A Cost-Effective Nano-Sized Curcumin Delivery System with High Drug Loading Capacity Prepared via Flash Nanoprecipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
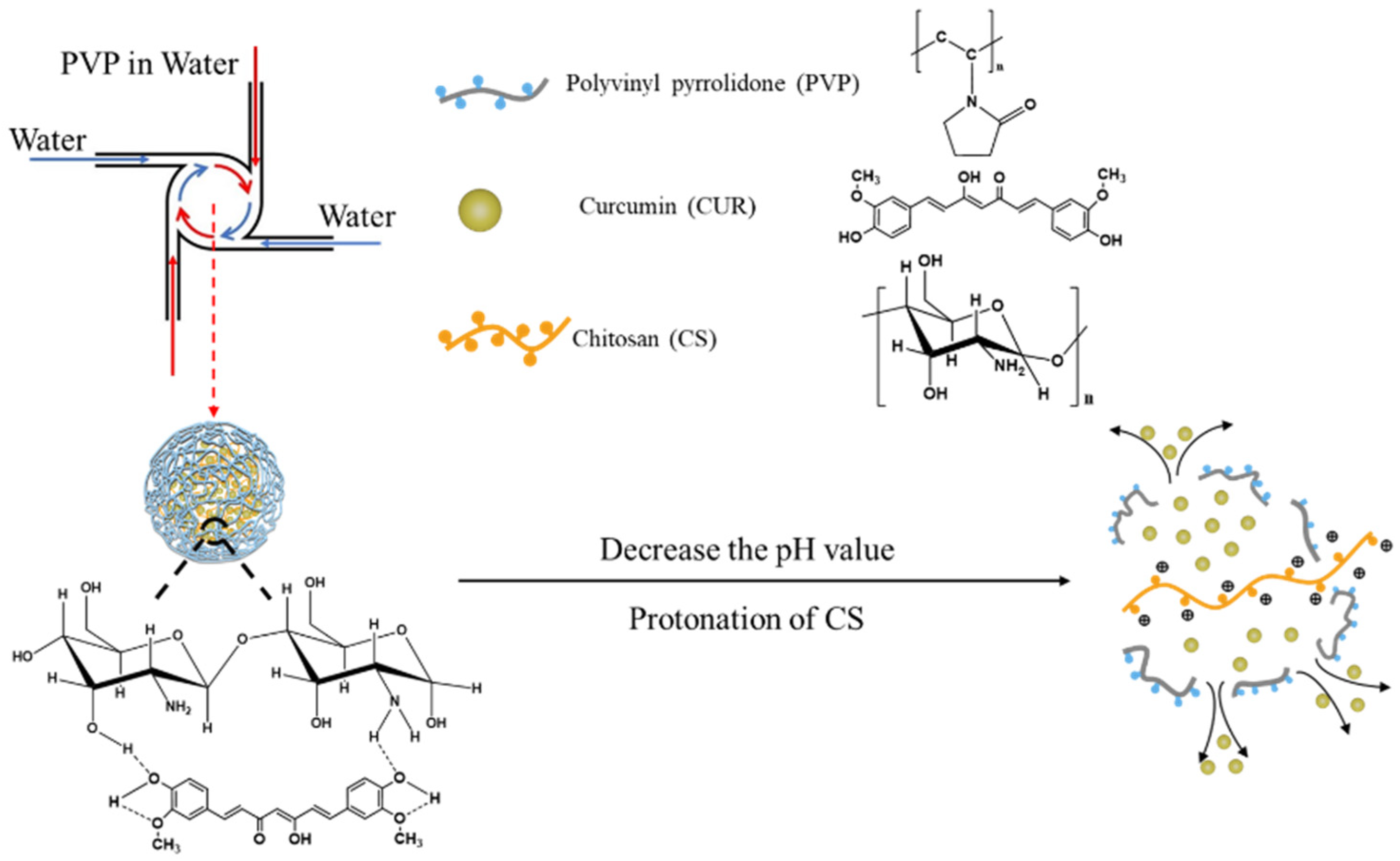
2.2. Preparation of CUR-loaded NPs
2.3. Encapsulation Efficiency and Drug Loading Capacity
2.4. Characterization
2.5. Stability Test
2.6. In Vitro Release
3. Results
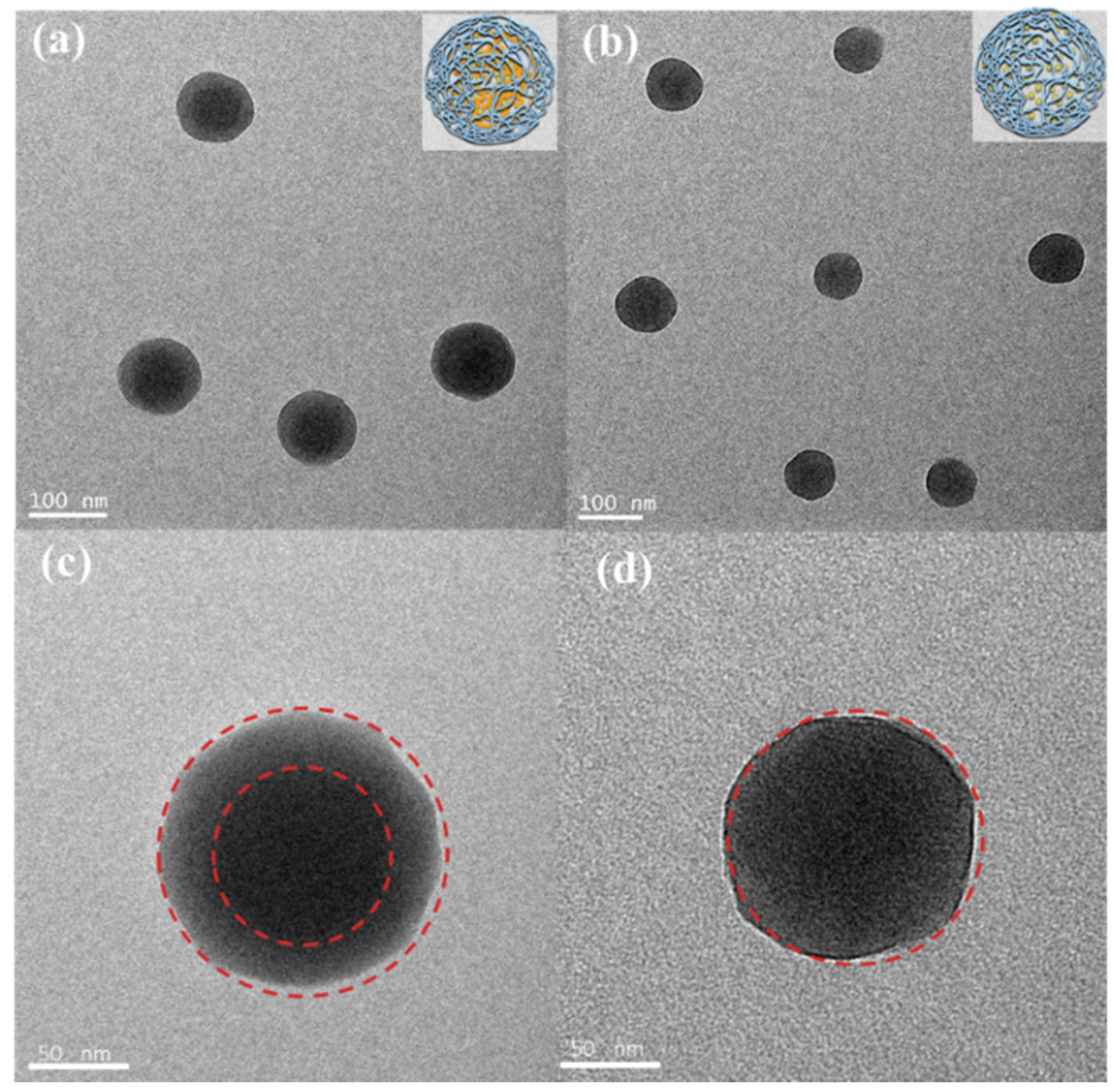
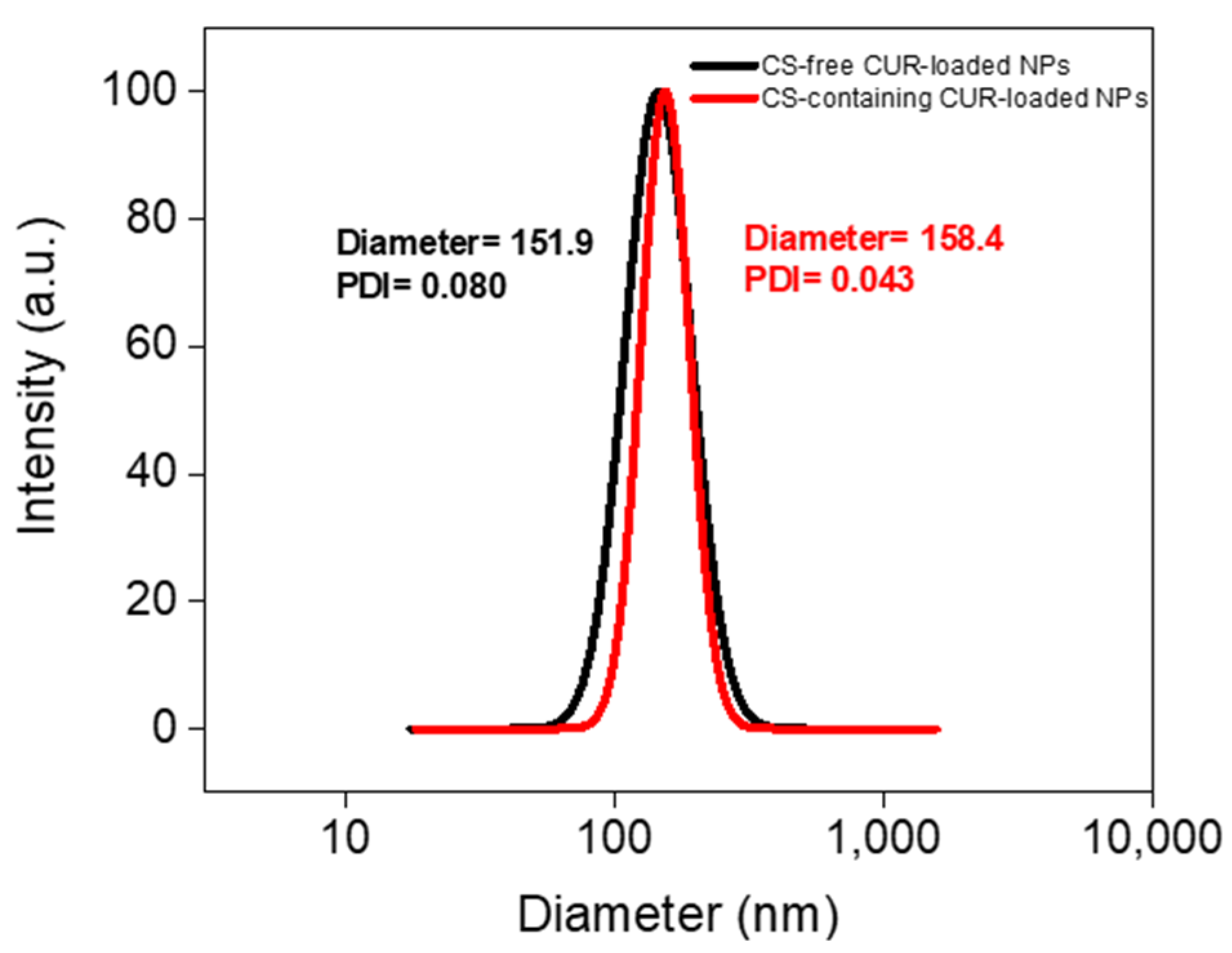
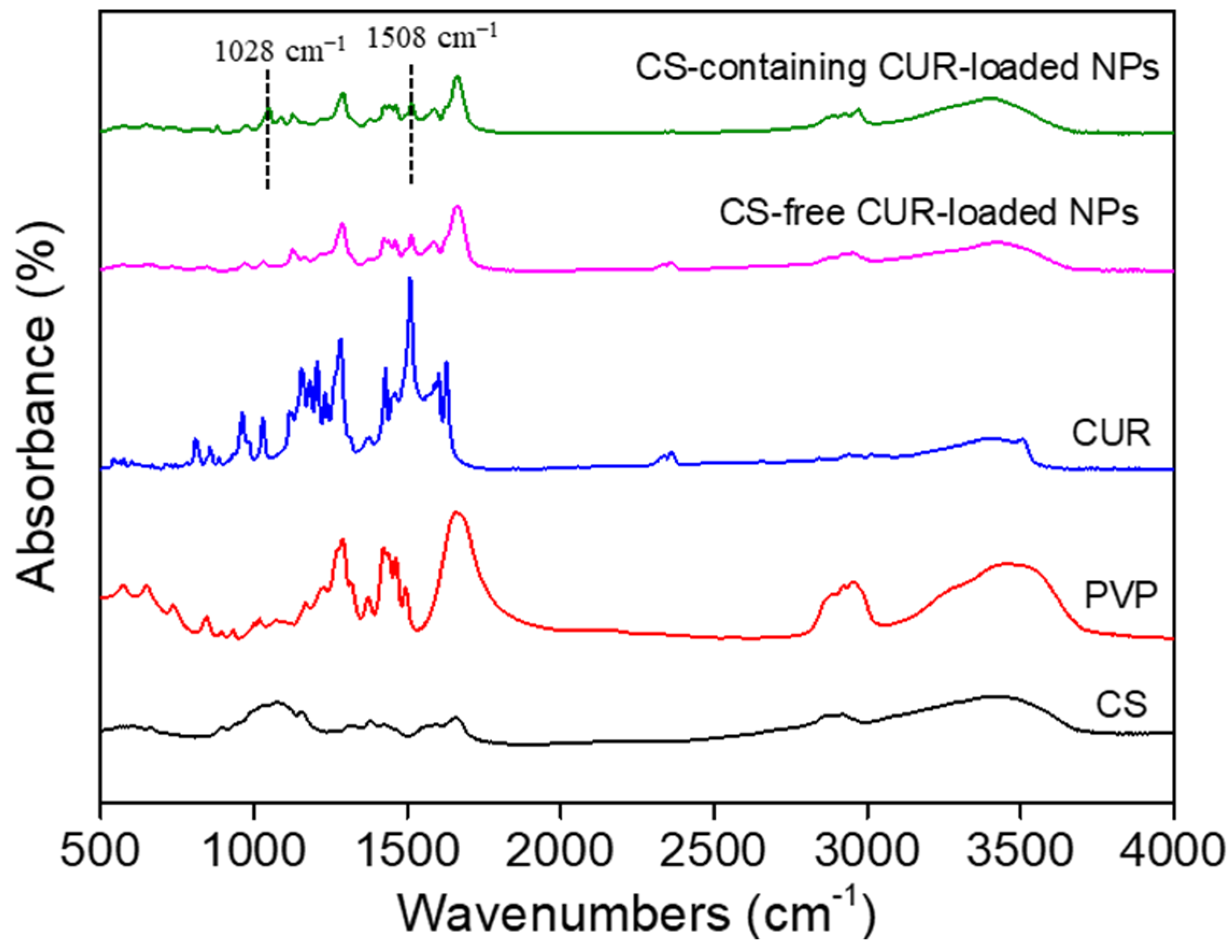
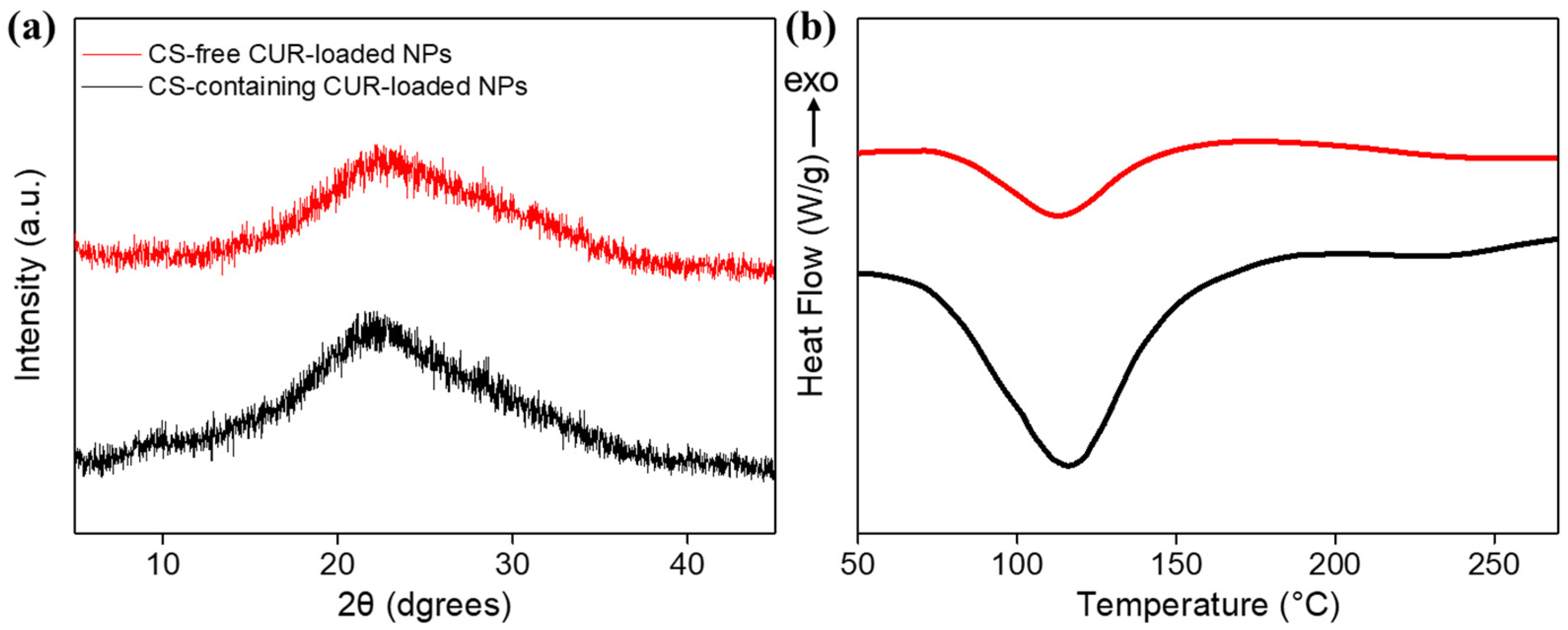
3.1. Structural Characterization of CUR-Loaded NPs
3.2. Encapsulation Efficiency (EE) and Drug Loading Capacity (DLC)
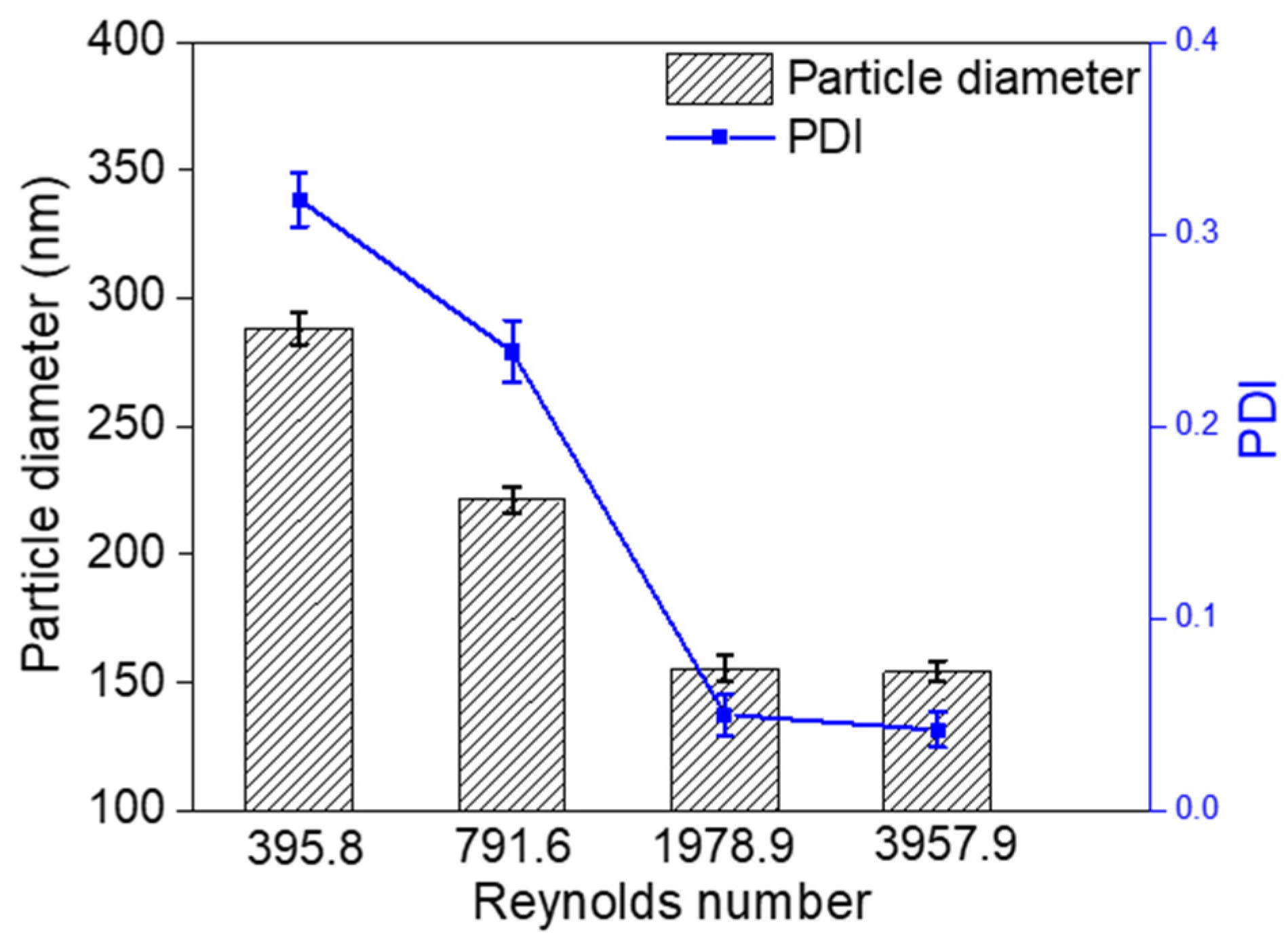
3.3. Effect of Reynolds Number on Particle Size
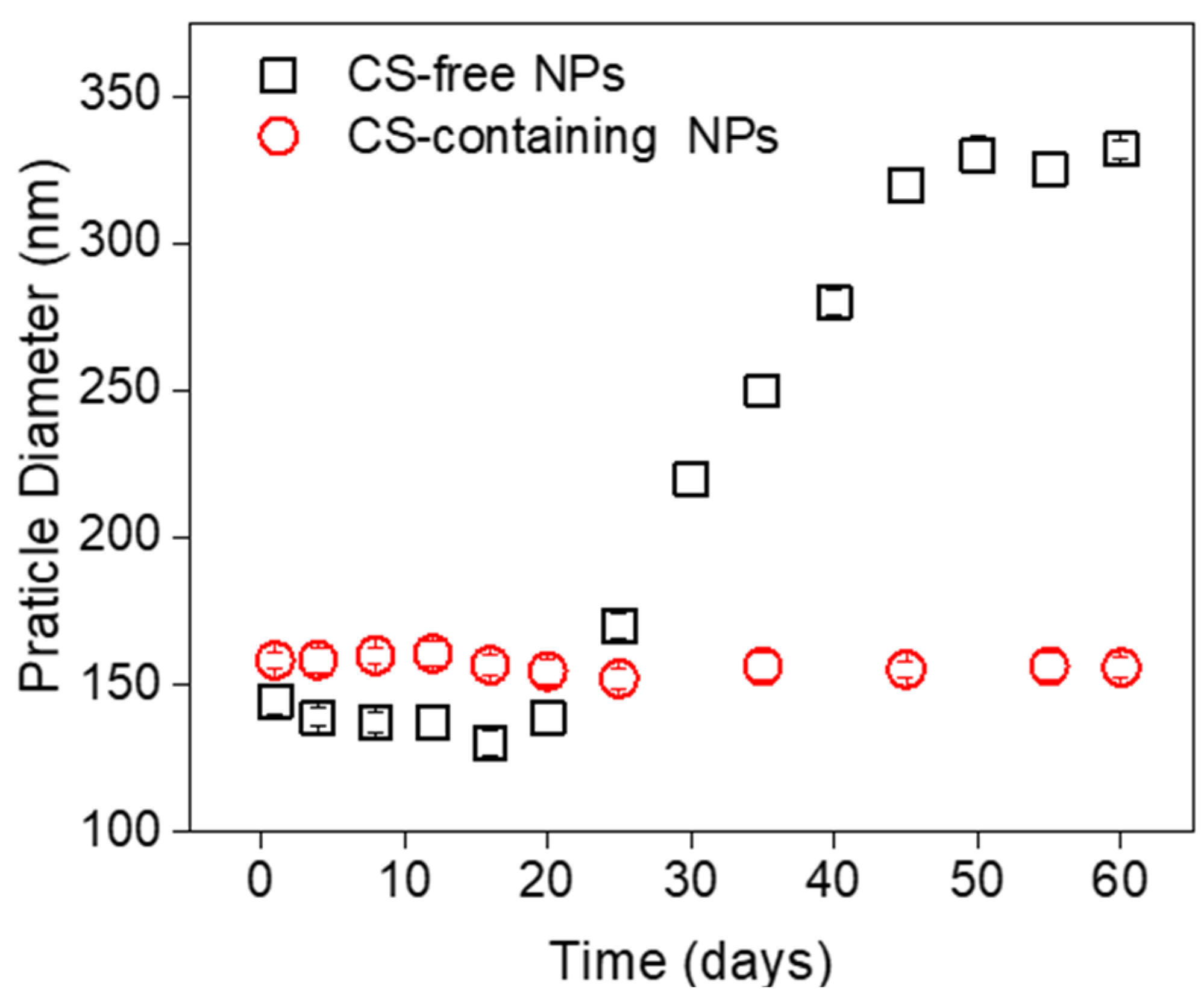
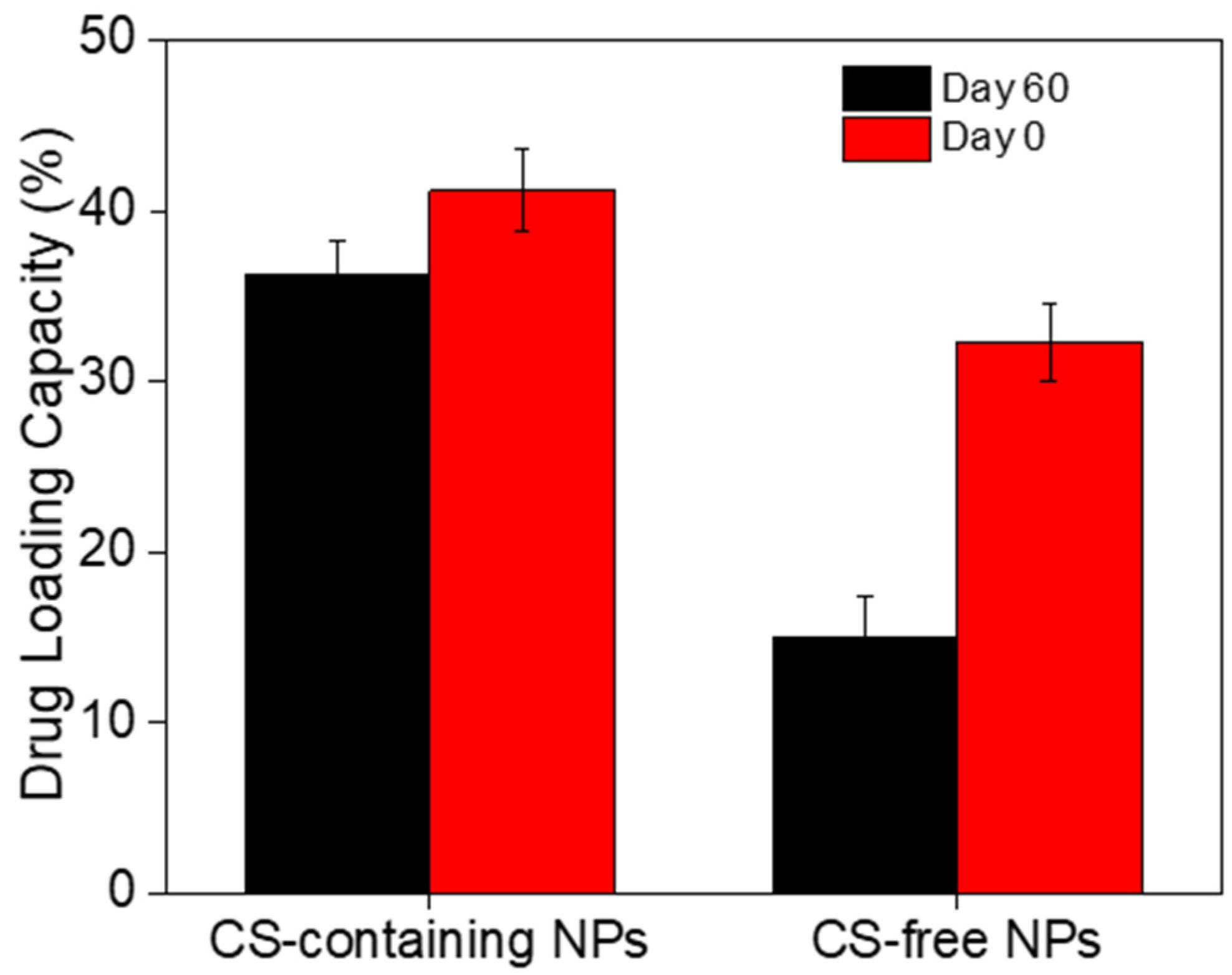
3.4. Stability of the CUR-loaded NPs
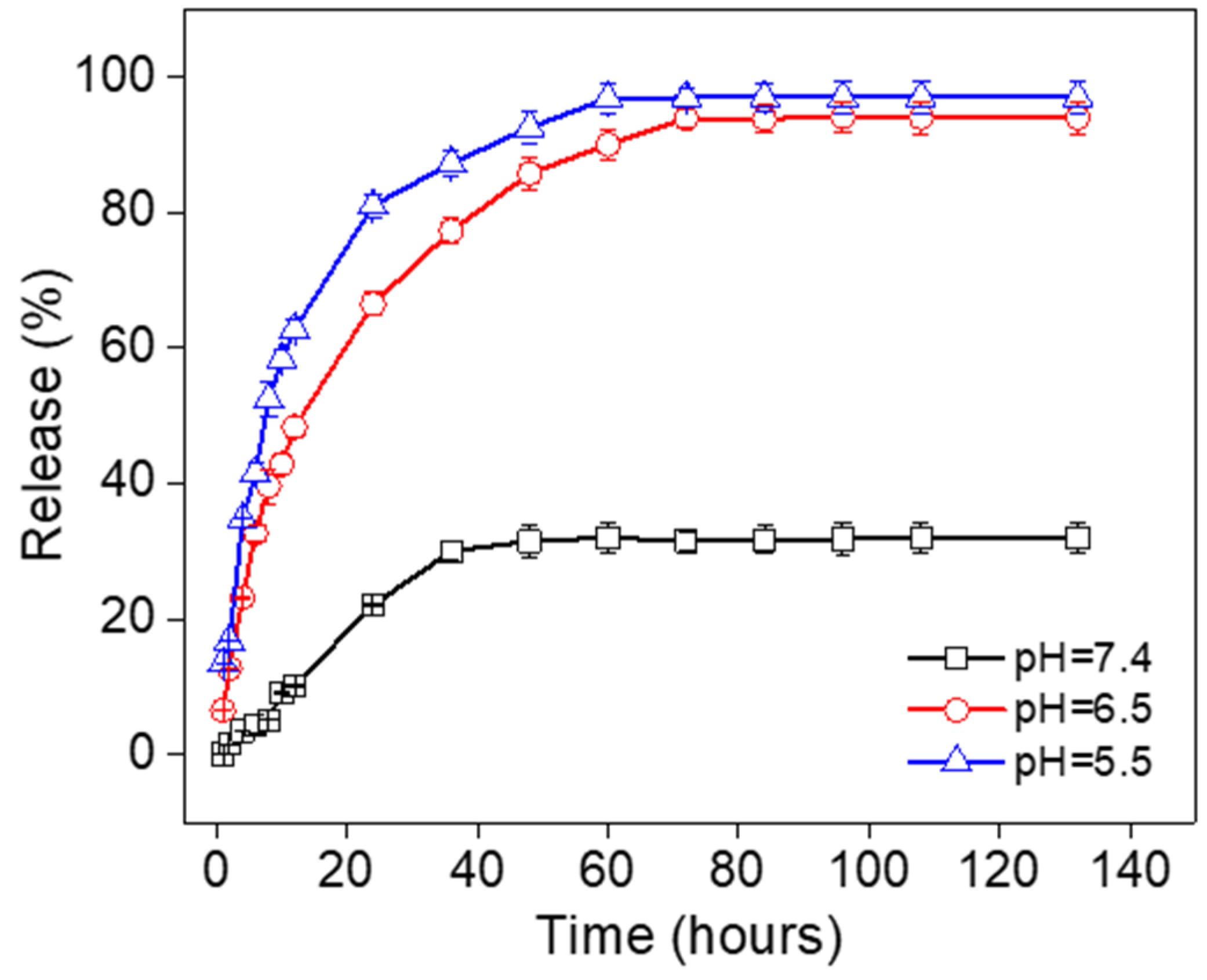
3.5. In Vitro Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Poor Aqueous Solubility—An Industry Wide Problem in Drug Discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. Ph-Responsive and Targeted Delivery of Curcumin Via Phenylboronic Acid-Functionalized Zno Nanoparticles for Breast Cancer Therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin Inhibits