Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Geometric and Electronic Structures of Janus WSSe Bilayer
3.2. Redox Potential of the Photoexcited Carriers in Janus WSSe Bilayer
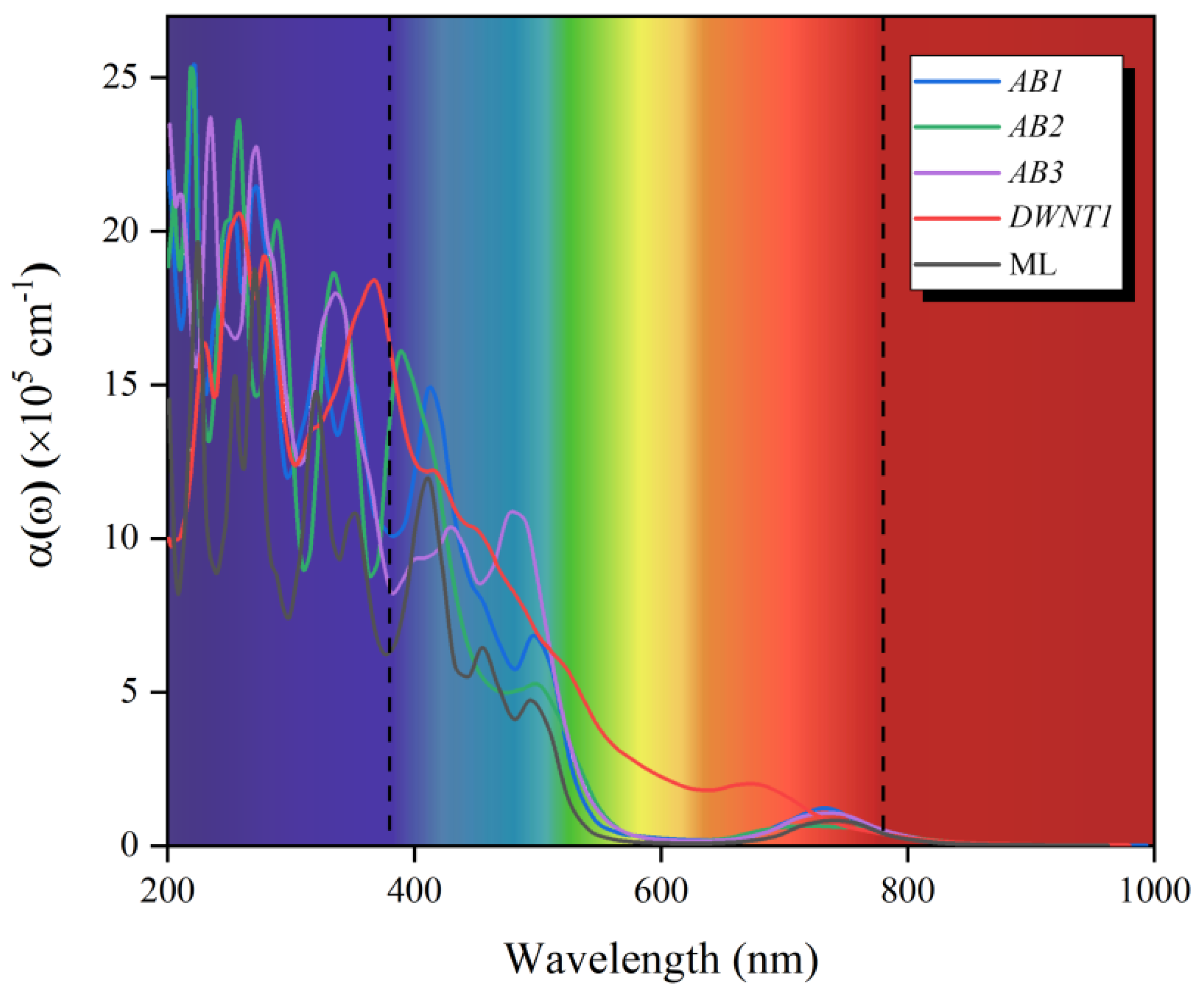
3.3. Optical Absorption and STH Efficiency of Janus WSSe Bilayer
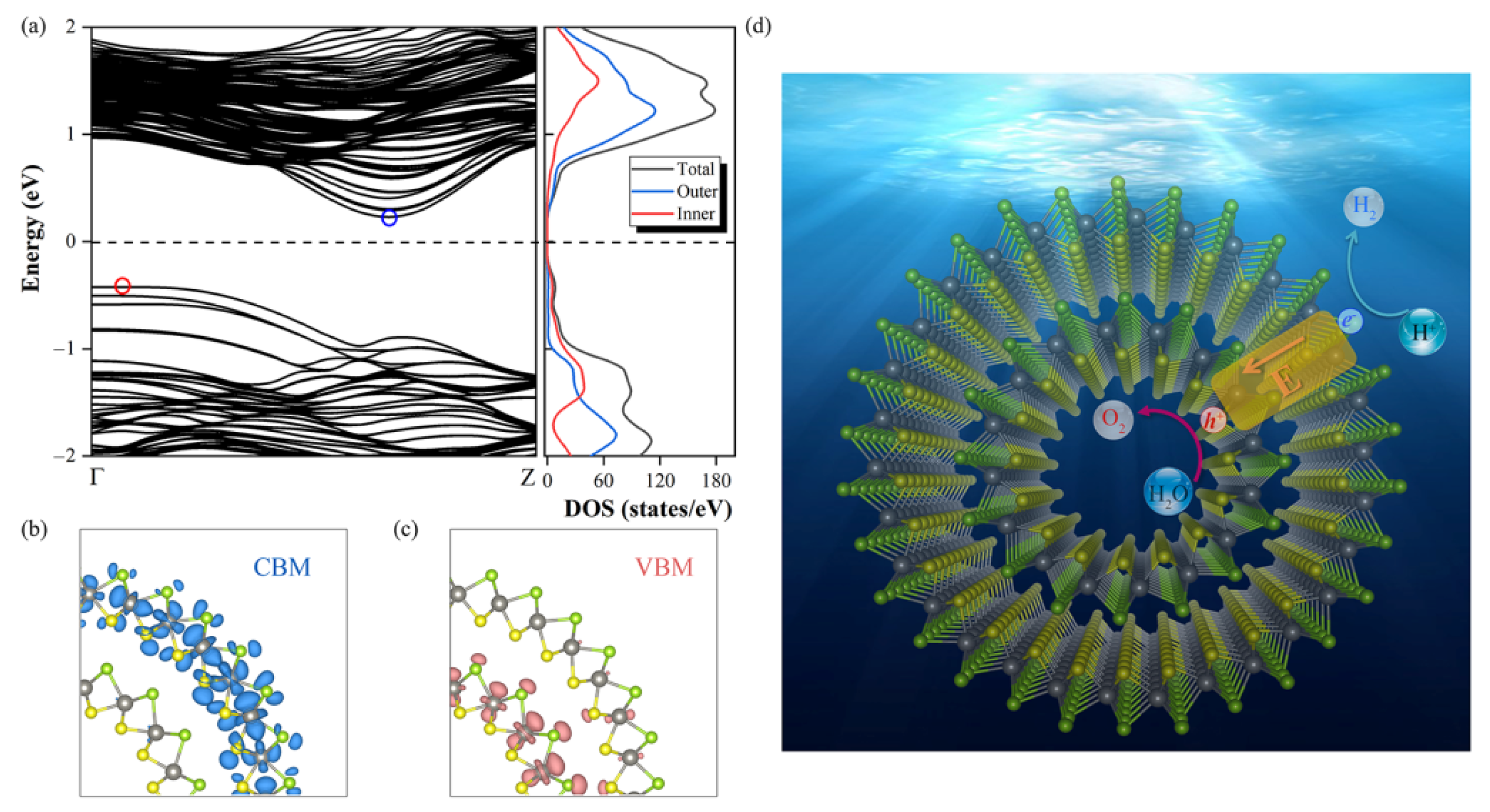
3.4. Geometric and Electronic Structures, and Photocatalytic Properties of Janus WSSe Double-Walled Nanotube
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Liu, E.; Jin, C.; Xu, C.; Fan, J.; Hu, X. Facile strategy to fabricate Ni2P/g-C3N4 heterojunction with excellent photocatalytic hydrogen evolution activity. Int. J. Hydrog. Energy 2018, 43, 21355–21364. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Zhao, P.; Ma, Y.; Lv, X.; Li, M.; Huang, B.; Dai, Y. Two-dimensional III2-VI3 materials: Promising photocatalysts for overall water splitting under infrared light spectrum. Nano Energy 2018, 51, 533–538. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, J. Proposed photosynthesis method for producing hydrogen from dissociated water molecules using incident near-infrared light. Phys. Rev. Lett. 2014, 112, 018301. [Google Scholar] [CrossRef]
