of Fe-cu binary oxide nanoparticles for the removal of hexavalent chromium from aqueous solution. Water Sci. Technol. 2016, 74, 165–175. [Google Scholar]
- Wen, Z.; Zhang, Y.; Cheng, G.; Wang, Y.; Chen, R. Simultaneous removal of As (V)/Cr (VI) and acid orange 7 (AO7) by nanosized ordered magnetic mesoporous Fe-Ce bimetal oxides: Behavior and mechanism. Chemosphere 2019, 218, 1002–1013. [Google Scholar] [CrossRef]
- Madzokere, T.C.; Karthigeyan, A. Heavy metal ion effluent discharge containment using magnesium oxide (MgO) nanoparticles. Mater. Today Proc. 2017, 4, 9–18. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Chen, J.P. Adsorption of fluoride by Fe–Mg–La triple-metal composite: Adsorbent preparation, illustration of performance and study of mechanisms. Chem. Eng. J. 2015, 262, 839–846. [Google Scholar] [CrossRef]
- Yusoff, A.H.; Salimi, M.N.; Jamlos, M.F. A review: Synthetic strategy control of magnetite nanoparticles production. Adv. Nano Res. 2018, 6, 1. [Google Scholar]
- Abimanyu, H.; Kim, C.S.; Ahn, B.S.; Yoo, K.S. Synthesis of dimethyl carbonate by transesterification with various MgO–CeO2 mixed oxide catalysts. Catal. Lett. 2007, 118, 30–35. [Google Scholar] [CrossRef]
- Abebe, B.; Ananda Murthy, H.C. Synthesis and characterization of Ti-Fe oxide nanomaterials for lead removal. J. Nanomater. 2018, 2018, 9651039. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kaviyarasu, K.; Vijaya, J.J.; Siddhardha, B.; Jeyaraj, B.; Kennedy, J.; Maaza, M. Evaluation on the heterostructured CeO2/Y2O3 binary metal oxide nanocomposites for UV/Vis light induced photocatalytic degradation of Rhodamine-B dye for textile engineering application. J. Alloys Compd. 2017, 727, 1324–1337. [Google Scholar] [CrossRef]
- Liu, H.; Deng, S.; Li, Z.; Yu, G.; Huang, J. Preparation of Al–Ce hybrid adsorbent and its application for defluoridation of drinking water. J. Hazard. Mater. 2010, 179, 424–430. [Google Scholar] [CrossRef]
- Tsade, H.; Abebe, B.; Murthy, H.A. Nano sized Fe–Al oxide mixed with natural maize cob sorbent for lead remediation. Mater. Res. Express 2019, 6, 085043. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.A.; Dessie, Y. Synthesis and characterization of Ti–Fe oxide nanomaterials: Adsorption–degradation of methyl orange dye. Arabian J. Sci. Eng. 2020, 45, 4609–4620. [Google Scholar] [CrossRef]
