Molybdenum–Tungsten Blue Nanoparticles as a Precursor for Ultrafine Binary Carbides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Molybdenum–Tungsten Blue Dispersions
2.3. Characterization of Molybdenum–Tungsten Blue Dispersions
3. Results
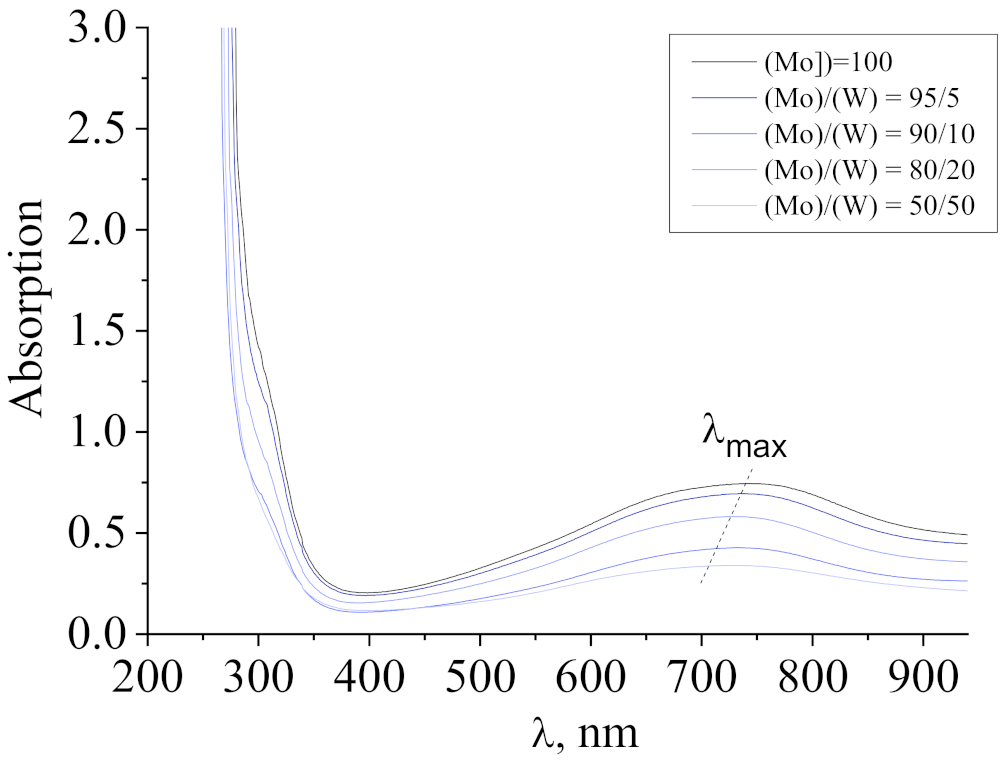
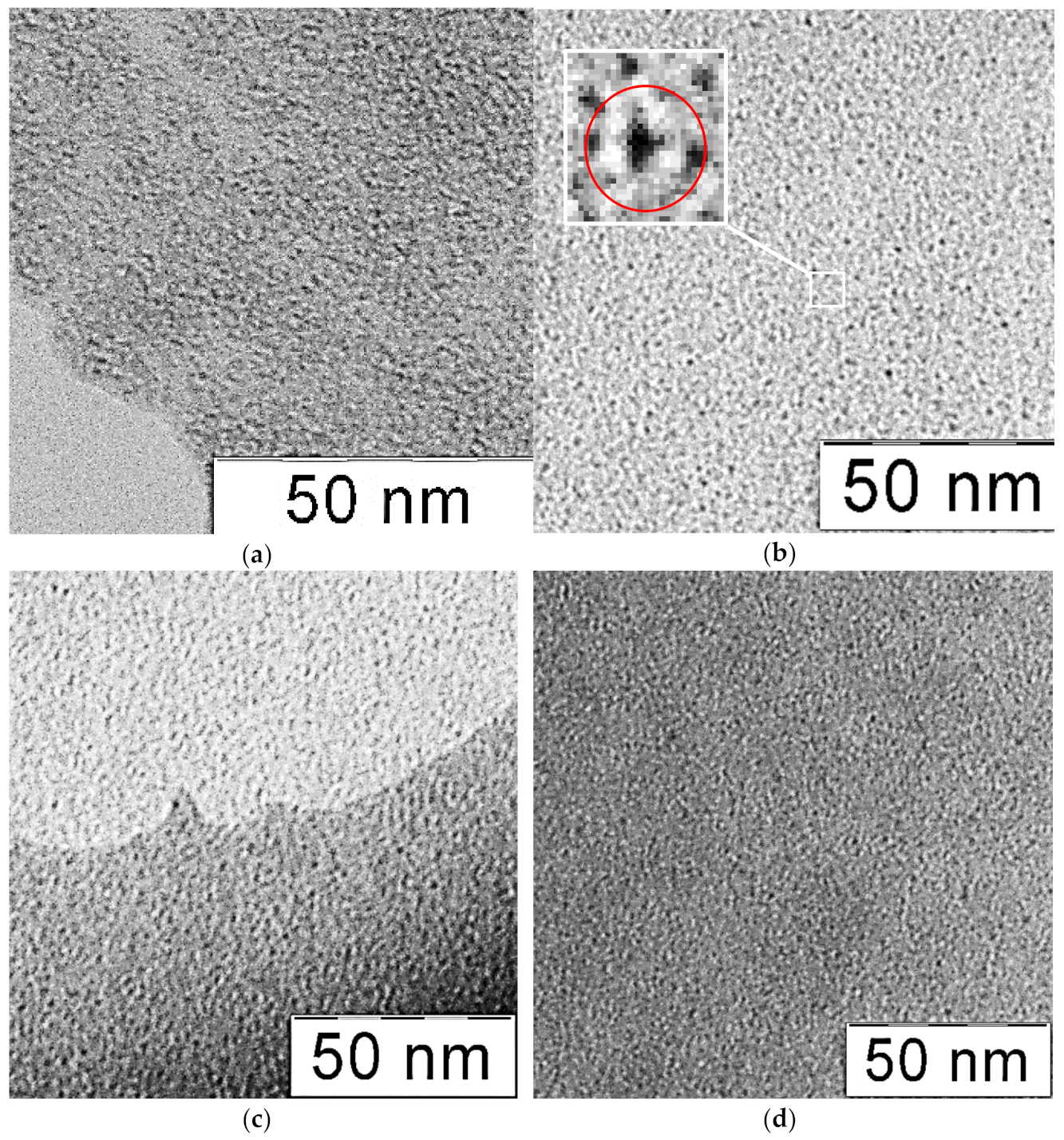
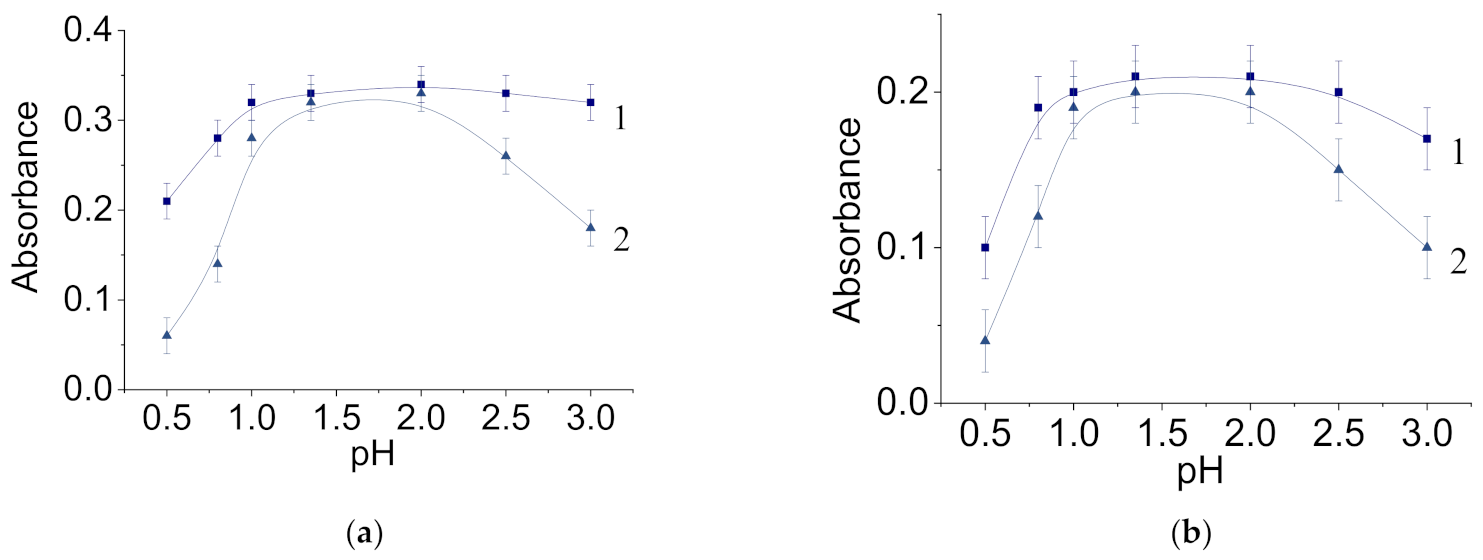
3.1. Characterization of Molybdenum–Tungsten Blue Nanoparticles
- the resulting polyoxometalate complexes differ in composition and structure from molybdenum oxide nanoclusters of the Mo154-x family;
- molybdenum oxide nanoclusters of the Mo154-x family are formed in the system, on the surface of which tungsten ions are adsorbed in a variable oxidation state; and
- the dispersed phase is represented by Mo154-x nanoclusters, and the system contains dissolved colored reduced forms of tungsten.