Proliferation, Invasion, Angiogenesis and Metastasis of Different Cancers through Interaction with Multiple Cell Signaling Proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The Story So Far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Chen, F.P.; Li, B.S.; Tang, C.H. Nanocomplexation between Curcumin and Soy Protein Isolate: Influence on Curcumin Stability/Bioaccessibility and in Vitro Protein Digestibility. J. Agric. Food Chem. 2015, 63, 3559–3569. [Google Scholar] [CrossRef]
- Iannazzo, D.; Ettari, R.; Giofre, S.; Eid, A.H.; Bitto, A. Recent Advances in Nanotherapeutics for Multiple Myeloma. Cancers 2020, 12, 3144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable Resveratrol-Cyclodextrin Complex Loaded Biodegradable Nanoparticles for Enhanced Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Maximenko, A.; Depciuch, J.; Lopuszynska, N.; Stec, M.; Swiatkowska-Warkocka, Z.; Bayev, V.; Zielinski, P.M.; Baran, J.; Fedotova, J.; Weglarz, W.P.; et al. Fe3o4@Sio2@Au Nanoparticles for Mri-Guided Chemo/Nir Photothermal Therapy of Cancer Cells. RSC Adv. 2020, 10, 26508–26520. [Google Scholar] [CrossRef]
- Depciuch, J.; Miszczyk, J.; Maximenko, A.; Zielinski, P.M.; Rawojc, K.; Panek, A.; Olko, P.; Parlinska-Wojtan, M. Gold Nanopeanuts as Prospective Support for Cisplatin in Glioblastoma Nano-Chemo-Radiotherapy. Int. J. Mol. Sci. 2020, 21, 9082. [Google Scholar] [CrossRef]
