Real Time Observation of Lithium Insertion into Pre-Cycled Conversion-Type Materials
Abstract
1. Introduction
2. Materials and Methods
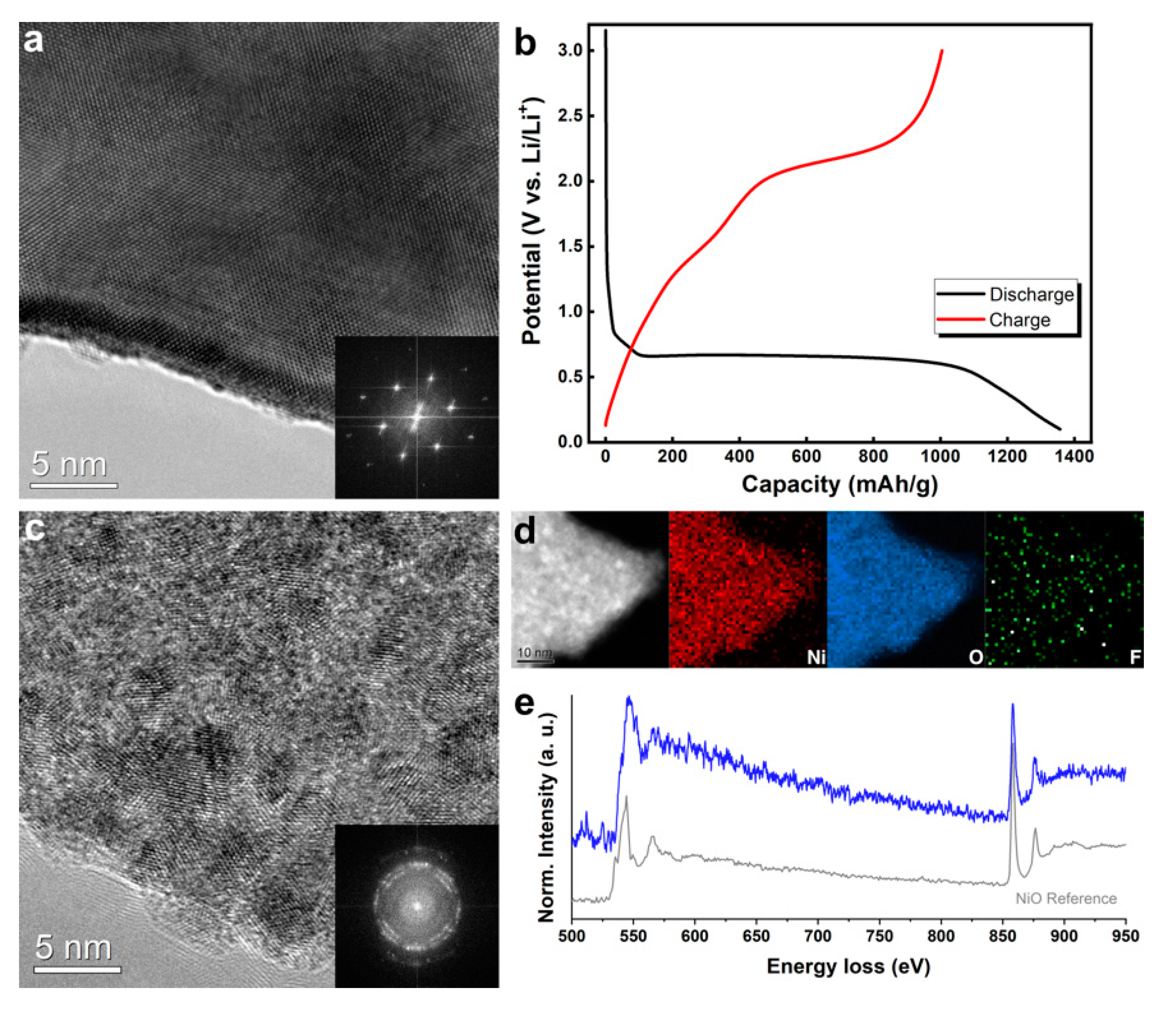
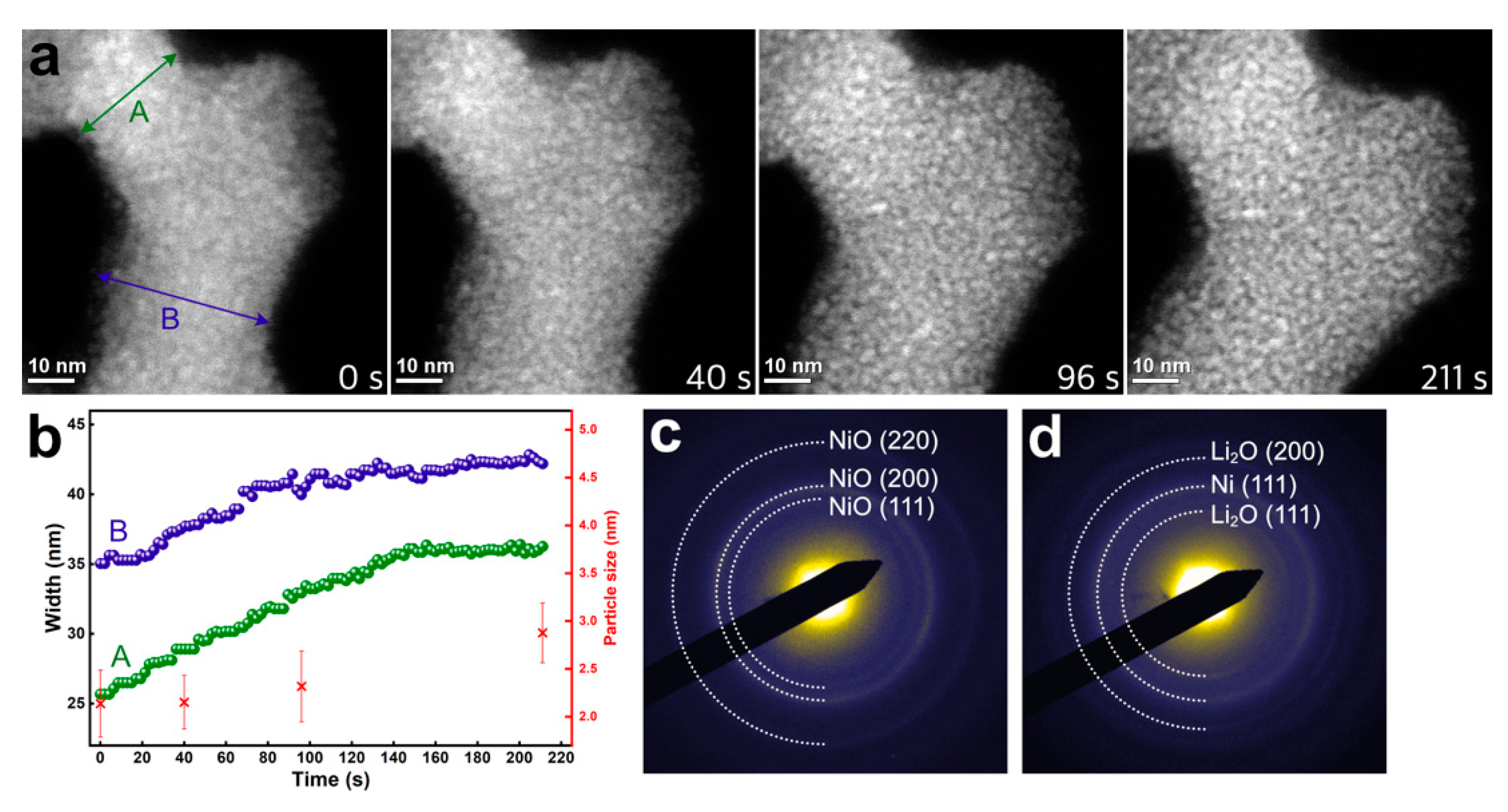
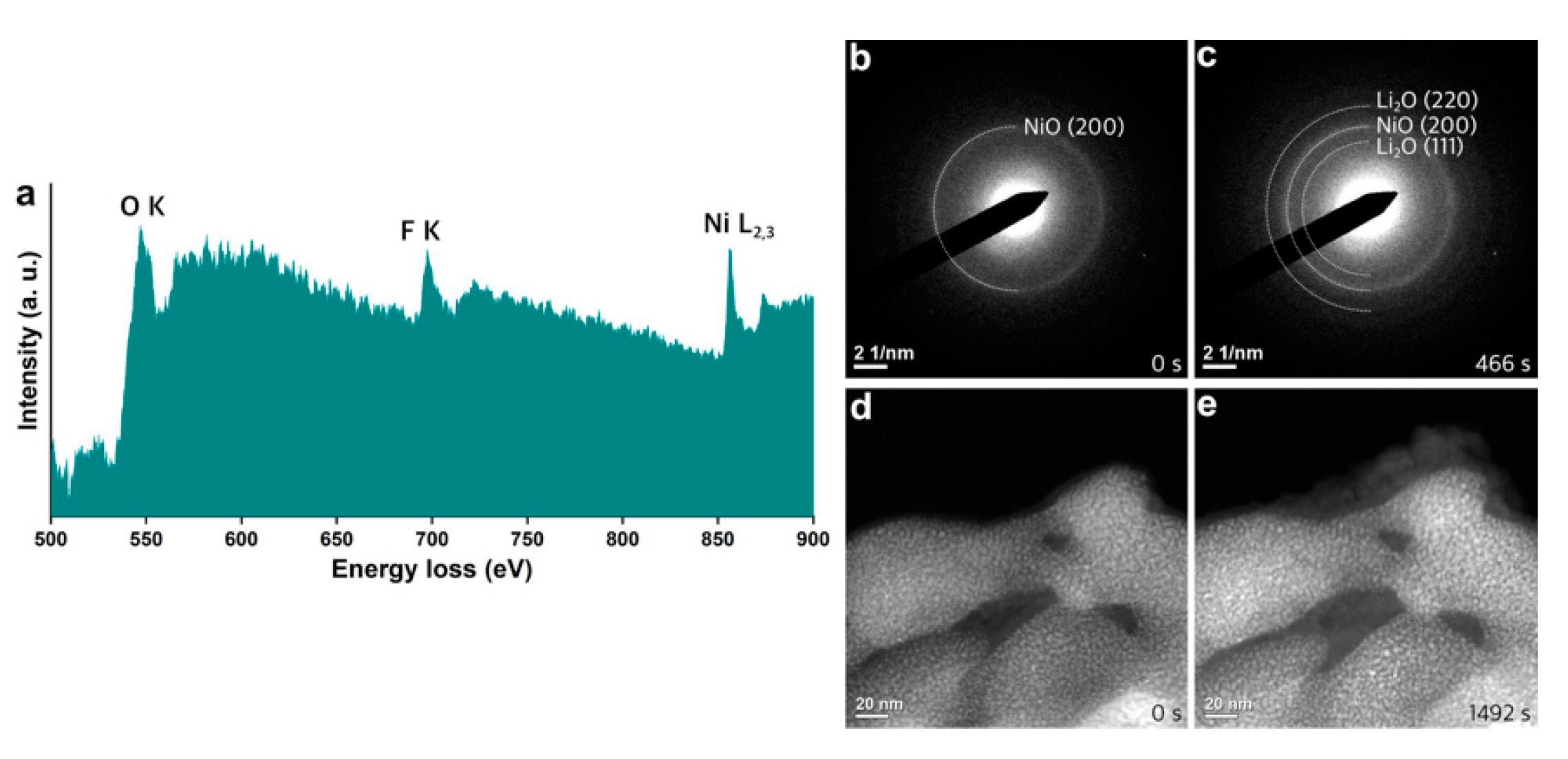
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-Sized Transition-Metal Oxides as Negative-Electrode Materials for Lithium-Ion Batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.-M. High Rate Capabilities Fe3O4-Based Cu Nano-Architectured Electrodes for Lithium-Ion Battery Applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef]
- Chae, B.-M.; Oh, E.-S.; Lee, Y.-K. Conversion Mechanisms of Cobalt Oxide Anode for Li-Ion Battery: In situ X-Ray Absorption Fine Structure Studies. J. Power Sources 2015, 274, 748–754. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.-F.; Yang, Y.; Sanchez Casalongue, H.; Robinson, J.T.; Liang, Y.; Cui, Y.; Dai, H. Mn3O4−Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef]
- Xiao, A.W.; Lee, H.J.; Capone, I.; Robertson, A.; Wi, T.-U.; Fawdon, J.; Wheeler, S.; Lee, H.-W.; Grobert, N.; Pasta, M. Understanding the Conversion Mechanism and Performance of Monodisperse Fef2 Nanocrystal Cathodes. Nat. Mater. 2020, 19, 644–654. [Google Scholar] [CrossRef]