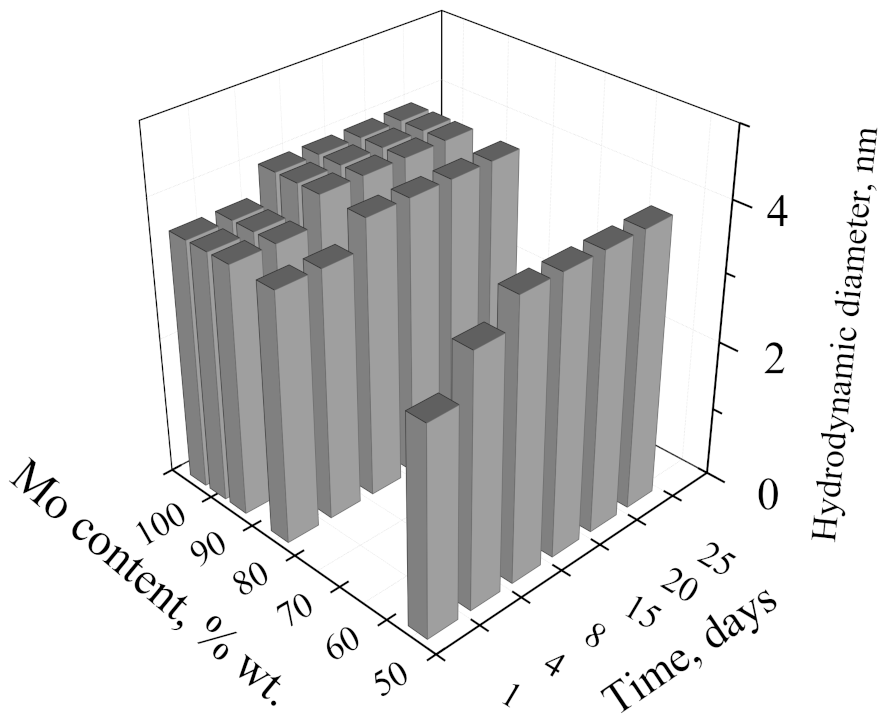
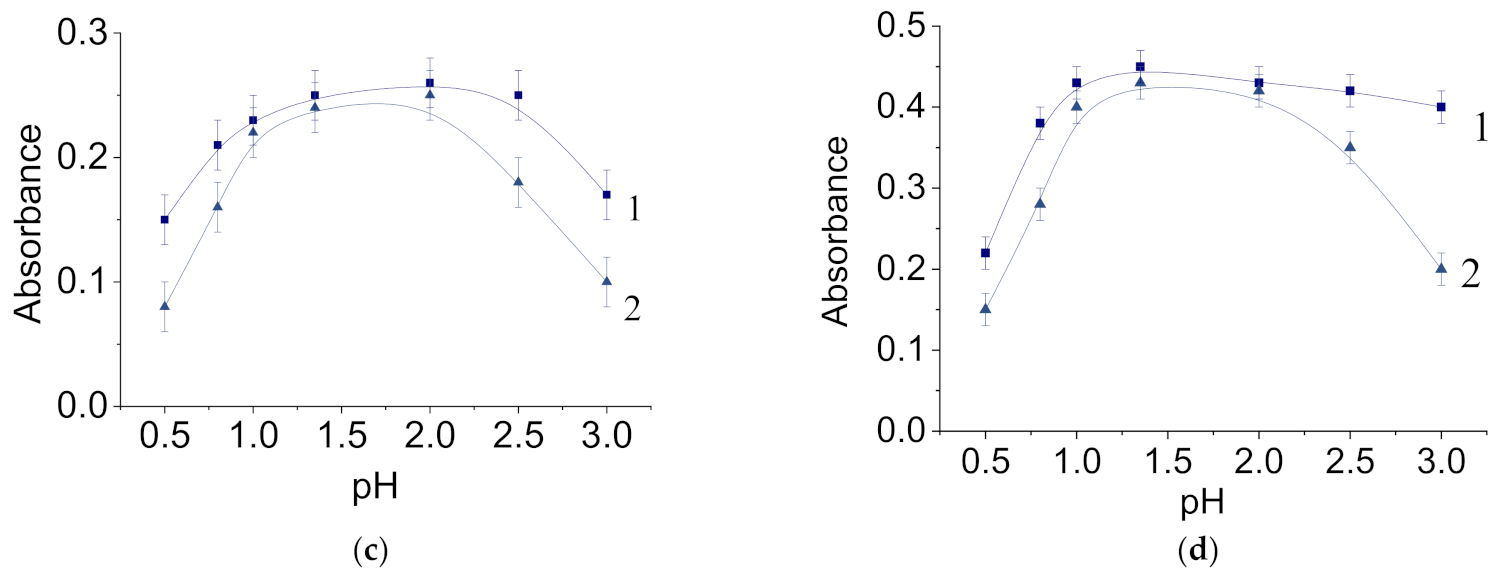
3.2. Properties of Molybdenum–Tungsten Blue Dispersions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Ren. Sust. Energy Rev. 2018, 75, 1101–1129. [Google Scholar] [CrossRef]
- Lamic, A.F.; Shin, C.H.; Djéga-Mariadassou, G.; Potvin, C. Characterization of New Bimetallic Oxycarbide (MoWC0.5O0.6) for Bifunctional Isomerization of n-Heptane. Catal. Lett. 2006, 107, 89–94. [Google Scholar] [CrossRef]
- Mehdad, A.; Jentoft, R.E.; Jentoft, F.C. Passivation Agents and Conditions for Mo2C and W2C: Effect on Catalytic Activity for Toluene Hydrogenation. J. Catal. 2017, 347, 89–101. [Google Scholar] [CrossRef]
- Peng, X.; Ge, X.; Wang, H.; Liu, Z.; Fisher, A.; Wang, X. Novel Molybdenum Carbide–Tungsten Carbide Composite Nanowires and Their Electrochemical Activation for Efficient and Stable Hydrogen Evolution. Adv. Fun. Mat. 2015, 25, 1520–1526. [Google Scholar]
- Garcia-Esparza, A.T.; Cha, D.; Ou, Y.; Kubota, J.; Domen, K.; Takanabe, K. Tungsten carbide nanoparticles as efficient cocatalysts for photocatalytic overall water splitting. ChemSusChem 2013, 6, 168–181. [Google Scholar] [CrossRef]
- Mehdad, A.; Jentoft, R.E.; Jentoft, F.C. Single-phase mixed molybdenum-niobium carbides: Synthesis, characterization and multifunctional catalytic behavior in toluene conversion. J. Catal. 2017, 351, 161–173. [Google Scholar] [CrossRef]
- Leroy Covington, K.M.; Islam, A.W.; Roberts, K.L. Synthesis and characterization of nanostructured molybdenum & tungsten carbide materials, and study of diffusion model. Pol. J. Chem. Tech. 2012, 14, 28–34. [Google Scholar]
- Regmi, Y.N.; Wan, C.; Duffee, K.D.; Leonard, B.M. Nanocrystalline Mo2C as a bifunctional water splitting electrocatalyst. Chem. Cat. Chem. 2015, 7, 3911–3916. [Google Scholar] [CrossRef]
