SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap that Rapidly Eliminates SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
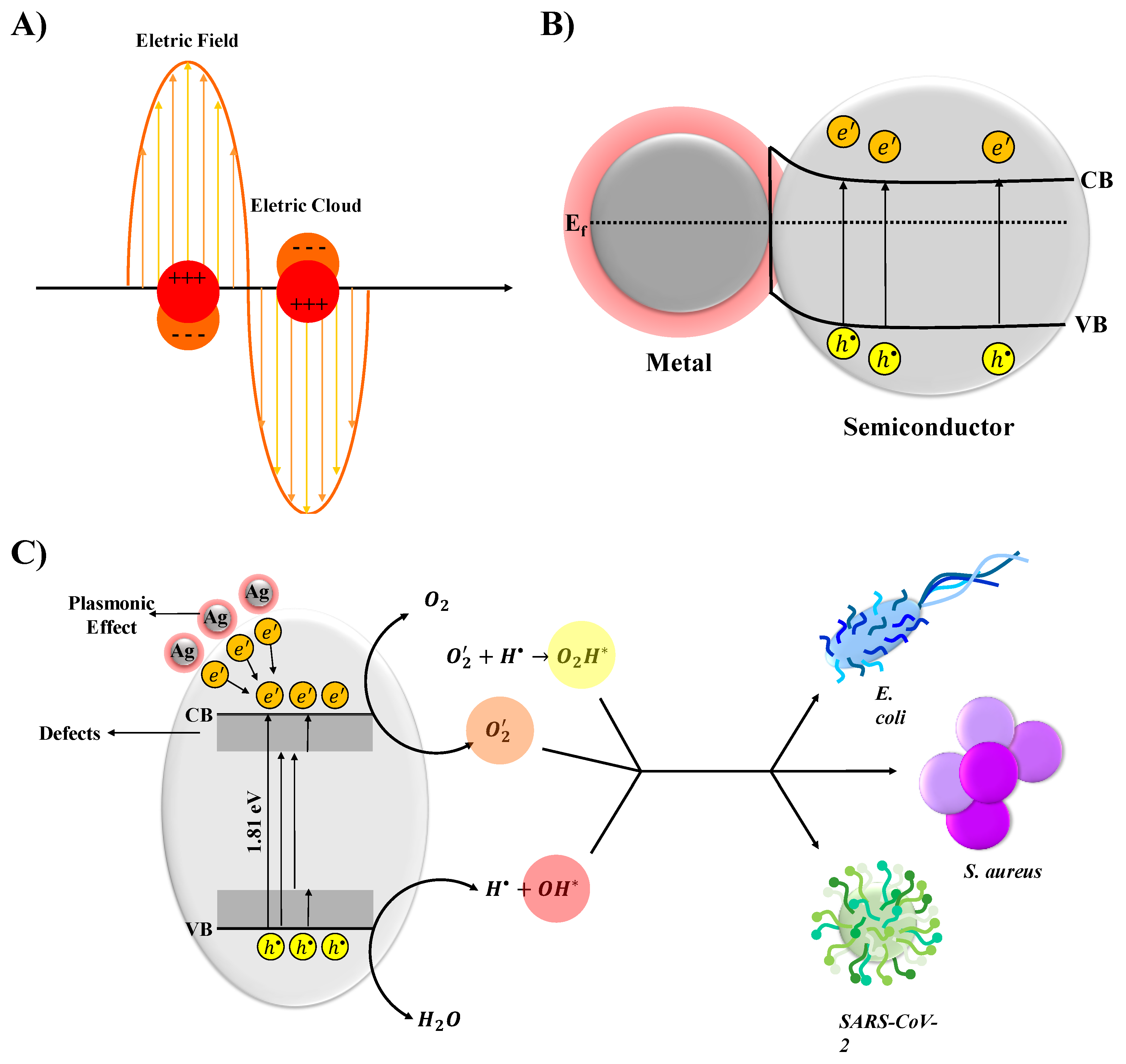
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Infection Prevention and Control during Health Care when Novel Coronavirus (nCoV) Infection is Suspected—Interim Guidance; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Rolain, J.-M.; Lagier, J.-C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; DiTaranto, N.; Cioffi, N. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef]
- Tharayil, A.; Rajakumari, R.; Chirayil, C.J.; Thomas, S.; Kalarikkal, N. A short review on nanotechnology interventions against COVID-19. Emergent Mater. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Luo, W.; Majumder, M.S.; Liu, D.; Poirier, C.; Mandl, K.D.; Lipsitch, M.; Santillana, M. The role of absolute humidity on transmission rates of the COVID-19 outbreak. medRxiv 2020. [Google Scholar] [CrossRef]
- Chin, A.; Chu, J.; Perera, M.; Hui, K.; Yen, H.-L.; Chan, M.; Peiris, M.; Poon, L. Stability of SARS-CoV-2 in different environmental conditions. medRxiv 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Lloyd-Smith, J.O.; De Wit, E.; Munster, V.J.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Maroufi, P.; Momen-Heravi, M.; Dao, S.; Köse, Ş.; Ganbarov, K.; Pagliano, P.; Esposito, S.; Kafil, H.S. Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez. Med. 2020, 28, 185–191. [Google Scholar] [PubMed]
- Derraik, J.G.B.; Anderson, W.A.; Connelly, E.A.; Anderson, Y.C. Rapid Review of SARS-CoV-1 and SARS-CoV-2 Viability, Susceptibility to Treatment, and the Disinfection and Reuse of PPE, Particularly Filtering Facepiece Respirators. Int. J. Environ. Res. Public Health 2020, 17, 6117. [Google Scholar] [CrossRef] [PubMed]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.L.M.; Ducker, W.A. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. CCOVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; MacLachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Mollazadeh-Bajestani, M.; Moztarzadeh, F.; Uludağ, H.; Hardy, J.G.; Mozafari, M. An overview of the use of biomaterials, nanotechnology, and stem cells for detection and treatment of COVID-19: Towards a framework to address future global pandemics. Emergent Mater. 2021, 1–16. [Google Scholar] [CrossRef]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Obj. 2020, 24, 100620. [Google Scholar] [CrossRef]