- Svechkarev, D.; Kyrychenko, A.; Payne, W.M.; Mohs, A.M. Probing the Self-Assembly Dynamics and Internal Structure of Amphiphilic Hyaluronic Acid Conjugates by Fluorescence Spectroscopy and Molecular Dynamics Simulations. Soft Matter 2018, 14, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Sun, X.; Gao, J.; Zhou, B. Chitosan Coordination Driven Self-Assembly for Effective Delivery of Curcumin. Int. J. Biol. Macromol. 2020, 165, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Prud’homme, R.K. Flash Nanoprecipitation of Organic Actives and Block Copolymers Using a Confined Impinging Jets Mixer. Aust. J. Chem. 2003, 56, 1021–1024. [Google Scholar] [CrossRef]
- Fu, Z.; Li, L.; Wang, Y.; Chen, Q.; Zhao, F.; Dai, L.; Chen, Z.; Liu, D.; Guo, X. Direct Preparation of Drug-Loaded Mesoporous Silica Nanoparticles by Sequential Flash Nanoprecipitation. Chem. Eng. J. 2020, 382, 122905. [Google Scholar] [CrossRef]
- Wan, K.Y.; Wong, S.N.; Wong, K.W.; Chow, S.F.; Chow, A.H.L. Interplay between Amphiphilic Stabilizers and Cholesterol in the Stabilization of Itraconazole Nanoparticles Prepared by Flash Nanoprecipitation. Mol. Pharm. 2019, 16, 195–204. [Google Scholar] [CrossRef]
