Field-Induced Assembly of sp-sp2 Carbon Sponges
Abstract
:1. Introduction
2. Materials and Methods
3. Results
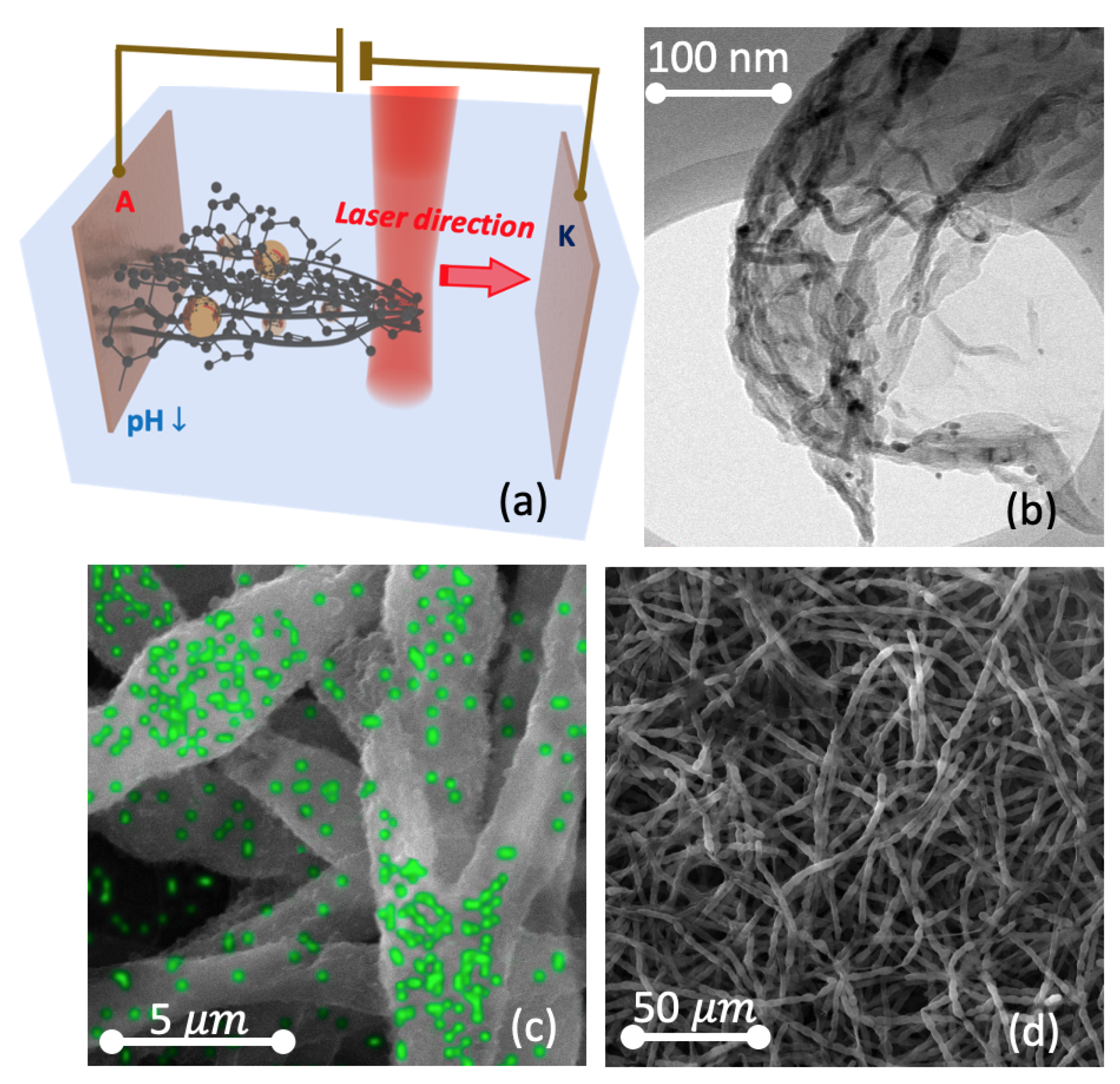
3.1. The Manifestation of a Self-Assembly Effect during the Carbon Growth
3.2. The Raman Characterization
3.3. Photoluminescence and Absorbance Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| NPs | Nanoparticles |
| CNT | Carbon nanotube |
| IDs | Induces dipoles |
| EDAX | Energy-dispersive X-ray analyser |
| DI | Distilled water |
| VIS-NIR | visible - near infrared spectral range |
| DCL | double containment layer |
| PL | photoluminescence |
| THz | terahertz |
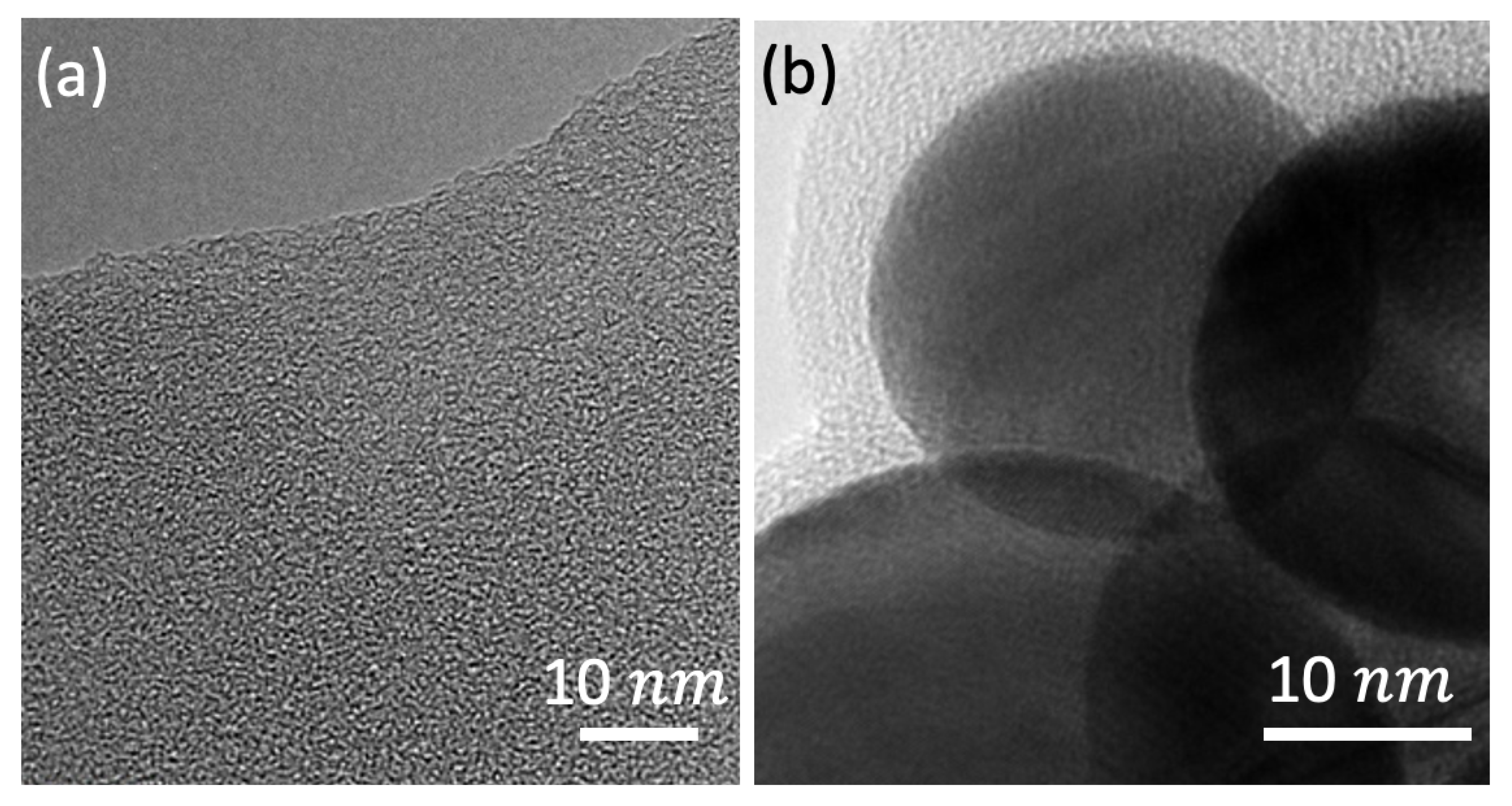
Appendix A. The Shungite Precursor and Gold Nanoparticles
References
- Bianco, A. A carbon science perspective in 2018: Current achievements and future challenges. Carbon 2018, 132, 785–801. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, C.; Cao, Y.; Han, Q.; Parker, C.B.; Glass, J.T. Robust and High-Performance Electrodes via Crumpled Au-CNT Forests for Stretchable Supercapacitors. Matter 2020, 2, 1307–1323. [Google Scholar] [CrossRef] [Green Version]
- Weinhold, L.; Chatterjee, S.; Klar, P.J. Modifying Graphene’s lattice dynamics by hot electron injection from single gold nanoparticles. Commun. Phys. 2019, 2, 18. [Google Scholar] [CrossRef]
- Kutrovskaya, S.; Osipov, A.; Baryshev, S.; Zasedatelev, A.; Samyshkin, V.; Demirchyan, S.; Pulci, O.; Grassano, D.; Gontrani, L.; Hartmann, R.R.; et al. Excitonic fine structure in emission of linear carbon chains. Nanoletter 2020, 20, 6502–6509. [Google Scholar] [CrossRef] [PubMed]