- Ponrouch, A.; Taberna, P.-L.; Simon, P.; Palacín, M.R. On the Origin of the Extra Capacity at Low Potential in Materials for Li Batteries Reacting Through Conversion Reaction. Electrochim. Acta 2012, 61, 13–18. [Google Scholar] [CrossRef]
- Wang, F.; Robert, R.; Chernova, N.A.; Pereira, N.; Omenya, F.; Badway, F.; Hua, X.; Ruotolo, M.; Zhang, R.; Wu, L.; et al. Conversion Reaction Mechanisms in Lithium Ion Batteries: Study of the Binary Metal Fluoride Electrodes. J. Am. Chem. Soc. 2011, 133, 18828–18836. [Google Scholar] [CrossRef]
- He, K.; Xin, H.L.; Zhao, K.; Yu, X.; Nordlund, D.; Weng, T.-C.; Li, J.; Jiang, Y.; Cadigan, C.A.; Richards, R.M.; et al. Transitions from Near-Surface to Interior Redox upon Lithiation in Conversion Electrode Materials. Nano Lett. 2015, 15, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, S.; Li, J.; Yu, X.; Meng, Q.; Zhu, Y.; Hu, E.; Sun, K.; Yun, H.; Yang, X.-Q.; et al. Visualizing Non-Equilibrium Lithiation of Spinel Oxide Via in Situ Transmission Electron Microscopy. Nat. Commun. 2016, 7, 11441. [Google Scholar] [CrossRef]
- Su, Q.; Xie, D.; Zhang, J.; Du, G.; Xu, B. In Situ Transmission Electron Microscopy Observation of the Conversion Mechanism of Fe2O3/Graphene Anode during Lithiation–Delithiation Processes. ACS Nano 2013, 7, 9115–9121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Q.; Zhang, Y.; Peng, L.; Yu, G.; Marschilok, A.C.; Wu, L.; Su, D.; Takeuchi, K.J.; Takeuchi, E.S.; et al. Size-Dependent Kinetics During Non-Equilibrium Lithiation of Nano-Sized Zinc Ferrite. Nat. Commun. 2019, 10, 93. [Google Scholar] [CrossRef]