- Bastos, L.; Monteiro, W.; Zacharias, M.; da Cruz, G.; Rodrigues, J.A. Preparation and characterization of Mo/W bimetallic carbides by using different synthesis methods. Catal. Lett. 2008, 120, 48–55. [Google Scholar] [CrossRef]
- Kushkhov, K.B.; Kardanov, A.L.; Adamokova, M.N. Electrochemical synthesis of binary molybdenum–tungsten carbides (Mo,W)2C from tungstate–molybdate–carbonate melts. Russ. Metall. 2013, 2, 79–85. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Zhang, B.; Anbalgam, K.; Thomas, T.; Yang, M. Synthesis and application of nano-structured metal nitrides and carbides: A review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Alaba, P.A.; Abbas, A.; Huang, J.; Wan Daud, W.M.A. Molybdenum carbide nanoparticle: Understanding the surface properties and reaction mechanism for energy production towards a sustainable future. Ren. Sust. Energy Rev. 2018, 91, 287–300. [Google Scholar] [CrossRef]
- Giordano, C.; Erpen, C.; Yao, W.; Antonietti, M. Synthesis of Mo and W carbide and nitride nanoparticles via a simple “urea glass” route. Nano. Lett. 2008, 8, 59–63. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.; Xie, S.; Hua, W.; Zhang, Y.; Ren, N.; Xu, H.; Tang, Y. Synthesis of nanoporous molybdenum carbide nanowires based on organic- inorganic hybrid nanocomposites with sub-nanometer periodic structures. Chem. Mater. 2009, 21, 5560–5562. [Google Scholar] [CrossRef]
- Mehdad, A.; Jentoft, R.E.; Jentoft, F.C. Single-phase mixed molybdenum–tungsten carbides: Synthesis, characterization and catalytic activity for toluene conversion. Catal. Today 2019, 323, 112–122. [Google Scholar] [CrossRef]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self-assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Botar, B.; Ellern, A.; Kögerler, P. Mapping the formation areas of giant molybdenum blue clusters: A spectroscopic study. Dalton Trans. 2012, 41, 8951–8959. [Google Scholar] [CrossRef]