- Khan, M.M.; Khan, W.; Ahamed, M.; Alhazaa, A.N. Microstructural properties and enhanced photocatalytic performance of Zn doped CeO 2 nanocrystals. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manyasree, D.; Kiranmayi, P.; Kumar, R. Synthesis, characterization and antibacterial activity of aluminium oxide nanoparticles. Int. J. Pharm. Pharm. Sci. 2018, 10, 32–35. [Google Scholar]
- Sahai, A.; Goswami, N.; Kaushik, S.D.; Tripathi, S. Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci. 2016, 390, 974–983. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Janet, C.M.; Viswanathan, B.; Viswanath, R.P.; Varadarajan, T.K. Characterization and photoluminescence properties of MgO microtubes synthesized from hydromagnesite flowers. J. Phys. Chem. C 2007, 111, 10267–10272. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889. [Google Scholar]
- Farahmandjou, M.; Soflaee, F. Low temperature synthesis of α-Fe2O3 nano-rods using simple chemical route. J. Nanostructures 2014, 4, 413–418. [Google Scholar]
- Lobato, N.C.C.; Mansur, M.B.; Ferreira, A.D.M. Characterization and chemical stability of hydrophilic and hydrophobic magnetic nanoparticles. Mater. Res. 2017, 20, 736–746. [Google Scholar] [CrossRef]
- Huang, X.; Schmucker, A.; Dyke, J.; Hall, S.M.; Retrum, J.; Stein, B.; Remmes, N.; Baxter, D.V.; Dragnea, B.; Bronstein, L.M. Magnetic nanoparticles with functional silanes: Evolution of well-defined shells from anhydride containing silane. J. Mater. Chem. 2009, 19, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Rufus, A.; Sreeju, N.; Philip, D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016, 6, 94206–94217. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Ghosh, S.; Islam, S.M. Flower-like AgNPs@ m-MgO as an excellent catalyst for CO2 fixation and acylation reactions under ambient conditions. New J. Chem. 2018, 42, 14194–14202. [Google Scholar] [CrossRef]
- Mahadevaiah, R.; Lalithamba, H.S.; Shekarappa, S.; Hanumanaika, R. Synthesis of Nα-protected formamides from amino acids using MgO nano catalyst: Study of molecular docking and antibacterial activity. Sci. Iran. 2017, 24, 3002–3013. [Google Scholar] [CrossRef]
- Sutapa, I.W.; Wahab, A.W.; Taba, P.; La Nafie, N. Synthesis and structural profile analysis of the MgO nanoparticles produced through the sol-gel method followed by annealing process. Orient. J. Chem. 2018, 34, 1016. [Google Scholar] [CrossRef]