- Hwang, S.; Meng, Q.; Chen, P.-F.; Kisslinger, K.; Cen, J.; Orlov, A.; Zhu, Y.; Stach, E.A.; Chu, Y.-H.; Su, D. Strain Coupling of Conversion-type Fe3O4 Thin Films for Lithium Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 7813–7816. [Google Scholar] [CrossRef]
- Dupont, L.; Grugeon, S.; Laruelle, S.; Tarascon, J.-M. Structure, Texture and Reactivity Versus Lithium of Chromium-Based Oxides Films as Revealed by TEM Investigations. J. Power Sources 2007, 164, 839–848. [Google Scholar] [CrossRef]
- Débart, A.; Dupont, L.; Poizot, P.; Leriche, J.-B.; Tarascon, J.-M. A Transmission Electron Microscopy Study of the Reactivity Mechanism of Tailor-Made CuO Particles toward Lithium. J. Electrochem. Soc. 2001, 148, A1266–A1274. [Google Scholar] [CrossRef]
- Grugeon, S.; Laruelle, S.; Dupont, L.; Tarascon, J.-M. An Update on the Reactivity of Nanoparticles Co-based Compounds towards Li. Solid State Sci. 2003, 5, 895–904. [Google Scholar] [CrossRef]
- Débart, A.; Dupont, L.; Patrice, R.; Tarascon, J.-M. Reactivity of Transition Metal (Co, Ni, Cu) Sulphides versus Lithium: The Intriguing Case of the Copper Sulphide. Solid State Sci. 2006, 8, 640–651. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Wang, Q.; Wang, J.; Chen, L.; Gu, M. Fast Lithiation of NiO Investigated by in Situ Transmission Electron Microscopy. Appl. Phys. Lett. 2019, 115, 143902. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Harris, J.T.; Korgel, B.A.; Wang, C.; Nix, W.D.; Cui, Y. In Situ TEM of Two-Phase Lithiation of Amorphous Silicon Nanospheres. Nano Lett. 2013, 13, 758–764. [Google Scholar] [CrossRef]
- Boebinger, M.G.; Yeh, D.; Xu, M.; Miles, B.C.; Wang, B.; Papakyriakou, M.; Lewis, J.A.; Kondekar, N.P.; Cortes, F.J.Q.; Hwang, S.; et al. Avoiding Fracture in a Conversion Battery Material through Reaction with Larger Ions. Joule 2018, 2, 1783–1799. [Google Scholar] [CrossRef]
- Hwang, S.; Yao, Z.; Zhang, L.; Fu, M.; He, K.; Mai, L.; Wolverton, C.; Su, D. Multistep Lithiation of Tin Sulfide: An Investigation Using in SituElectron Microscopy. ACS Nano 2018, 12, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, K.; Meng, Q.; Li, X.; Zhu, Y.; Hwang, S.; Sun, K.; Gan, H.; Zhu, Y.; Mo, Y.; et al. Kinetic Phase Evolution of Spinel Cobalt Oxide during Lithiation. ACS Nano 2016, 10, 9577–9585. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Lin, F.; Zhu, Y.; Yu, X.; Li, J.; Lin, R.; Nordlund, D.; Weng, T.-C.; Richards, R.M.; Yang, X.-Q.; et al. Sodiation Kinetics of Metal Oxide Conversion Electrodes: A Comparative Study with Lithiation. Nano Lett. 2015, 15, 5755–5763. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Reddy, M.V.; Yanwu, Z.; Lit, C.S.; Hoong, T.C.; Subba Rao, G.V.; Chowdari, B.V.R.; Wee, A.T.S.; Lim, C.T.; Sow, C.-H. Fabrication of NiO Nanowall Electrodes for High Performance Lithium Ion Battery. Chem. Mater. 2008, 20, 3360–3367. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, B.; Zhang, C.Q.; Li, Y.; Yuan, Y.F.; Wu, H.M. Electrochemical Properties of NiO–Ni Nanocomposite as Anode Material for Lithium Ion Batteries. J. Power Sources 2006, 161, 541–544. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Su, D. Real Time Observation of Lithium Insertion into Pre-Cycled Conversion-Type Materials. Nanomaterials 2021, 11, 728. https://doi.org/10.3390/nano11030728
Hwang S, Su D. Real Time Observation of Lithium Insertion into Pre-Cycled Conversion-Type Materials. Nanomaterials. 2021; 11(3):728. https://doi.org/10.3390/nano11030728
Chicago/Turabian StyleHwang, Sooyeon, and Dong Su. 2021. "Real Time Observation of Lithium Insertion into Pre-Cycled Conversion-Type Materials" Nanomaterials 11, no. 3: 728. https://doi.org/10.3390/nano11030728
APA StyleHwang, S., & Su, D. (2021). Real Time Observation of Lithium Insertion into Pre-Cycled Conversion-Type Materials. Nanomaterials, 11(3), 728. https://doi.org/10.3390/nano11030728