- Rabia, A.; Tumino, F.; Milani, A.; Russo, V.; Bassi, A.L.; Achilli, S.; Fratesi, G.; Onida, G.; Manini, N.; Sun, Q.; et al. Scanning tunneling microscopy and Raman spectroscopy of polymeric sp–sp2 carbon atomic wires synthesized on the Au(111) surface. Nanoscale 2019, 11, 18191–18196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Chen, S.; Xiang, H.; Gong, X.G. Hybrid crystalline sp2-sp3 carbon as a high-efficiency solar cell absorber. J. Carbon 2016, 1098, 246–252. [Google Scholar] [CrossRef]
- Kazaryan, A.; Kharisov, G.G. Synthesis and Research of Luminescent Properties of Carbon Nanoparticles; 2017; pp. 1–51. Available online: https://www.researchgate.net/publication/316171909_Synthesis_and_research_of_luminescent_properties_of_carbon_nanoparticles (accessed on 15 March 2021). [CrossRef]
- Sin, J.S.; Pak, H.C.; Kim, K.I.; Ri, K.C.; Ju, D.Y.; Kim, N.H.; Sin, C.S. An electric double layer of colloidal particles in salt-free concentrated suspensions including non-uniform size effects and orientational ordering of water dipoles. J. Phys. Chem. Chem. Phys. 2016, 18, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wu, N. Electric-field assisted assembly of colloidal particles into ordered nonclose-packed arrays. Langmuir 2017, 33, 5769–5776. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Nicholson, P.S. Role of Ionic Depletion in Deposition during Electrophoretic Deposition. J. Am. Ceram. Soc. 1999, 11, 3031–3036. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. Review on Fundamentals and Applications of Electrophoretic Deposition. Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Colombo, L.; Piseri, P.; Ravagnan, L.; Milani, P. Growth of sp-sp2 nanostructures in a carbon plasma. Phys. Rev. B. 2007, 76, 134119–134123. [Google Scholar] [CrossRef] [Green Version]
- Rozhkova, N.; Goryunov, A. Natural graphene-based shungite nanocarbon. Carbon Nanomater. Sourceb. 2016, 76, 153176–153178. [Google Scholar]
- Arakelyan, S.M.; Veiko, V.P.; Kutrovskaya, S.V.; Kucherik, A.O.; Osipov, A.V.; Vartanyan, T.A.; Itina, T.E. Reliable and well-controlled synthesis of noble metal nanoparticles by continuous wave laser ablation in different liquids for deposition of thin films with variable optical properties. J. Nanopart. Res. 2016, 18, 155–158. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutrovskaya, S.; Samyshkin, V.; Lelekova, A.; Povolotskiy, A.; Osipov, A.; Arakelian, S.; Kavokin, A.V.; Kucherik, A. Field-Induced Assembly of sp-sp2 Carbon Sponges. Nanomaterials 2021, 11, 763. https://doi.org/10.3390/nano11030763
Kutrovskaya S, Samyshkin V, Lelekova A, Povolotskiy A, Osipov A, Arakelian S, Kavokin AV, Kucherik A. Field-Induced Assembly of sp-sp2 Carbon Sponges. Nanomaterials. 2021; 11(3):763. https://doi.org/10.3390/nano11030763
Chicago/Turabian StyleKutrovskaya, Stella, Vlad Samyshkin, Anastasia Lelekova, Alexey Povolotskiy, Anton Osipov, Sergey Arakelian, Alexey Vitalievich Kavokin, and Alexey Kucherik. 2021. "Field-Induced Assembly of sp-sp2 Carbon Sponges" Nanomaterials 11, no. 3: 763. https://doi.org/10.3390/nano11030763