- Podder, S.; Halder, S.; Roychowdhury, A.; Das, D.; Ghosh, C.K. Superb hydroxyl radical-mediated biocidal effect induced antibacterial activity of tuned ZnO/chitosan type II heterostructure under dark. J. Nanoparticle Res. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Alkhouri, N.; Zein, N.N. Protease inhibitors: Silver bullets for chronic hepatitis C infection? Clevel. Clin. J. Med. 2012, 79, 213–222. [Google Scholar] [CrossRef][Green Version]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; De Conti, R.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Tremiliosi, G.C.; Simoes, L.G.P.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.B.; Durigon, E.L.; Machado, R.R.G.; Medina, D.S.; Ribeiro, L.K.; Rosa, I.L.V.; et al. Engineering polycotton fiber surfaces, with antimicrobial activity against S. aureus, E. Coli, C. albicans and SARS-CoV-2. Jpn. J. Med. Sci. 2020, 1, 47–58. [Google Scholar]
- Assis, M.; Cordoncillo, E.; Torres-Mendieta, R.; Beltrán-Mir, H.; Minguez-Vega, G.; Oliveira, R.; Leite, E.R.; Foggi, C.C.; Vergani, C.E.; Longo, E.; et al. Towards the scale-up of the formation of nanoparticles on α-Ag2WO4 with bactericidal properties by femtosecond laser irradiation. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.; Filho, F.C.G.; Pimentel, D.S.; Robeldo, T.; Gouveia, A.F.; Castro, T.F.D.; Fukushima, H.C.S.; De Foggi, C.C.; Da Costa, J.P.C.; Borra, R.C.; et al. Ag Nanoparticles/AgX (X=Cl, Br and I) Composites with Enhanced Photocatalytic Activity and Low Toxicological Effects. ChemistrySelect 2020, 5, 4655–4673. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Kafshdooz, L.; Razban, Z.; Tbrizi, A.D.; Rasoulpour, S.; Khalilov, R.; Kavetskyy, T.; Saghfi, S.; Nasibova, A.N.; Kaamyabi, S.; et al. An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnology 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as a Disinfectant. In Reviews of Environmental Contamination and Toxicology; Springer New York: New York, NY, USA, 2007; pp. 23–45. ISBN 978-0-387-69163-3. [Google Scholar]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Zhou, X.; Li, Z.; Zhu, H.; Du, F.; Yuan, X.; Yao, Q.; Xie, J. Embedding ultrasmall Ag nanoclusters in Luria-Bertani extract via light irradiation for enhanced antibacterial activity. Nano Res. 2020, 13, 203–208. [Google Scholar] [CrossRef]
- Monerris, M.; Broglia, M.F.; Yslas, E.I.; Barbero, C.A.; Rivarola, C.R. Highly effective antimicrobial nanocomposites based on hydrogel matrix and silver nanoparticles: Long-lasting bactericidal and bacteriostatic effects. Soft Matter 2019, 15, 8059–8066. [Google Scholar] [CrossRef]
- Luo, S.; Fan, L.; Yang, K.; Zhong, Z.; Wu, X.; Ren, T. In situ and controllable synthesis of Ag NPs in tannic acid-based hyperbranched waterborne polyurethanes to prepare antibacterial polyurethanes/Ag NPs composites. RSC Adv. 2018, 8, 36571–36578. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Asharani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.G.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. BioMetals 2008, 22, 235–242. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Galdiero, S.; Rai, M.; Gade, A.