- Liu, T. An unusually slow self-assembly of inorganic ions in dilute aqueous solution. J. Am. Chem. Soc. 2003, 125, 312–313. [Google Scholar] [CrossRef]
- Liu, T.; Diemann, E.; Müller, A. Hydrophilic inorganic macro-ions in solution: Unprecedented self-assembly emerging from historical “blue waters”. J. Chem. Educ. 2007, 84, 526–532. [Google Scholar] [CrossRef]
- Müller, A.; Roy, S. En route from the mystery of molybdenum blue via related manipulatable building 523 blocks to aspects of materials science. Coord. Chem. Rev. 2003, 245, 153–166. [Google Scholar] [CrossRef]
- Gavrilova, N.; Myachina, M.; Harlamova, D.; Nazarov, V. Synthesis of Molybdenum Blue Dispersions 5 Using Ascorbic Acid as Reducing Agent. Colloids Interfaces 2020, 4, 24–38. [Google Scholar] [CrossRef]
- Nakamura, I.; Miras, H. Investigating the formation of “Molybdenum Blues” with gel electrophoresis an mass spectrometry. J. Am. Chem. Soc. 2015, 137, 6524–6530. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Chandel, S.; Mallick, A.; Sreejith, S.S.; Ghosh, N.; Roy, S. Studying the crystallization of polyoxometalates from colloidal softoxometalates. Cryst. Growth Des. 2018, 18, 4068–4075. [Google Scholar] [CrossRef]
- Grzhegorzhevskii, K.V.; Zelenovsky, P.S.; Koryakova, O.V.; Ostroushko, A.A. Thermal destruction of giant polyoxometalate nanoclusters: A vibrational spectroscopy study. Inorg. Chim. Acta 2019, 489, 287–300. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Bögge, H.; Schmidtmann, M.; Roy, S.; Berkle, A. Changeable pore sizes allowing effective and specific recognition by a molybdenum-oxide based “Nanosponge”: En route to sphere-surface and nanoporous-cluster chemistry. Angew. Chem. 2002, 114, 3756–3761. [Google Scholar] [CrossRef]