- Yin, W.-J.; Wen, B.; Nie, G.-Z.; Wei, X.-L.; Liu, L.-M. Tunable dipole and carrier mobility for a few layer Janus MoSSe structure. J. Mater. Chem. C 2018, 6, 1693–1700. [Google Scholar] [CrossRef]
- Shang, C.; Xu, B.; Lei, X.; Yu, S.; Chen, D.; Wu, M.; Sun, B.; Liu, G.; Ouyang, C. Bandgap tuning in MoSSe bilayers: Synergistic effects of dipole moment and interlayer distance. Phys. Chem. Chem. Phys. 2018, 20, 20919–20926. [Google Scholar] [CrossRef]
- Peng, R.; Ma, Y.; Huang, B.; Dai, Y. Two-dimensional Janus PtSSe for photocatalytic water splitting under the visible or infrared light. J. Mater. Chem. A 2019, 7, 603–610. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Yu, G.; Shen, D.; Wang, Y.; Kan, E. Visible-Light-Absorption in Graphitic C3N4 Bilayer: Enhanced by Interlayer Coupling. J. Phys. Chem. Lett. 2012, 3, 3330–3334. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Liao, X.; Jin, H.; Wei, Y. Investigation of Stacking Effects of Bilayer MoSSe on Photocatalytic Water Splitting. J. Phys. Chem. C 2019, 123, 22570–22577. [Google Scholar] [CrossRef]
- Long, C.; Dai, Y.; Gong, Z.-R.; Jin, H. Robust type-II band alignment in Janus-MoSSe bilayer with extremely long carrier lifetime induced by the intrinsic electric field. Phys. Rev. B 2019, 99, 115316. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Tang, X.; Shang, J.; Kou, L. Janus WSSe Monolayer: An Excellent Photocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 29335–29343. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Xia, C.; Xiong, W.; Du, J.; Wang, T.; Peng, Y.; Li, J. Universality of electronic characteristics and photocatalyst applications in the two-dimensional Janus transition metal dichalcogenides. Phys. Rev. B 2018, 98, 165424. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Ye, H.; Yu, Z.; Liu, Y.; Su, B.; Xu, W. Structural and electronic properties of 2H phase Janus transition metal dichalcogenide bilayers. Superlattices Microst. 2019, 131, 8–14. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, C.; Yu, Y.; Zarkadoula, E.; Yoon, M.; Puretzky, A.A.; Liang, L.; Kong, X.; Gu, Y.; Strasser, A.; et al. Low Energy Implantation into Transition-Metal Dichalcogenide Monolayers to Form Janus Structures. ACS Nano 2020, 14, 3896–3906. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, H.; Long, C.; Wang, T.; Peng, R.; Huang, B.; Dai, Y. MoSSe nanotube: A promising photocatalyst with an extremely long carrier lifetime. J. Mater. Chem. A 2019, 7, 7885–7890. [Google Scholar] [CrossRef]
- Tang, Z.-K.; Wen, B.; Chen, M.; Liu, L.-M. Janus MoSSe Nanotubes: Tunable Band Gap and Excellent Optical Properties for Surface Photocatalysis. Adv. Theor. Simul. 2018, 1, 1800082. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Kovalenko, A.V.; Bandura, A.V. First-principles study on stability, structural and electronic properties of monolayers and nanotubes based on pure Mo(W)S(Se)2 and mixed (Janus) Mo(W)SSe dichalcogenides. Phys. E 2020, 115, 113681. [Google Scholar] [CrossRef]
- Ju, L.; Shang, J.; Tang, X.; Kou, L. Tunable Photocatalytic Water Splitting by the Ferroelectric Switch in a 2D AgBiP2Se6 Monolayer. J. Am. Chem. Soc. 2020, 142, 1492–1500. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Shang, J.; Tang, X.; Kou, L. Janus transition metal dichalcogenides: A superior platform for photocatalytic water splitting. J. Phys. Mater. 2020, 3, 022004. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Wang, H.; Wang, Y. A Janus MoSSe monolayer: A potential wide solar-spectrum water-splitting photocatalyst with a low carrier recombination rate. J. Mater. Chem. A 2018, 6, 2295–2301. [Google Scholar] [CrossRef]