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, M.; Gaikwad, S.; Ingle, A. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [PubMed]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4114. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, Z.; Zhang, J.; Liang, C.; Dai, K. Ag SPR-promoted 2D porous g-C3N4/Ag2MoO4 composites for enhanced photocatalytic performance towards methylene blue degradation. Appl. Surf. Sci. 2018, 459, 271–280. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Gao, E.; Sun, S.; Zhang, L. Photocatalysis Coupled with Thermal Effect Induced by SPR on Ag-Loaded Bi2WO6 with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 25898–25903. [Google Scholar] [CrossRef]
- Mitsushio, M.; Miyashita, K.; Higo, M. Sensor properties and surface characterization of the metal-deposited SPR optical fiber sensors with Au, Ag, Cu, and Al. Sensors Actuators A Phys. 2006, 125, 296–303. [Google Scholar] [CrossRef]
- Vasa, P.; Lienau, C. Strong Light–Matter Interaction in Quantum Emitter/Metal Hybrid Nanostructures. ACS Photon. 2018, 5, 2–23. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.W.; Moon, S.Y.; Park, J.Y. The effect of hot electrons and surface plasmons on heterogeneous catalysis. J. Phys. Condens. Matter 2016, 28, 254002. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Wen, L.; Xu, R.; Obergfell, M.; Mi, Y.; Zhan, Z.; Nasori, N.; Demsar, J.; Lei, Y. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yi, S.-C.; Oh, S.-G. Preparation and antibacterial effects of Ag–SiO2 thin films by sol–gel method. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Tang, H.; Xiao, Y.; Qiu, S.; Li, S.; Cao, S. Hierarchically-structured SiO2-Ag@TiO2 hollow spheres with excellent photocatalytic activity and recyclability. J. Hazard. Mater. 2018, 354, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Yuan, Q.; Li, Y.; Tu, J.; Zhao, L.; Li, N.; Li, X. Synthesis of Fe3O4@SiO2–Ag magnetic nanocomposite based on small-sized and highly dispersed silver nanoparticles for catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2012, 383, 96–102. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New Application of Z-Scheme Ag3PO4/g-C3N4 Composite in Converting CO2 to Fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xuan, Y. Enhanced solar thermal conversion and thermal conduction of MWCNT-SiO2/Ag binary nanofluids. Appl. Energy 2018, 212, 809–819. [Google Scholar] [CrossRef]
- Gu, G.; Xu, J.; Wu, Y.; Chen, M.; Wu, L. Synthesis and antibacterial property of hollow SiO2/Ag nanocomposite spheres. J. Colloid Interface Sci. 2011, 359, 327–333. [Google Scholar] [CrossRef]
- Flores, J.; Torres, V.; Popa, M.; Crespo, D.; Calderon-Moreno, J. Preparation of core–shell nanospheres of silica–silver: SiO2@Ag. J. Non-Crystalline Solids 2008, 354, 5435–5439. [Google Scholar] [CrossRef]