- Liu., Q.; Wang, X. Polyoxometalate clusters: Sub-nanometer building blocks for construction of advanced materials. Matter 2020, 2, 816–841. [Google Scholar] [CrossRef]
- Shishido, S.; Ozeki, T. The pH dependent nuclearity variation of {Mo154−x}-type polyoxomolybdates and tectonic effect on their aggregations. J. Am. Chem. Soc. 2008, 130, 10588–10595. [Google Scholar] [CrossRef]
- Zheng, Z.; Yuan, Z.; Li, S.; Li, H. Big to small: Ultrafine Mo2C particles derived from giant polyoxomolybdate clusters for hydrogen evolution reaction. Small 2019, 15, 1–11. [Google Scholar]
- Conte, M.; Liu, X.; Murphy, D.M.; Taylor, S.H.; Whiston, K.; Hutchings, G.J. Insights into the reaction mechanism of cyclohexane oxidation catalyzed by molybdenum blue nanorings. Catal. Lett. 2016, 146, 126–135. [Google Scholar] [CrossRef]
- Lunk, H.-J.; Ziemer, B.; Salmen, M.; Heidemann, D. What is behind ‘tungsten blue oxides’? Int. J. Refract. Met. Hard Mat. 1993, 12, 17–26. [Google Scholar] [CrossRef]
- Scaffer, C.; Merca, A.; Bogge, H.; Todea, A.M.; Kistler, M.L.; Tianbo, L.; Thouvenot, R. Unprecedented and Differently Applicable Pentagonal Units in a Dynamic Library: A Keplerate of the Type {(W)W5}12{Mo2}30. Angew. Chem. Int. Ed. 2009, 48, 149–153. [Google Scholar] [CrossRef]
- Tytko, K.; Glemser, О. Isopolymolybdates and isopolytungstates. Adv. Inorg. Chem. Radiochem. 1976, 19, 239–315. [Google Scholar]
- Juesholt, M.; Christiansen, T.L.; Jensen, K.M. Mechanisms for tungsten oxide nanoparticle formation in solvothermal synthesis: From polyoxometalates to crystalline materials. J. Phys. Chem. C 2019, 123, 5110–5119. [Google Scholar] [CrossRef]