- Harrison, A.; Vuong, T.T.; Zeevi, M.P.; Hittel, B.J.; Wi, S.; Tang, C. Rapid Self-Assembly of Metal/Polymer Nanocomposite Particles as Nanoreactors and Their Kinetic Characterization. Nanomaterials 2019, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Levit, S.L.; Yang, H.; Tang, C. Rapid Self-Assembly of Polymer Nanoparticles for Synergistic Codelivery of Paclitaxel and Lapatinib Via Flash Nanoprecipitation. Nanomaterials 2020, 10, 561. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, K.; Li, L.; Zhao, F.; Wang, Y.; Wang, M.; Shen, Y.; Cui, H.; Liu, D.; Guo, X. Spherical and Spindle-Like Abamectin-Loaded Nanoparticles by Flash Nanoprecipitation for Southern Root-Knot Nematode Control: Preparation and Characterization. Nanomaterials 2018, 8, 449. [Google Scholar] [CrossRef]
- Dou, J.; Zhao, F.; Fan, W.; Chen, Z.; Guo, X. Preparation of Non-Spherical Vaterite Caco3 Particles by Flash Nano Precipitation Technique for Targeted and Extended Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 57, 101768. [Google Scholar] [CrossRef]
- Zeng, Z.; Dong, C.; Zhao, P.; Liu, Z.; Liu, L.; Mao, H.-Q.; Leong, K.W.; Gao, X.; Chen, Y. Scalable Production of Therapeutic Protein Nanoparticles Using Flash Nanoprecipitation. Adv. Healthc. Mater. 2019, 8, 1801010. [Google Scholar] [CrossRef]