- Lambert, S.; Cellier, C.; Grange, P.; Pirard, J.-P.; Heinrichs, B. Synthesis of Pd/SiO2, Ag/SiO2, and Cu/SiO2 cogelled xerogel catalysts: Study of metal dispersion and catalytic activity. J. Catal. 2004, 221, 335–346. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Jiao, Y.; Tian, Y.; Wang, Y.; Jiang, Z. Biomimetic Synthesis of TiO2–SiO2–Ag Nanocomposites with Enhanced Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2013, 5, 3824–3832. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Wu, L. Novel Method to Fabricate SiO2/Ag Composite Spheres and Their Catalytic, Surface-Enhanced Raman Scattering Properties. J. Phys. Chem. C 2007, 111, 11692–11698. [Google Scholar] [CrossRef]
- Abduraimova, A.; Molkenova, A.; Duisembekova, A.; Mulikova, T.; Kanayeva, D.; Atabaev, T. Cetyltrimethylammonium Bromide (CTAB)-Loaded SiO2–Ag Mesoporous Nanocomposite as an Efficient Antibacterial Agent. Nanomaterials 2021, 11, 477. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Fang, Y.; Zhu, Z. Boosting antibacterial activity with mesoporous silica nanoparticles supported silver nanoclusters. J. Colloid Interface Sci. 2019, 555, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Fullenkamp, D.E.; Rivera, J.G.; Gong, Y.-K.; Lau, K.A.; He, L.; Varshney, R.; Messersmith, P.B. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials 2012, 33, 3783–3791. [Google Scholar] [CrossRef]
- Otari, S.V.; Patil, R.M.; Waghmare, S.R.; Ghosh, S.J.; Pawar, S.H. A novel microbial synthesis of catalytically active Ag–alginate biohydrogel and its antimicrobial activity. Dalton Trans. 2013, 42, 9966–9975. [Google Scholar] [CrossRef]
- Rawat, K.A.; Majithiya, R.P.; Rohit, J.V.; Basu, H.; Singhal, R.K.; Kailasa, S.K. Mg2+ ion as a tuner for colorimetric sensing of glyphosate with improved sensitivity via the aggregation of 2-mercapto-5-nitrobenzimidazole capped silver nanoparticles. RSC Adv. 2016, 6, 47741–47752. [Google Scholar] [CrossRef]
- Hassanien, R.; Al-Hinai, M.; Al-Said, S.A.F.; Little, R.; Šiller, L.; Wright, N.G.; Houlton, A.; Horrocks, B.R. Preparation and Characterization of Conductive and Photoluminescent DNA-Templated Polyindole Nanowires. ACS Nano 2010, 4, 2149–2159. [Google Scholar] [CrossRef]
- Shoeb, M.; Mobin, M.; Rauf, M.A.; Owais, M.; Naqvi, A.H. In Vitro and in Vivo Antimicrobial Evaluation of Graphene–Polyindole (Gr@PIn) Nanocomposite against Methicillin-Resistant Staphylococcus aureus Pathogen. ACS Omega 2018, 3, 9431–9440. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xu, J.; Liu, X.; Hu, Q.; Huang, J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J. Mater. Chem. B 2013, 1, 3477–3485. [Google Scholar] [CrossRef]