- Gavrilova, N.; Dyakonov, V.; Myachina, M.; Nazarov, V.; Skudin, V. Synthesis of Mo2C by thermal decomposition of molybdenum blue nanoparticles. Nanomaterials 2020, 10, 2053. [Google Scholar] [CrossRef]
- Gavrilova, N.; Myachina, M.; Dyakonov, V.; Nazarov, V.; Skudin, V. Synthesis of microporous Mo2C-W2C binary carbides by thermal decomposition of molybdenum–tungsten blues. Nanomaterials 2020, 10, 2428. [Google Scholar] [CrossRef] [PubMed]
- Azmat, S.; Jan, T.; Ilyas, S.Z.; Hassan, A.; Habib, I.; Mahmood, Q.; Mahmood, A. Solar light photocatalytic performance of WO3 nanostructures: Waste water treatment. Mater. Res. Express. 2018, 5, 115025–115049. [Google Scholar] [CrossRef]
- Guzman, G.; Yebka, B.; Livage, J.; Julien, C. Lithium intercalation studies in hydrated molybdenum oxides. Solid State Ion. 1996, 86, 407–413. [Google Scholar] [CrossRef]
- Bazhenova, M.D.; Gavrilova, N.N.; Nazarov, V.V. Some colloidochemical properties of molybdenum blues synthesized using glucose as a reducing agent. Colloid J. 2015, 77, 1–5. [Google Scholar] [CrossRef]
- Myachina, M.A.; Gavrilova, N.N.; Nazarov, V.V. Formation of molybdenum blue particles via the reduction of a molybdate solution with hydroquinone. Colloid J. 2019, 81, 541–546. [Google Scholar] [CrossRef]
- Gavrilova, N.N.; Nazarov, V.V.; Skudin, V.V. Synthesis of membrane catalysts based on Mo2C. Kinet. Catal. 2015, 56, 670–680. [Google Scholar] [CrossRef]
- Gavrilova, N.N.; Myachina, M.A.; Ardashev, D.V.; Nazarov, V.V.; Skudin, V.V. Sol–gel synthesis of membrane Мo2С/Al2O3 catalysts with different architectures and their catalytic activity in the reaction of carbon dioxide conversion of methane. Kinet. Catal. 2018, 59, 635–643. [Google Scholar] [CrossRef]
- Lyklema, J. Fundamentals of Interface and Colloid Science V. 5. Soft Colloids, 4th ed.; Elsevier: London, UK, 2005; pp. 3–38. [Google Scholar]