- Chow, S.F.; Wan, K.Y.; Cheng, K.K.; Wong, K.W.; Sun, C.C.; Baum, L.; Chow, A.H.L. Development of Highly Stabilized Curcumin Nanoparticles by Flash Nanoprecipitation and Lyophilization. Eur. J. Pharm. Biopharm. 2015, 94, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.L.; Baum, L. Highly Stabilized Curcumin Nanoparticles Tested in an in Vitro Blood-Brain Barrier Model and in Alzheimer’s Disease Tg2576 Mice. Aaps J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. Effects of Amphiphilic Diblock Copolymer on Drug Nanoparticle Formation and Stability. Biomaterials 2013, 34, 10238–10248. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, C.; Liu, Y.; Prud’homme, R.K.; Fox, R.O. Mixing in a Multi-Inlet Vortex Mixer (Mivm) for Flash Nano-Precipitation. Chem. Eng. Sci. 2008, 63, 2829–2842. [Google Scholar] [CrossRef]
- Borodko, Y.; Habas, S.E.; Koebel, M.; Yang, P.D.; Frei, H.; Somorjai, G.A. Probing the Interaction of Poly( Vinylpyrrolidone) with Platinum Nanocrystals by Uv-Raman and Ftir. J. Phys. Chem. B 2006, 110, 23052–23059. [Google Scholar] [CrossRef]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and Correlation of Drug Polymer Miscibility and Molecular Interactions by Various Approaches for the Preparation of Amorphous Solid Dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepagan, V.G.; Rani, V.V.D.