- Liang, X.; Sun, M.; Li, L.; Qiao, R.; Chen, K.; Xiao, Q.; Xu, F. Preparation and antibacterial activities of polyaniline/Cu0.05Zn0.95O nanocomposites. Dalton Trans. 2012, 41, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 22196—Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- ISO. ISO 21702—Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Pe-tersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 2016; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Tinio, J.V.G.; Simfroso, K.T.; Peguit, A.D.M.V.; Candidato, R.T. Influence of OH−Ion Concentration on the Surface Morphology of ZnO-SiO2Nanostructure. J. Nanotechnol. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Santos, P.L.; Bonacin, J.A.; Passos, R.R.; Pocrifka, L.A. Rice Husk Reuse in the Preparation of SnO2/SiO2Nanocomposite. Mater. Res. 2015, 18, 639–643. [Google Scholar] [CrossRef]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- He, X.; Zhang, R.; Yang, C.; Rong, Y.; Huang, G. Study on orientation in EVA/Fe3O4 composite hot-melt adhesives. Int. J. Adhes. Adhes. 2013, 44, 9–14. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Soheilmoghaddam, M.; Pour, R.H.; Adelnia, H.; Mohamad, Z. Mechanical, thermal and flammability properties of ethylene-vinyl acetate (EVA)/sepiolite nanocomposites. Polym. Test. 2014, 37, 117–122. [Google Scholar] [CrossRef]
- Van Hoang, V. Molecular Dynamics Simulation of Amorphous SiO2Nanoparticles. J. Phys. Chem. B 2007, 111, 12649–12656. [Google Scholar] [CrossRef]
- Borowicz, P.; Taube, A.; Rzodkiewicz, W.; Latek, M.; Gierałtowska, S. Raman Spectra of High-κDielectric Layers Investigated with Micro-Raman Spectroscopy Comparison with Silicon Dioxide. Sci. World J. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Chernev, B.S.; Hirschl, C.; Eder, G.C. Non-Destructive Determination of Ethylene Vinyl Acetate Cross-Linking in Photovoltaic (PV) Modules by Raman Spectroscopy. Appl. Spectrosc. 2013, 67, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, Z.; Zhou, Y.; Lei, Z.; Liu, Y.; Feng, W.; Zhang, Z.; Chen, H. Solvent-free electrically conductive Ag/ethylene vinyl acetate (EVA) composites for paper-based printable electronics. RSC Adv. 2019, 9, 19501–19507. [Google Scholar] [CrossRef]
- Peike, C.; Kaltenbach, T.; Weiß, K.-A.; Koehl, M. Non-destructive degradation analysis of encapsulants in PV modules by Raman Spectroscopy. Sol. Energy Mater. Sol. Cells 2011, 95, 1686–1693. [Google Scholar] [CrossRef]
- Shimoyama, M.; Maeda, H.; Matsukawa, K.; Inoue, H.; Ninomiya, T.; Ozaki, Y. Discrimination of ethylene/vinyl acetate copolymers with different composition and prediction of the vinyl acetate content in the copolymers using Fourier-transform Raman spectroscopy and multivariate data analysis. Vib. Spectrosc. 1997, 14, 253–259. [Google Scholar] [CrossRef]
- Kuna, L.; Eder, G.C.; Leiner, C.; Peharz, G. Reducing shadowing losses with femtosecond-laser-written deflective optical elements in the bulk of EVA encapsulation. Prog. Photovoltaics Res. Appl. 2014, 23, 1120–1130. [Google Scholar] [CrossRef]