- Qiao, M.; Liu, J.; Wang, Y.; Li, Y.; Chen, Z. PdSeO3 Monolayer: Promising Inorganic 2D Photocatalyst for Direct Overall Water Splitting Without Using Sacrificial Reagents and Cocatalysts. J. Am. Chem. Soc. 2018, 140, 12256–12262. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Fu, C.F.; Sun, J.; Luo, Q.; Li, X.; Hu, W.; Yang, J. Intrinsic Electric Fields in Two-dimensional Materials Boost the Solar-to-Hydrogen Efficiency for Photocatalytic Water Splitting. Nano Lett. 2018, 18, 6312–6317. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Y.; Zhang, S.; Jin, H.; Huang, B.; Dai, Y. GeSe@SnS: Stacked Janus structures for overall water splitting. J. Mater. Chem. A 2019, 7, 12060–12067. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, S.; Du, Y.; Hu, Z. The origin of the enhanced photocatalytic activity of carbon nitride nanotubes: A first-principles study. J. Mater. Chem. A 2017, 5, 4827–4834. [Google Scholar] [CrossRef]
- Ju, L.; Dai, Y.; Wei, W.; Li, M.; Liang, Y.; Huang, B. One-dimensional cadmium sulphide nanotubes for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2018, 20, 1904–1913. [Google Scholar] [CrossRef]
- Ju, L.; Dai, Y.; Wei, W.; Liang, Y.; Huang, B. Potential of one-dimensional blue phosphorene nanotubes as a water splitting photocatalyst. J. Mater. Chem. A 2018, 6, 21087–21097. [Google Scholar] [CrossRef]
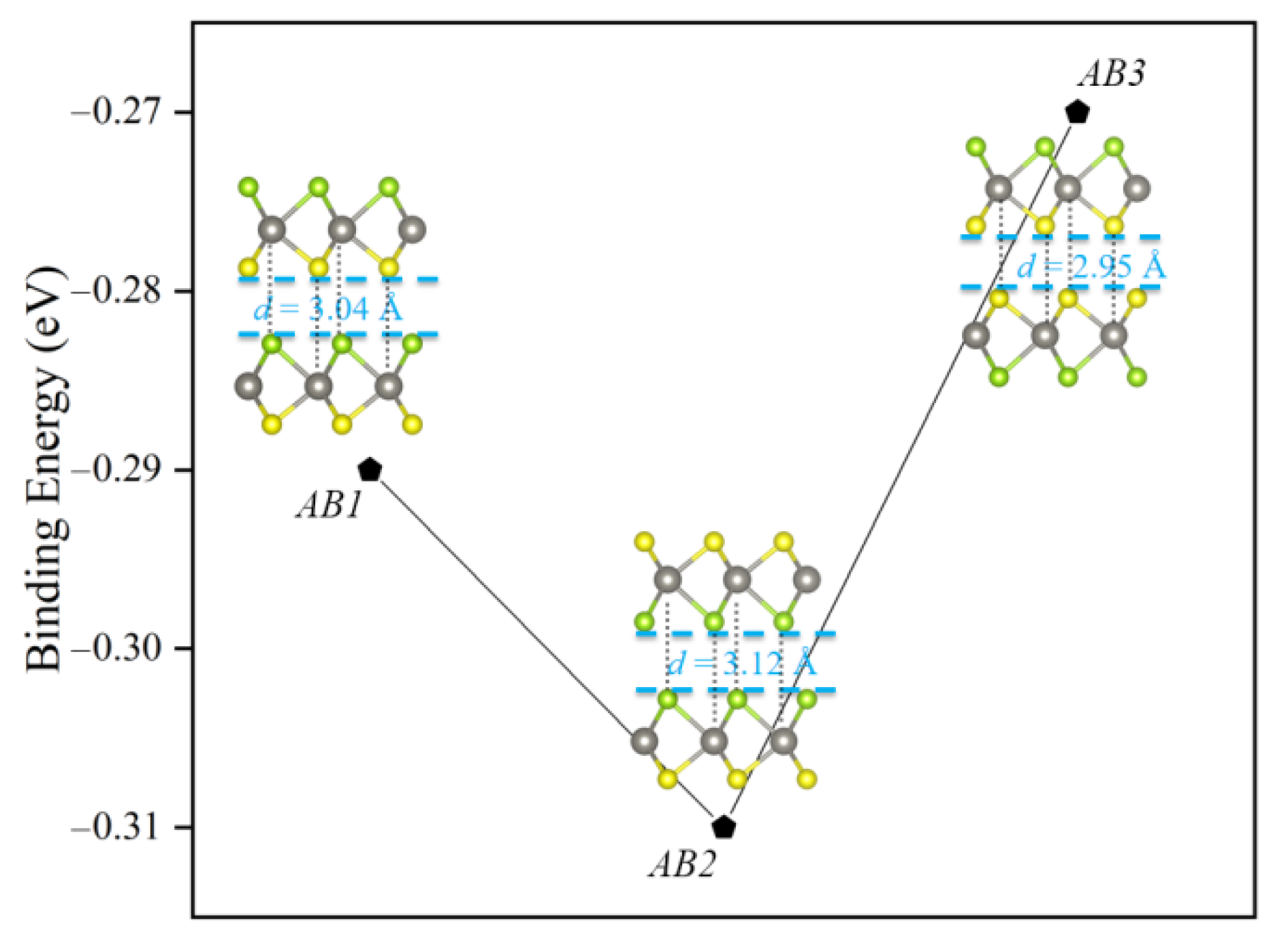
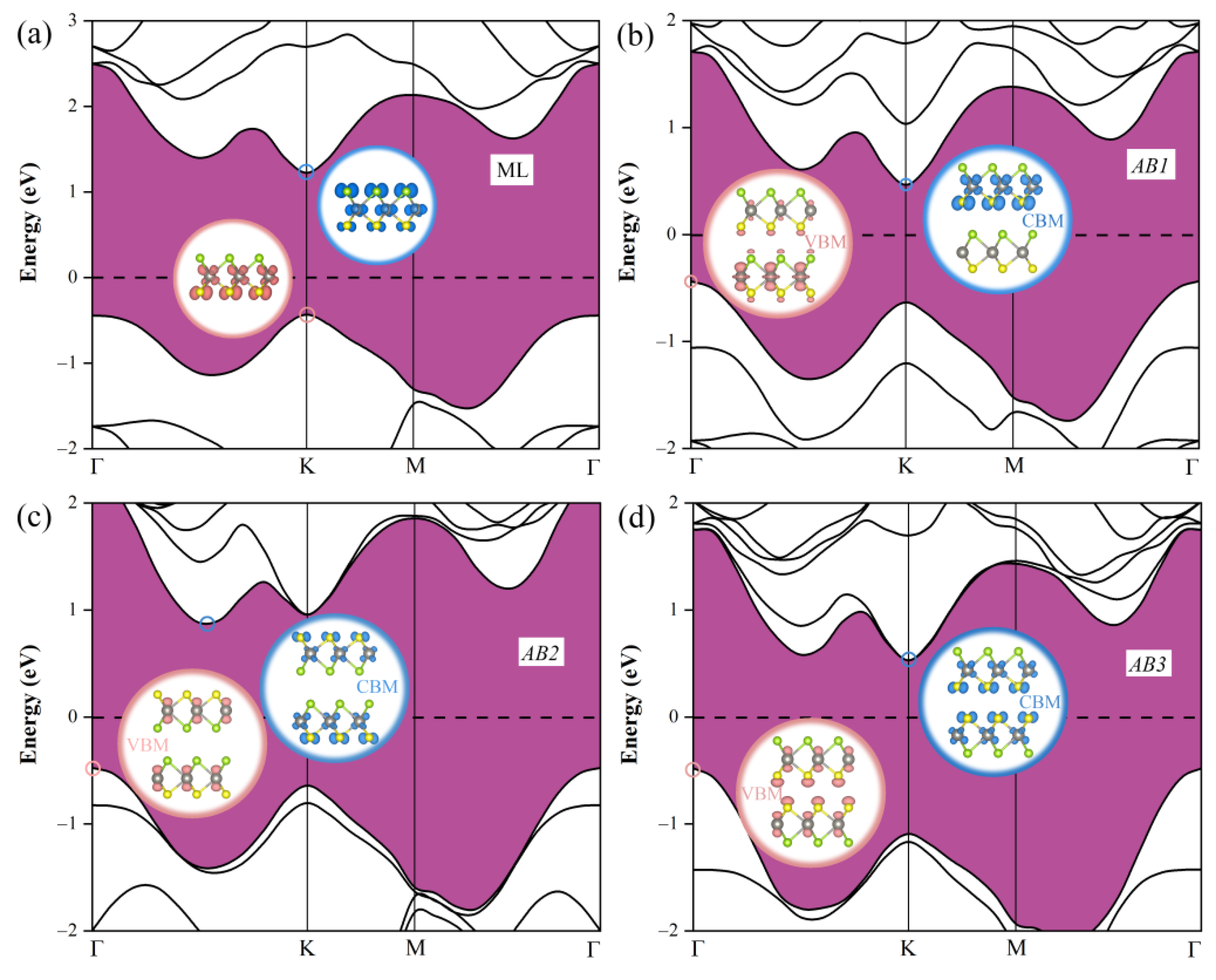