- Hingston, F.J.; Posner, A.M.; Quirk, J.P. Anion adsorption by goethite and gibbsite. J. Soil Sci. 1972, 23, 177–192. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and arsenite removal by zerovalent iron: Kinetics, redox transformation, and implications for in situ groundwater remediation. Environ. Sci. Technol. 2001, 35, 1487–1492. [Google Scholar] [CrossRef]
- Stumm, W. Chemistry of the Solide Water Interface; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Zeng, H.; Zhai, L.; Qiao, T.; Yu, Y.; Zhang, J.; Li, D. Efficient removal of As (V) from aqueous media by magnetic nanoparticles prepared with Iron-containing water treatment residuals. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Hang, C.; Li, Q.; Gao, S.; Shang, J.K. As (III) and As (V) adsorption by hydrous zirconium oxide nanoparticles synthesized by a hydrothermal process followed with heat treatment. Ind. Eng. Chem. Res. 2012, 51, 353–361. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Ndungu, P. Enhanced As (III) and As (V) adsorption from aqueous solution by a clay based hybrid sorbent. Front. Chem. 2020, 7, 913. [Google Scholar] [CrossRef]
- Mahmood, T.; Aslam, M.; Naeem, A.; Siddique, T.; Din, S.U. Adsorption of As (III) from aqueous solution onto iron impregnated used tea activated carbon: Equilibrium, kinetic and thermodynamic study. J. Chil. Chem. Soc. 2018, 63, 3855–3866. [Google Scholar] [CrossRef]
- Esposito, A.; Pagnanelli, F.; Lodi, A.; Solisio, C.; Veglio, F. Biosorption of heavy metals by Sphaerotilusnatans: An equilibrium study at different pH and biomass concentrations. Hydrometallurgy 2001, 62, 129–141. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Mahtab, M.S.; Farooqi, I.H. Enhanced lead (II) removal with low energy consumption in an electrocoagulation column employing concentric electrodes: Process optimisation by RSM using CCD. Int. J. Environ. Anal. Chem. 2021, 1–18. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Khownpurk, P.; Chandra-Ambhorn, W. As (III) removal under the presence of competitive anions using the calcined ground oyster shell as the adsorbent. Sep. Sci. Technol. 2020, 55, 395–405. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- O’reilly, S.E.; Strawn, D.G.; Sparks, D.L. Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Sci. Soc. Am. J. 2001, 65, 67–77. [Google Scholar] [CrossRef]