- Hirschl, C.; Biebl–Rydlo, M.; DeBiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater. Sol. Cells 2013, 116, 203–218. [Google Scholar] [CrossRef]
- Sakthisabarimoorthi, A.; Dhas, S.M.B.; Jose, M. Nonlinear optical properties of Ag@SiO2 core-shell nanoparticles investigated by continuous wave He-Ne laser. Mater. Chem. Phys. 2018, 212, 224–229. [Google Scholar] [CrossRef]
- Sun, D.-H.; Lu, P.; Zhang, J.-L.; Liu, Y.-L.; Ni, J.-Z. Synthesis of the Fe3O4@SiO2@SiO2-Tb(PABA)3 luminomagnetic microspheres. J. Nanosci. Nanotechnol. 2011, 11, 9774–9779. [Google Scholar] [CrossRef] [PubMed]
- Ramalla, I.; Gupta, R.K.; Bansal, K. Effect on superhydrophobic surfaces on electrical porcelain insulator, improved technique at polluted areas for longer life and reliability. Int. J. Eng. Technol. 2015, 4, 509–519. [Google Scholar] [CrossRef]
- Liang, Y.; Ouyang, J.; Wang, H.; Wang, W.; Chui, P.; Sun, K. Synthesis and characterization of core–shell structured SiO2@YVO4:Yb3+, Er3+ microspheres. Appl. Surf. Sci. 2012, 258, 3689–3694. [Google Scholar] [CrossRef]
- Siddiqui, M.R.H.; Adil, S.; Assal, M.; Ali, R.; Al-Warthan, A.A. Synthesis and Characterization of Silver Oxide and Silver Chloride Nanoparticles with High Thermal Stability. Asian J. Chem. 2013, 25, 3405–3409. [Google Scholar] [CrossRef]
- Oje, A.I.; Ogwu, A.; Mirzaeian, M.; Tsendzughul, N. Electrochemical energy storage of silver and silver oxide thin films in an aqueous NaCl electrolyte. J. Electroanal. Chem. 2018, 829, 59–68. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. DYNA 2019, 86, 198–205. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez, A.; Menargues, S. TGA/FTIR study of the catalytic pyrolysis of ethylene–vinyl acetate copolymers in the presence of MCM-41. Polym. Degrad. Stab. 2005, 89, 145–152. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez, A.; Menargues, S. TGA/FTIR study of the evolution of the gases evolved in the catalytic pyrolysis of ethylene-vinyl acetate copolymers. Comparison among different catalysts. Polym. Degrad. Stab. 2005, 89, 454–460. [Google Scholar] [CrossRef]
- Hoang, T.; Chinh, N.T.; Trang, N.T.T.; Hang, T.T.X.; Thanh, D.T.M.; Hung, D.V.; Ha, C.-S.; Aufray, M. Effects of maleic anhydride grafted ethylene/vinyl acetate copolymer (EVA) on the properties of EVA/silica nanocomposites. Macromol. Res. 2013, 21, 1210–1217. [Google Scholar] [CrossRef]
- Poljanšek, I.; Fabjan, E.; Burja, K.; Kukanja, D. Emulsion copolymerization of vinyl acetate-ethylene in high pressure reactor-characterization by inline FTIR spectroscopy. Prog. Org. Coat. 2013, 76, 1798–1804. [Google Scholar] [CrossRef]