| Stacking Patterns | a = b (Å) | Eb (eV) | ΔΦ (eV) | Eg (eV) |
|---|---|---|---|---|
| AB1 | 3.253 | −0.29 | 1.37 | 0.90 |
| AB2 | 3.251 | −0.31 | 0 | 1.36 |
| AB3 | 3.254 | −0.27 | 0 | 1.01 |
| Configuration | ηabs (%) | ηcu (%) | ηSTH (%) | η’STH (%) |
|---|---|---|---|---|
| AB1 | 81.11 | 38.50 | 31.22 | 19.46 |
| AB2 | 55.59 | 20.38 | 11.33 | -- |
| AB3 | 54.69 | 10.01 | 5.47 | -- |
| DWNT1 | 76.38 | 64.44 | 49.22 | 28.48 |
| (15, 15) | 57.80 | 47.86 | 27.66 | 22.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.; Qin, J.; Shi, L.; Yang, G.; Zhang, J.; Sun, L. Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials 2021, 11, 705. https://doi.org/10.3390/nano11030705
Ju L, Qin J, Shi L, Yang G, Zhang J, Sun L. Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials. 2021; 11(3):705. https://doi.org/10.3390/nano11030705
Chicago/Turabian StyleJu, Lin, Jingzhou Qin, Liran Shi, Gui Yang, Jing Zhang, and Li Sun. 2021. "Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance" Nanomaterials 11, no. 3: 705. https://doi.org/10.3390/nano11030705
APA StyleJu, L., Qin, J., Shi, L., Yang, G., Zhang, J., & Sun, L. (2021). Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials, 11(3), 705. https://doi.org/10.3390/nano11030705