- Tuutijärvi, T.; Repo, E.; Vahala, R.; Sillanpää, M.; Chen, G. Effect of competing anions on arsenate adsorption onto maghemite nanoparticles. Chin. J. Chem. Eng. 2012, 20, 505–514. [Google Scholar] [CrossRef]
- Çiftçi, T.D.; Henden, E. Nickel/nickel boride nanoparticles coated resin: A novel adsorbent for arsenic(III) and arsenic(V) removal. Powder Technol. 2015, 269, 470–480. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Z.; Ma, J.; Qiu, Y.; Chen, J. Surfactant assisted Ce–Fe mixed oxide decorated multiwalled carbon nanotubes and their arsenic adsorption performance. J. Mater. Chem. A 2013, 1, 11355–11367. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Prasad, C.; Vijaya, Y.; Subbaiah, M.V. Application of ZnO nanorods as an adsorbent material for the removal of As (III) from aqueous solution: Kinetics, isotherms and thermodynamic studies. Int. J. Ind. Chem. 2018, 9, 17–25. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zheng, T.; Ma, J.; Zhang, G.; Ren, G.; Wang, L.; Liu, Y. Efficient oxidation and sorption of arsenite using a novel titanium (IV)-manganese (IV) binary oxide sorbent. J. Hazard. Mater. 2018, 353, 410–420. [Google Scholar] [CrossRef]
- Yu, X.Y.; Luo, T.; Jia, Y.; Zhang, Y.X.; Liu, J.H.; Huang, X.J. Porous hierarchically micro-/nanostructured MgO: Morphology control and their excellent performance in As (III) and As (V) removal. J. Phys. Chem. C 2018, 115, 22242–22250. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, J.; Chang, B.; Liu, T.; Zhang, F.; Jin, P.; Wang, W.; Wang, X. Removal of arsenic (III, V) by a granular Mn-oxide-doped Al oxide adsorbent: Surface characterization and performance. Environ. Sci. Pollut. Res. 2017, 24, 18505–18519. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Chen, L.; Huang, X.; Liu, J. Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem. Eng. J. 2014, 251, 25–34. [Google Scholar] [CrossRef]
- Tang, W.; Su, Y.; Li, Q.; Gao, S.; Shang, J.K. Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res. 2013, 47, 3624–3634. [Google Scholar] [CrossRef]

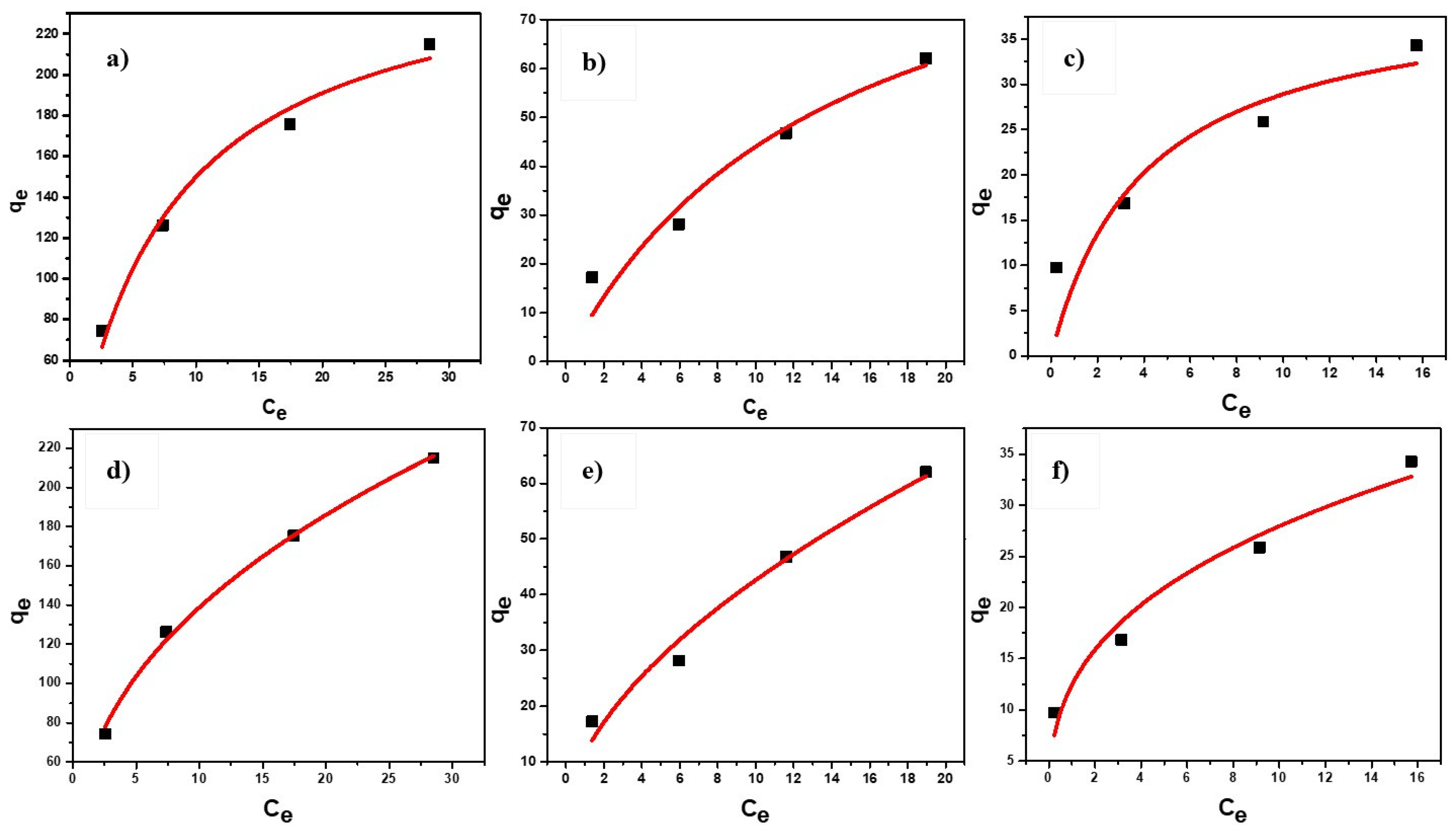

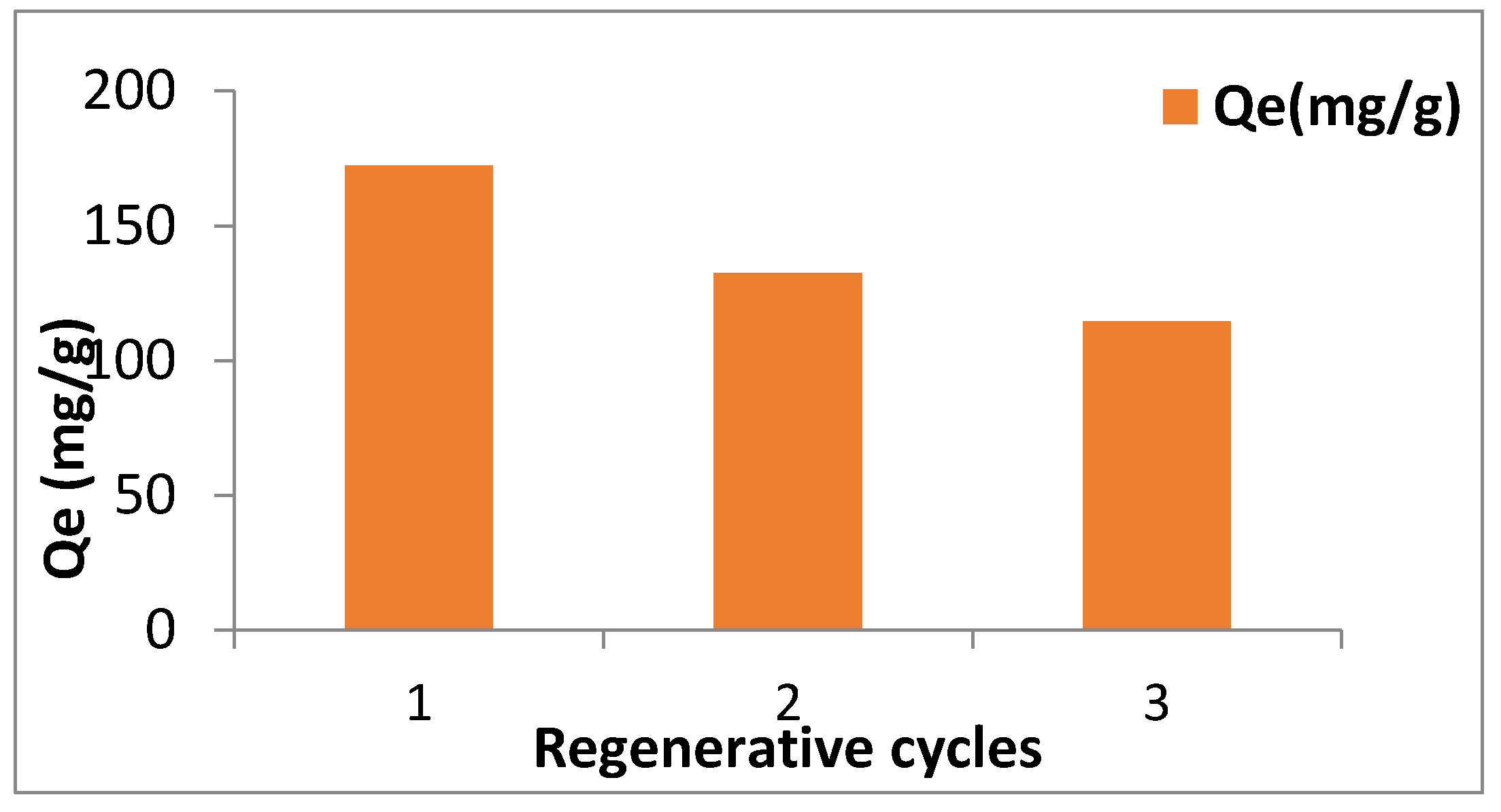
| Models | Adsorbent Dosage (g/L) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | b | R2 | Kf | n | R2 | ||
| Linear | 1 | 37.05 | 0.41221 | 0.9426 | 13.92659 | 2.287283 | 0.9616 |