- Iijima, M.; Omori, S.; Hirano, K.; Kamiya, H. Free-standing, roll-able, and transparent silicone polymer film prepared by using nanoparticles as cross-linking agents. Adv. Powder Technol. 2013, 24, 625–631. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, H.; Tian, K.; Li, R.; Xu, Z.; Jiao, J.; Cao, L.; Wu, Y. A combined interfacial and in-situ polymerization strategy to construct well-defined core-shell epoxy-containing SiO2-based microcapsules with high encapsulation loading, super thermal stability and nonpolar solvent tolerance. Int. J. Smart Nano Mater. 2016, 7, 221–235. [Google Scholar] [CrossRef][Green Version]
- Duquesne, S.; Jama, C.; Le Bras, M.; Delobel, R.; Recourt, P.; Gloaguen, J. Elaboration of EVA–nanoclay systems—characterization, thermal behaviour and fire performance. Compos. Sci. Technol. 2003, 63, 1141–1148. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Thomann, R.; Mülhaupt, R. Synthesis and thermal behaviour of layered silicate–EVA nanocomposites. Polymer 2001, 42, 4501–4507. [Google Scholar] [CrossRef]
- Shimoyama, M.; Hayano, S.; Matsukawa, K.; Inoue, H.; Ninomiya, T.; Ozaki, Y. Discrimination of ethylene/vinyl acetate copolymers with different composition and prediction of the content of vinyl acetate in the copolymers and their melting points by near-infrared spectroscopy and chemometrics. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1529–1537. [Google Scholar] [CrossRef]
- Watari, M.; Ozaki, Y. Calibration Models for the Vinyl Acetate Concentration in Ethylene-Vinyl Acetate Copolymers and its On-Line Monitoring by Near-Infrared Spectroscopy and Chemometrics: Use of Band Shifts Associated with Variations in the Vinyl Acetate Concentration to Improve the Models. Appl. Spectrosc. 2005, 59, 912–919. [Google Scholar] [CrossRef]
- Li, H.-Y.; Perret-Aebi, L.-E.; Théron, R.; Ballif, C.; Luo, Y.; Lange, R.F.M. Optical transmission as a fast and non-destructive tool for determination of ethylene-co-vinyl acetate curing state in photovoltaic modules. Prog. Photovoltaics Res. Appl. 2011, 21, 187–194. [Google Scholar] [CrossRef]
- Paul, K.K.; Ghosh, R.; Giri, P.K. Mechanism of strong visible light photocatalysis by Ag2O-nanoparticle-decorated monoclinic TiO2(B) porous nanorods. Nanotechnology 2016, 27, 315703. [Google Scholar] [CrossRef]
- Deng, A.; Zhu, Y. Synthesis of TiO2/SiO2/Ag/Ag2O and TiO2/Ag/Ag2O nanocomposite spheres with photocatalytic performance. Res. Chem. Intermed. 2018, 44, 4227–4243. [Google Scholar] [CrossRef]
- Shume, W.M.; Murthy, H.C.A.; Zereffa, E.A. A Review on Synthesis and Characterization of Ag2O Nanoparticles for Photocatalytic Applications. J. Chem. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Spreadborough, J.; Christian, J.W. High-temperature X-ray diffractometer. J. Sci. Instrum. 1959, 36, 116–118. [Google Scholar] [CrossRef]