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, Characterization, in Vitro Drug Release and Biological Studies of Curcumin Loaded Dextran Sulphate-Chitosan Nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; Mcclements, D.J. Core–Shell Biopolymer Nanoparticle Delivery Systems: Synthesis and Characterization of Curcumin Fortified Zein–Pectin Nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, C.; Francescangeli, O.; Tosi, G.; Muzzarelli, R.A.A. Susceptibility of Dibutyryl Chitin and Regenerated Chitin Fibres to Deacylation and Depolymerization by Lipases. Carbohydr. Polym. 2004, 56, 137–146. [Google Scholar] [CrossRef]
- Lau, C.; Mi, Y.L. A Study of Blending and Complexation of Poly(Acrylic Acid)/Poly(Vinyl Pyrrolidone). Polymer 2002, 43, 823–829. [Google Scholar] [CrossRef]
- Li, J.; Jiang, F.; Chi, Z.; Han, D.; Yu, L.; Liu, C. Development of Enteromorpha Prolifera Polysaccharide-Based Nanoparticles for Delivery of Curcumin to Cancer Cells. Int. J. Biol. Macromol. 2018, 112, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.; Saikia, P.M.; Dutta, R.K. Binding and Stabilization of Curcumin by Mixed Chitosan–Surfactant Systems: A Spectroscopic Study. J. Photochem. Photobiol. A Chem. 2012, 245, 18–27. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular Interactions, Characterization and Antimicrobial Activity of Curcumin–Chitosan Blend Films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Khezri, A.; Karimi, A.; Yazdian, F.; Jokar, M.; Mofradnia, S.R.; Rashedi, H.; Tavakoli, Z. Molecular Dynamic of Curcumin/Chitosan Interaction Using a Computational Molecular Approach: Emphasis on Biofilm Reduction. Int. J. Biol. Macromol. 2018, 114, 972–978. [Google Scholar] [CrossRef]
- Fu, Z.; Li, L.; Li, F.; Bhutto, R.A.; Niu, X.; Liu, D.; Guo, X. Facile Morphology Control During Rapid Fabrication of Nanosized Organosilica Particles. Ind. Eng. Chem. Res. 2020, 59, 14797–14805. [Google Scholar] [CrossRef]
- Chen, K.; Fu, Z.; Wang, M.; Lv, Y.; Wang, C.; Shen, Y.; Wang, Y.; Cui, H.; Guo, X. Preparation and Characterization of Size-Controlled Nanoparticles for High-Loading Lambda-Cyhalothrin Delivery through Flash Nanoprecipitation. J. Agric. Food Chem. 2018, 66, 8246–8252. [Google Scholar] [CrossRef]
- Fu, Z.N.; Li, L.; Wang, M.W.; Guo, X.H. Size Control of Drug Nanoparticles Stabilized by Mpeg-B-Pcl During Flash Nanoprecipitation. Colloid Polym. Sci. 2018, 296, 935–940. [Google Scholar] [CrossRef]