| 0.5 | 84.75 | 0.1186 | 0.8738 | 13.802 | 2.0492 | 0.9642 | |
| 0.1 | 263.2 | 0.13286 | 0.99 | 50.4761 | 3.460208 | 0.996 | |
| Non-linear | 1 | 40.54 | 0.24854 | 0.819 | 12.42526 | 2.83962 | 0.94786 |
| 0.5 | 105.15 | 0.07201 | 0.905 | 11.53966 | 1.76192 | 0.96806 | |
| 0.1 | 263.33 | 0.13199 | 0.974 | 52.44399 | 2.36656 | 0.99577 | |
| S. No. | Adsorbents | pH | Qe (mg g−1) | References |
|---|---|---|---|---|
| (1) | Fe (III)-Mg (II) oxide NPs | 6 | 263.2 [As (III)] | Present Study |
| (2) | Fe-Zr binary oxide | 7 | 46.1 [As (V)] | [3] |
| (3) | GNP/Fe–Mg Binary Oxide Composite | 7 | 103.9 [As (V)] | [16] |
| (4) | Nickel boride nanoparticle-coated resin | 6 | 23.4 [As (III)] | [64] |
| (5) | Ce-Fe mixed oxide MWCNT | 4 | 28.74 [As (V)] | [65] |
| (6) | ZnOnanorods | 7 | 52.63 [As (V)] | [66] |
| (7) | Ethylenediamine modified Fe3O4 NPs | 2 | 107 [As (V)] | [67] |
| (8) | Flower like porous MgO Np’s | 7 | 252.34 [As (III)] | [68] |
| (9) | Granular Mn-oxide-doped Al oxide (GMAO) | 7 | 48.52 [As (III)] | [69] |
| (10) | Magnetic nanoparticle-impregnated chitosan beads | 6.8 | 35.7 [As (V)] | [70] |
| (11) | Superparamagnetic Mg0.27Fe2.5O4 | 7 | 127.4 [As (III)] | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.U.; Zaidi, R.; Shaik, F.; Farooqi, I.H.; Azam, A.; Abuhimd, H.; Ahmed, F. Evaluation of Fe-Mg Binary Oxide for As (III) Adsorption—Synthesis, Characterization and Kinetic Modelling. Nanomaterials 2021, 11, 805. https://doi.org/10.3390/nano11030805
Khan SU, Zaidi R, Shaik F, Farooqi IH, Azam A, Abuhimd H, Ahmed F. Evaluation of Fe-Mg Binary Oxide for As (III) Adsorption—Synthesis, Characterization and Kinetic Modelling. Nanomaterials. 2021; 11(3):805. https://doi.org/10.3390/nano11030805
Chicago/Turabian StyleKhan, Saif Ullah, Rumman Zaidi, Feroz Shaik, Izharul Haq Farooqi, Ameer Azam, Hatem Abuhimd, and Faheem Ahmed. 2021. "Evaluation of Fe-Mg Binary Oxide for As (III) Adsorption—Synthesis, Characterization and Kinetic Modelling" Nanomaterials 11, no. 3: 805. https://doi.org/10.3390/nano11030805
APA StyleKhan, S. U., Zaidi, R., Shaik, F., Farooqi, I. H., Azam, A., Abuhimd, H., & Ahmed, F. (2021). Evaluation of Fe-Mg Binary Oxide for As (III) Adsorption—Synthesis, Characterization and Kinetic Modelling. Nanomaterials, 11(3), 805. https://doi.org/10.3390/nano11030805