- Rusanov, A.I. Micellization in Surfactant Solutions, 1st ed.; Taylor & Francis: London, UK, 1998; pp. 150–250. [Google Scholar]
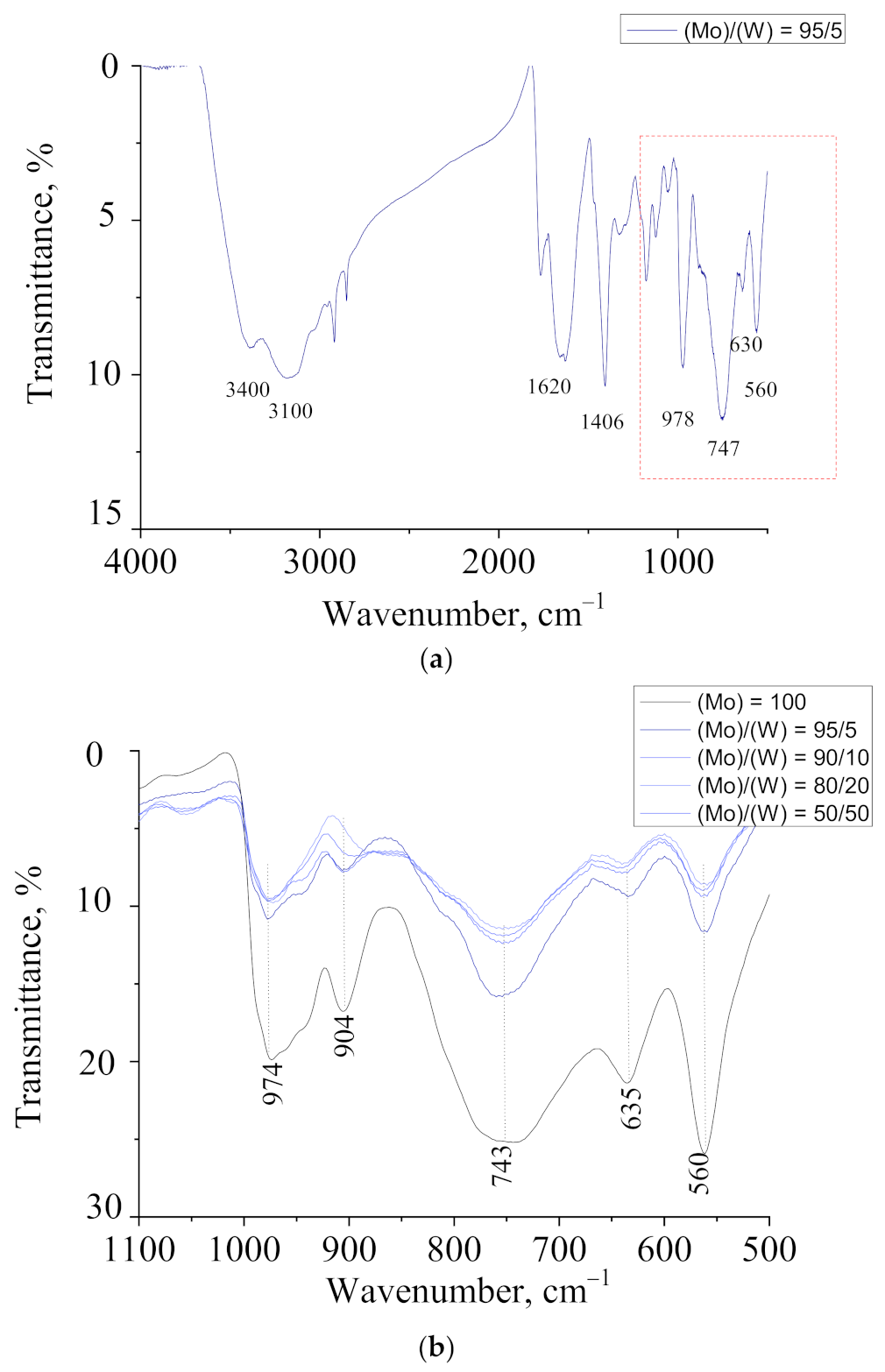
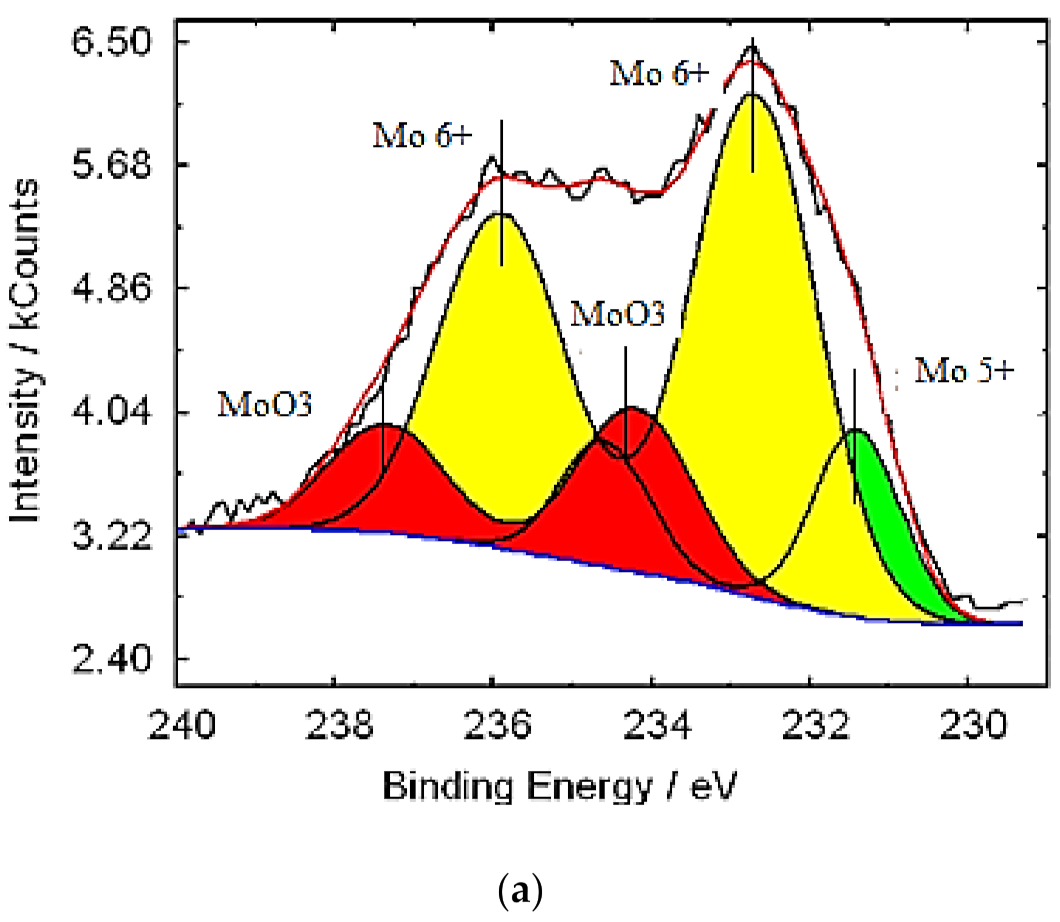
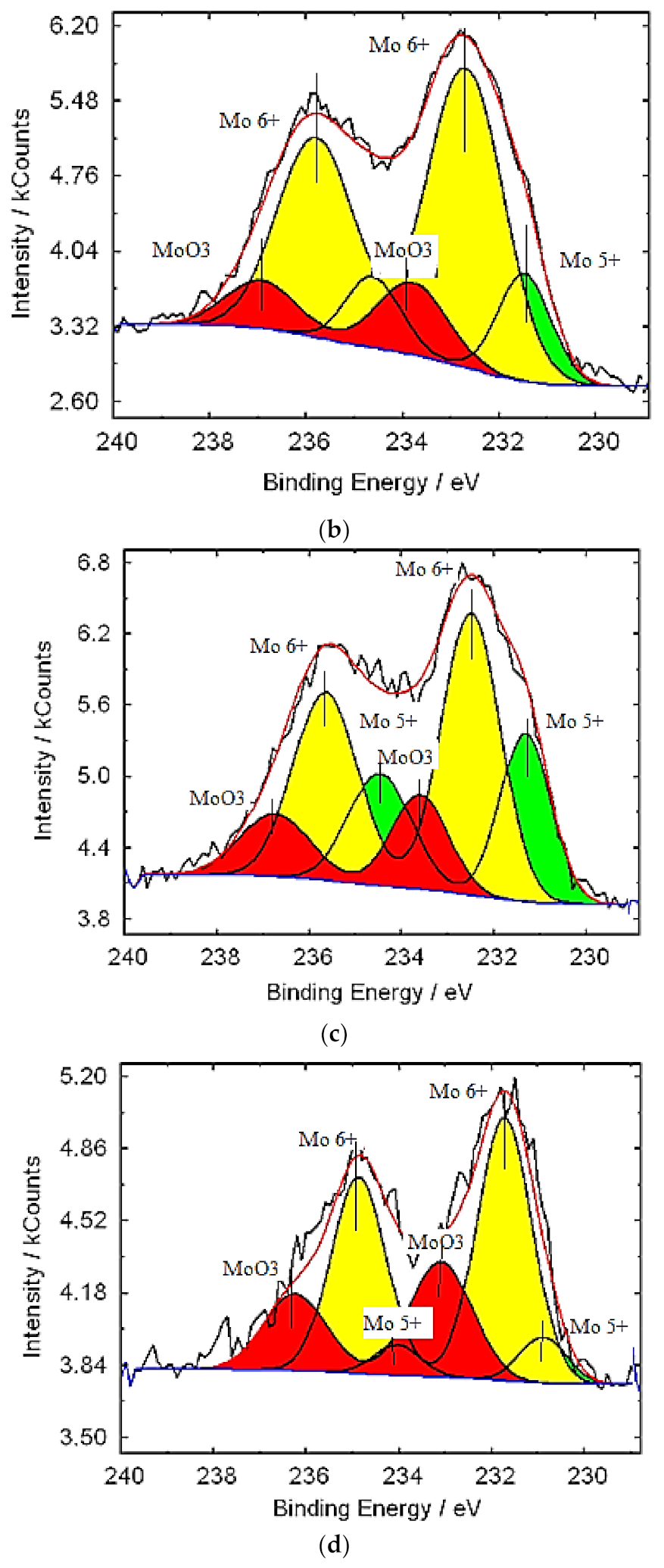
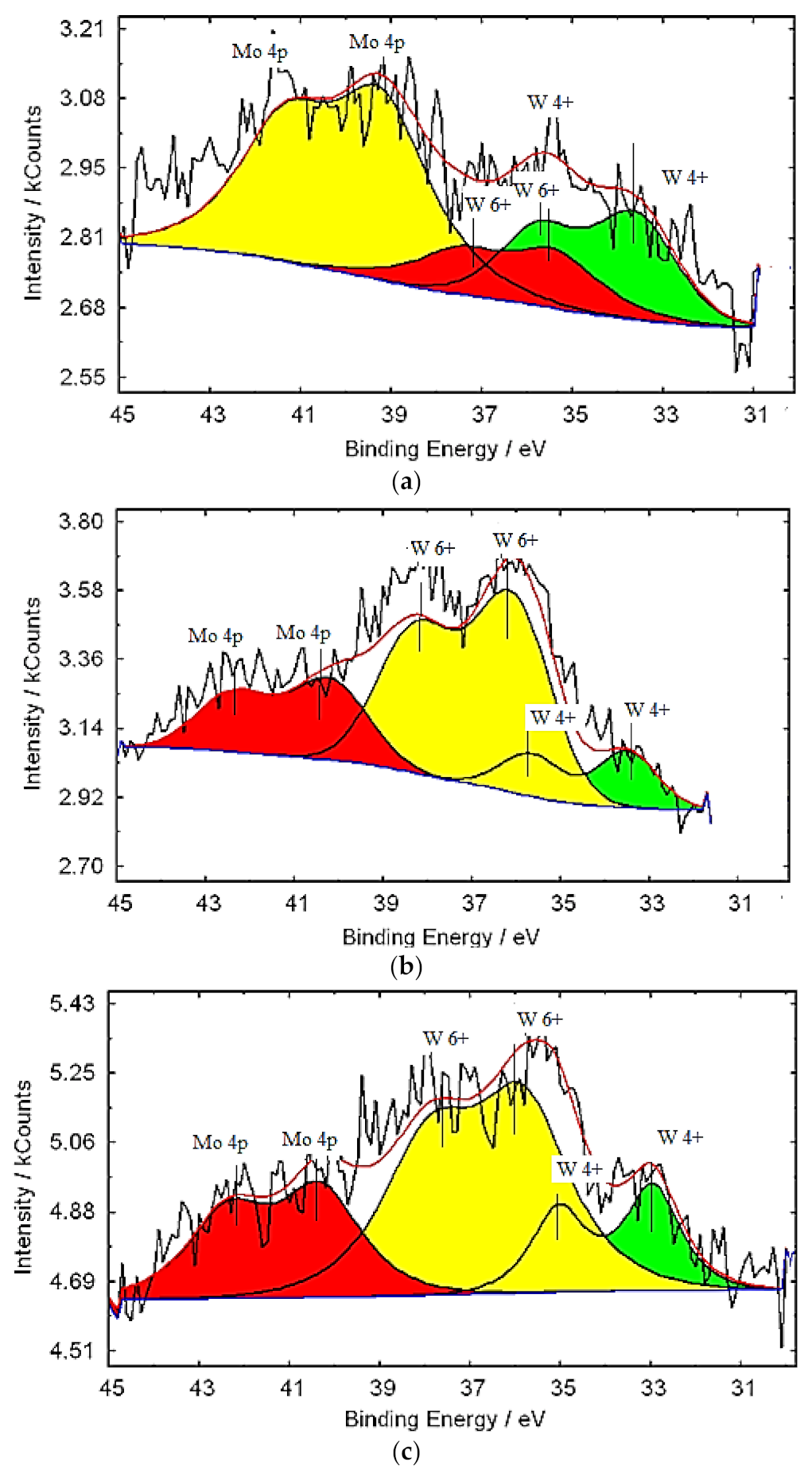
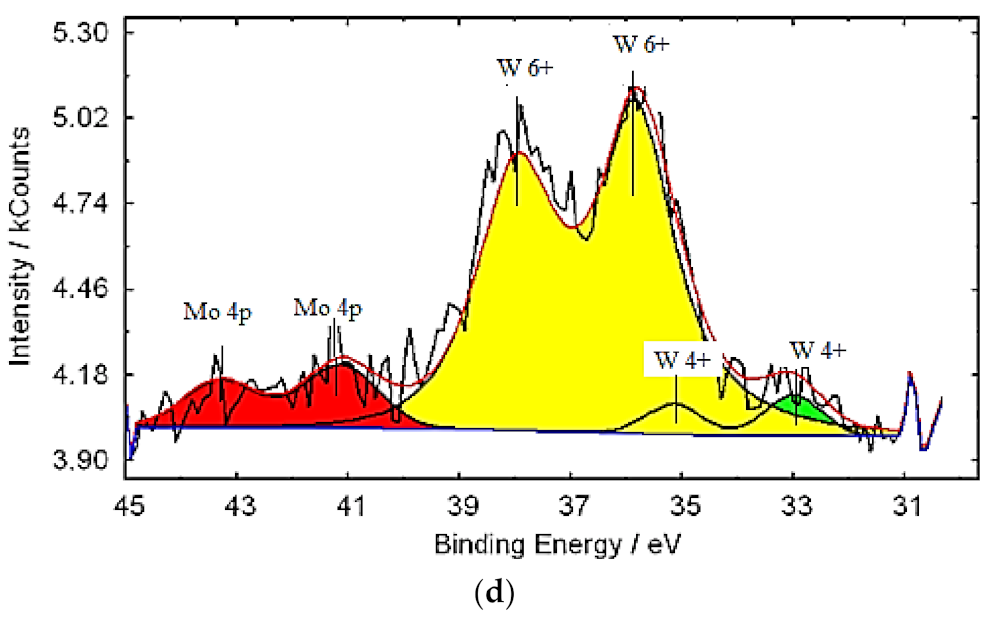
| Band Position (cm−1) | Assignment | Reference Data |
|---|---|---|
| 977 s 902 w | νMo=O | [37] |
| 737 s 634 m | ν(Mo–μ2O–Mo) or ν(Mo–μ3O–Mo) | [37] |
| 561 s | δ(O–Mo–O) | [37] |
| 1620 s | δH2O | [37] |
| 3400 s | ν(OH…H) | [37] |
| 1407 w | δNH4+ | [37] |
| Sample | Predominant Particle Size, nm (DLS) | Stability Time, Days | Particle Concentration, % wt. (MoO3–WO3) |
|---|---|---|---|
| (Mo)/(W) = 95/5 | 4.0 | >30 | 1.0 |
| (Mo)/(W) = 90/10 | 4.0 | >30 | 1.0 |
| (Mo)/(W) = 80/20 | 4.0 | >30 | 1.0 |
| (Mo)/(W) = 50/50 | 4.0 | >30 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myachina, M.; Gavrilova, N.; Poluboyarinova, K.; Nazarov, V. Molybdenum–Tungsten Blue Nanoparticles as a Precursor for Ultrafine Binary Carbides. Nanomaterials 2021, 11, 761. https://doi.org/10.3390/nano11030761
Myachina M, Gavrilova N, Poluboyarinova K, Nazarov V. Molybdenum–Tungsten Blue Nanoparticles as a Precursor for Ultrafine Binary Carbides. Nanomaterials. 2021; 11(3):761. https://doi.org/10.3390/nano11030761
Chicago/Turabian StyleMyachina, Maria, Natalia Gavrilova, Ksenia Poluboyarinova, and Victor Nazarov. 2021. "Molybdenum–Tungsten Blue Nanoparticles as a Precursor for Ultrafine Binary Carbides" Nanomaterials 11, no. 3: 761. https://doi.org/10.3390/nano11030761
APA StyleMyachina, M., Gavrilova, N., Poluboyarinova, K., & Nazarov, V. (2021). Molybdenum–Tungsten Blue Nanoparticles as a Precursor for Ultrafine Binary Carbides. Nanomaterials, 11(3), 761. https://doi.org/10.3390/nano11030761