- Liu, Y.; Kathan, K.; Saad, W.; Prud’homme, R.K. Ostwald Ripening of Beta-Carotene Nanoparticles. Phys. Rev. Lett. 2007, 98, 036102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.G.; Jiao, W.-Q.; Yang, H.; Liu, J.; Liu, D. Phenylboronic Acid-Conjugated Chitosan Nanoparticles for High Loading and Efficient Delivery of Curcumin. Carbohydr. Polym. 2021, 256, 117497. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Fu, Z.; Li, L.; Ma, E.; Guo, X. A Cost-Effective Nano-Sized Curcumin Delivery System with High Drug Loading Capacity Prepared via Flash Nanoprecipitation. Nanomaterials 2021, 11, 734. https://doi.org/10.3390/nano11030734
Chen Z, Fu Z, Li L, Ma E, Guo X. A Cost-Effective Nano-Sized Curcumin Delivery System with High Drug Loading Capacity Prepared via Flash Nanoprecipitation. Nanomaterials. 2021; 11(3):734. https://doi.org/10.3390/nano11030734
Chicago/Turabian StyleChen, Zhuo, Zhinan Fu, Li Li, Enguang Ma, and Xuhong Guo. 2021. "A Cost-Effective Nano-Sized Curcumin Delivery System with High Drug Loading Capacity Prepared via Flash Nanoprecipitation" Nanomaterials 11, no. 3: 734. https://doi.org/10.3390/nano11030734
APA StyleChen, Z., Fu, Z., Li, L., Ma, E., & Guo, X. (2021). A Cost-Effective Nano-Sized Curcumin Delivery System with High Drug Loading Capacity Prepared via Flash Nanoprecipitation. Nanomaterials, 11(3), 734. https://doi.org/10.3390/nano11030734