- Hui, S.; Chaki, T.K.; Chattopadhyay, S. Effect of silica-based nanofillers on the properties of a low-density polyethylene/ethylene vinyl acetate copolymer based thermoplastic elastomer. J. Appl. Polym. Sci. 2008, 110, 825–836. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Paslaru, E.; Munteanu, B.S.; Dumitriu, R.P.; Lungu, M.; Vasile, C. Polyurethane biocompatible silver bionanocomposites for biomedical applications. J. Nanoparticle Res. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- ISO. ISO 4892-2:2013 Plastics—Methods of Exposure to Laboratory Light Sources—Part 2: Xenon-arc Lamps; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Da Silva, J.S.; Machado, T.R.; Martins, T.A.; Assis, M.; Foggi, C.C.; Macedo, N.G.; Beltrán-Mir, H.; Cordoncillo, E.; Andrés, J.; Longo, E. α-AgVO3 Decorated by Hydroxyapatite (Ca10(PO4)6(OH)2): Tuning Its Photoluminescence Emissions and Bactericidal Activity. Inorg. Chem. 2019, 58, 5900–5913. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.; Robeldo, T.; Foggi, C.C.; Kubo, A.M.; Mínguez-Vega, G.; Condoncillo, E.; Beltran-Mir, H.; Torres-Mendieta, R.; Andrés, J.; Oliva, M.; et al. Ag Nanoparticles/α-Ag2WO4 Composite Formed by Electron Beam and Femtosecond Irradiation as Potent Antifungal and Antitumor Agents. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Z.; Yang, Y.; Peng, J.; Zheng, T. Effects and mechanism of SiO2 on photocatalysis and super hydrophilicity of TiO2 films prepared by sol-gel method. Mater. Res. Express 2018, 6, 046409. [Google Scholar] [CrossRef]
- Yao, C.; Zhu, J. Synthesis, Characterization and Photocatalytic Activity of Au/SiO2@TiO2 Core-Shell Microspheres. J. Braz. Chem. Soc. 2020, 31, 589–596. [Google Scholar] [CrossRef]
- Salgado, B.C.B.; Valentini, A. Evaluation of the Photocatalytic Activity of SiO2@TiO2 Hybrid Spheres in the Degradation of Methylene Blue and Hydroxylation of Benzene: Kinetic and Mechanistic STUDY. Braz. J. Chem. Eng. 2019, 36, 1501–1518. [Google Scholar] [CrossRef]
- Hou, Y.-X.; Abdullah, H.; Kuo, D.-H.; Leu, S.-J.; Gultom, N.S.; Su, C.-H. A comparison study of SiO2 /nano metal oxide composite sphere for antibacterial application. Compos. Part B Eng. 2018, 133, 166–176. [Google Scholar] [CrossRef]
- Shen, J.-N.; Ruan, H.-M.; Wu, L.-G.; Gao, C.-J. Preparation and characterization of PES–SiO2 organic–inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Zhang, J.; Zhang, H.; Liu, J. Preparation and antibacterial property of SiO2–Ag/PES hybrid ultrafiltration membranes. Desalin. Water Treat. 2013, 51, 3584–3590. [Google Scholar] [CrossRef]
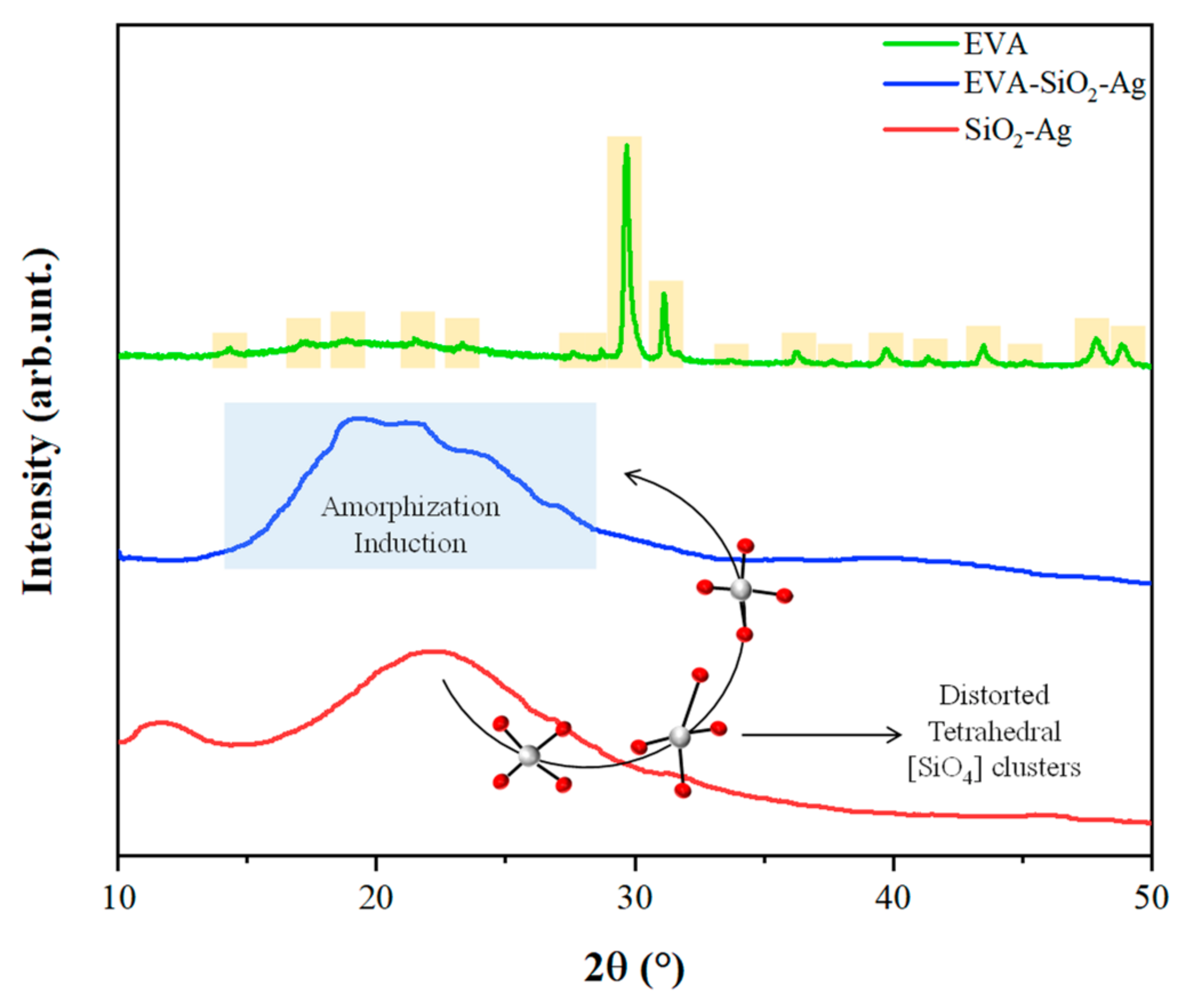
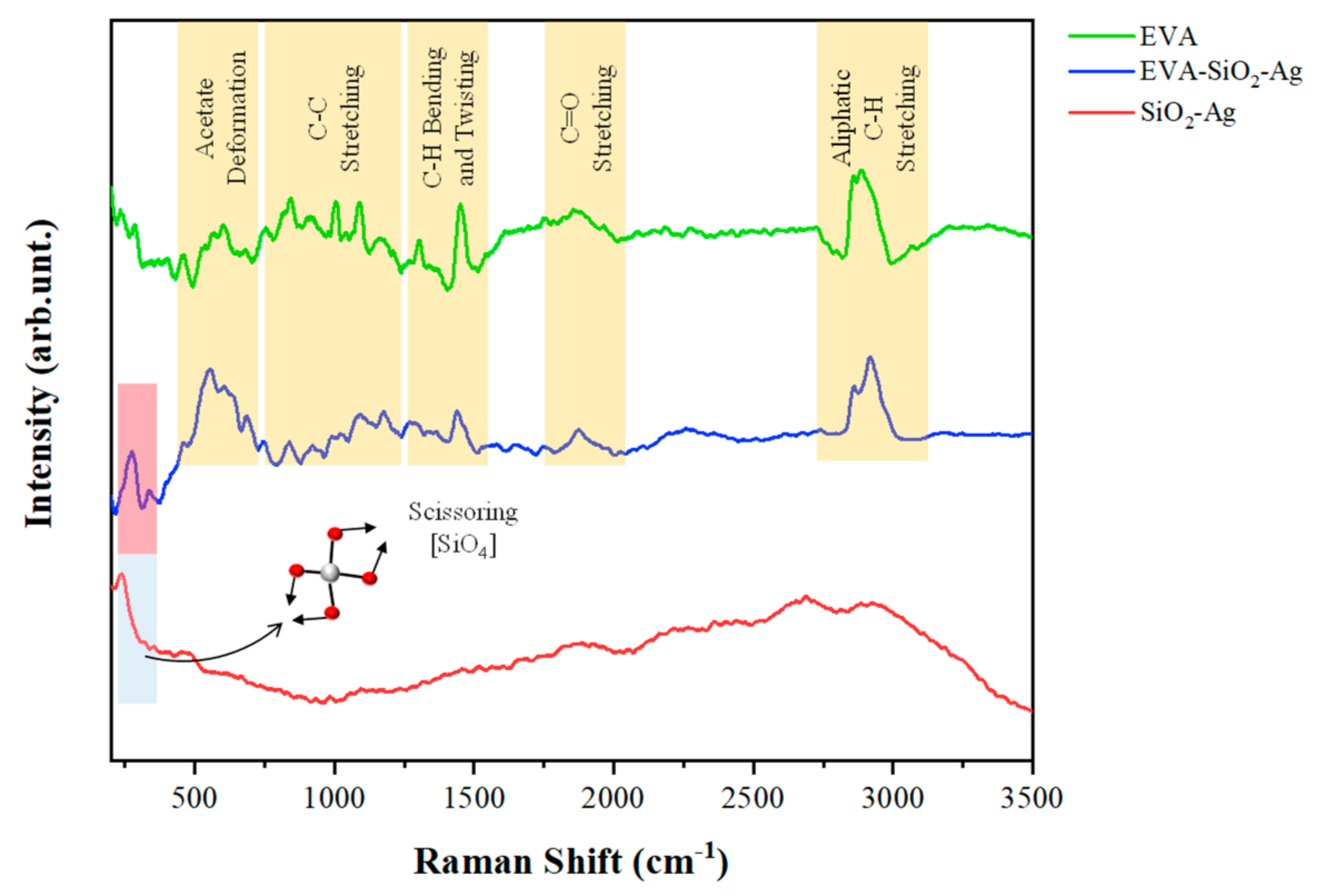
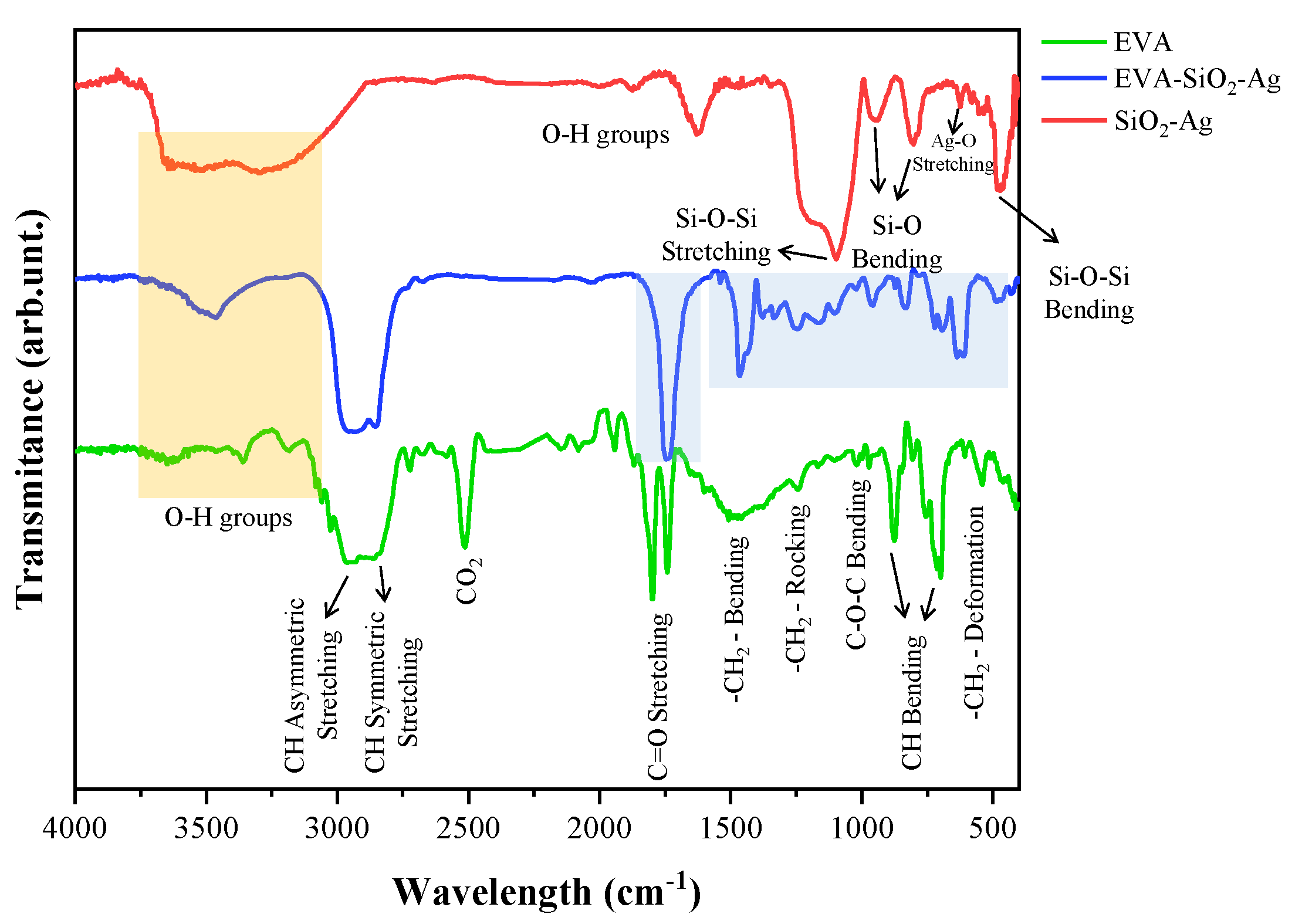
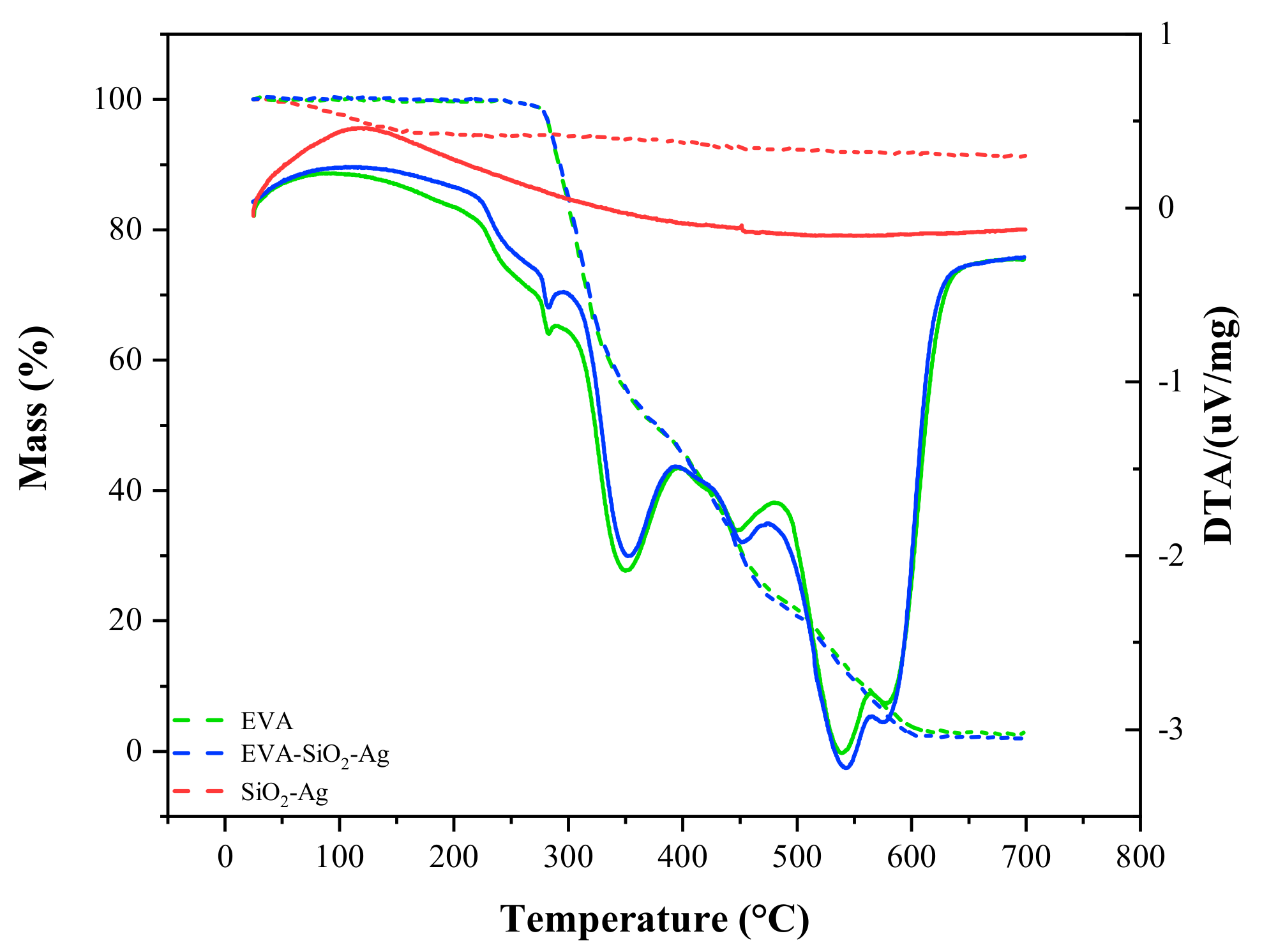
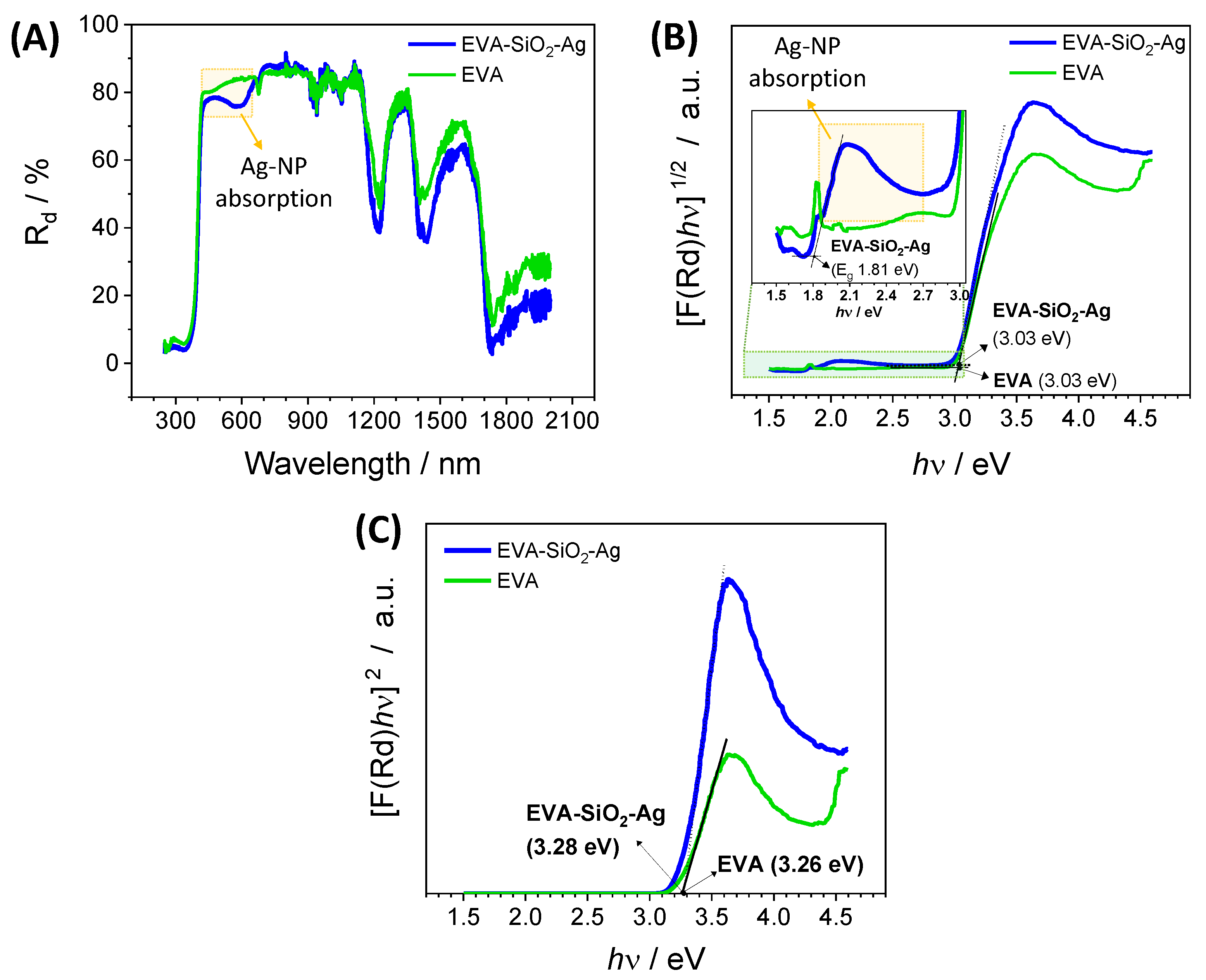
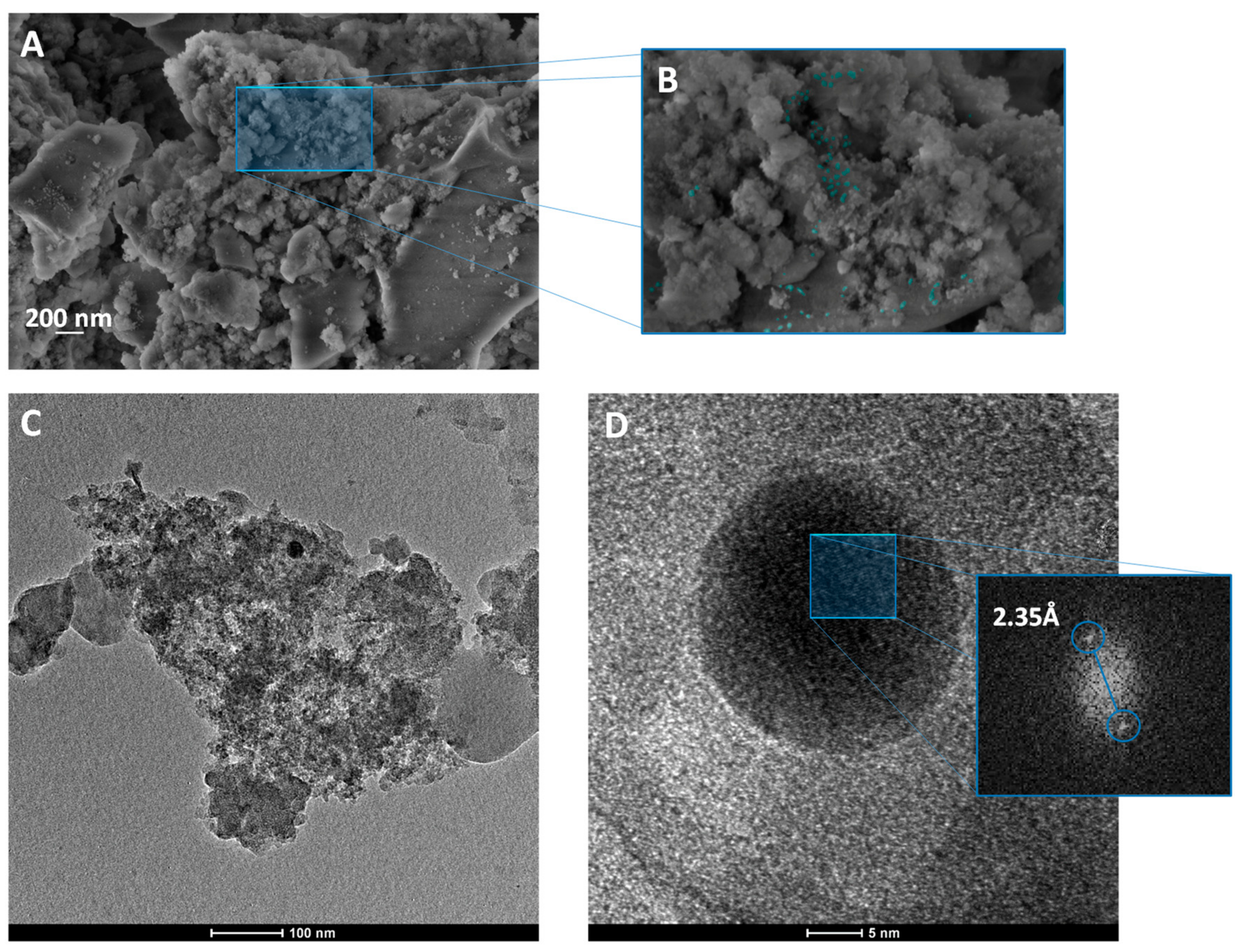
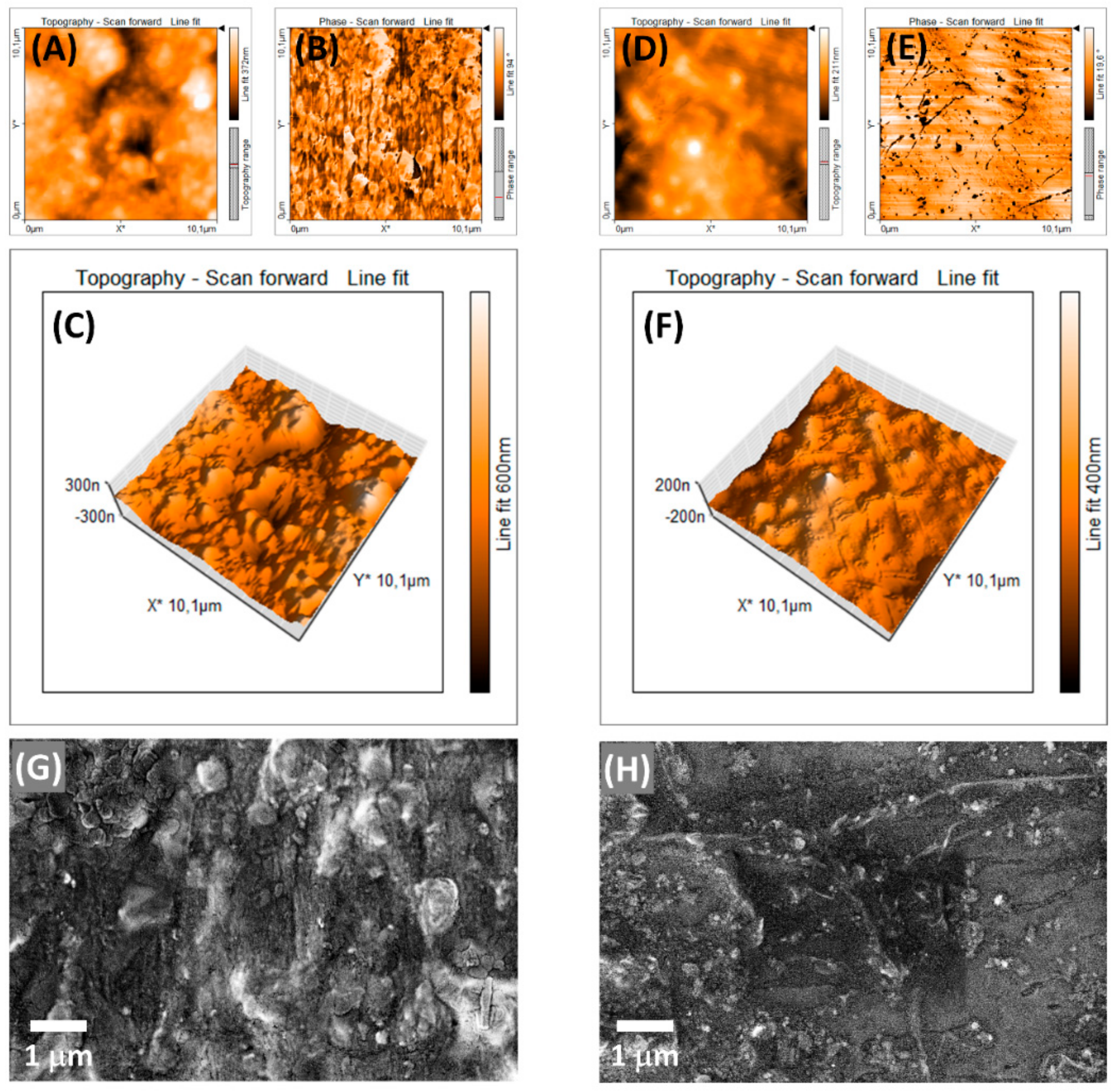
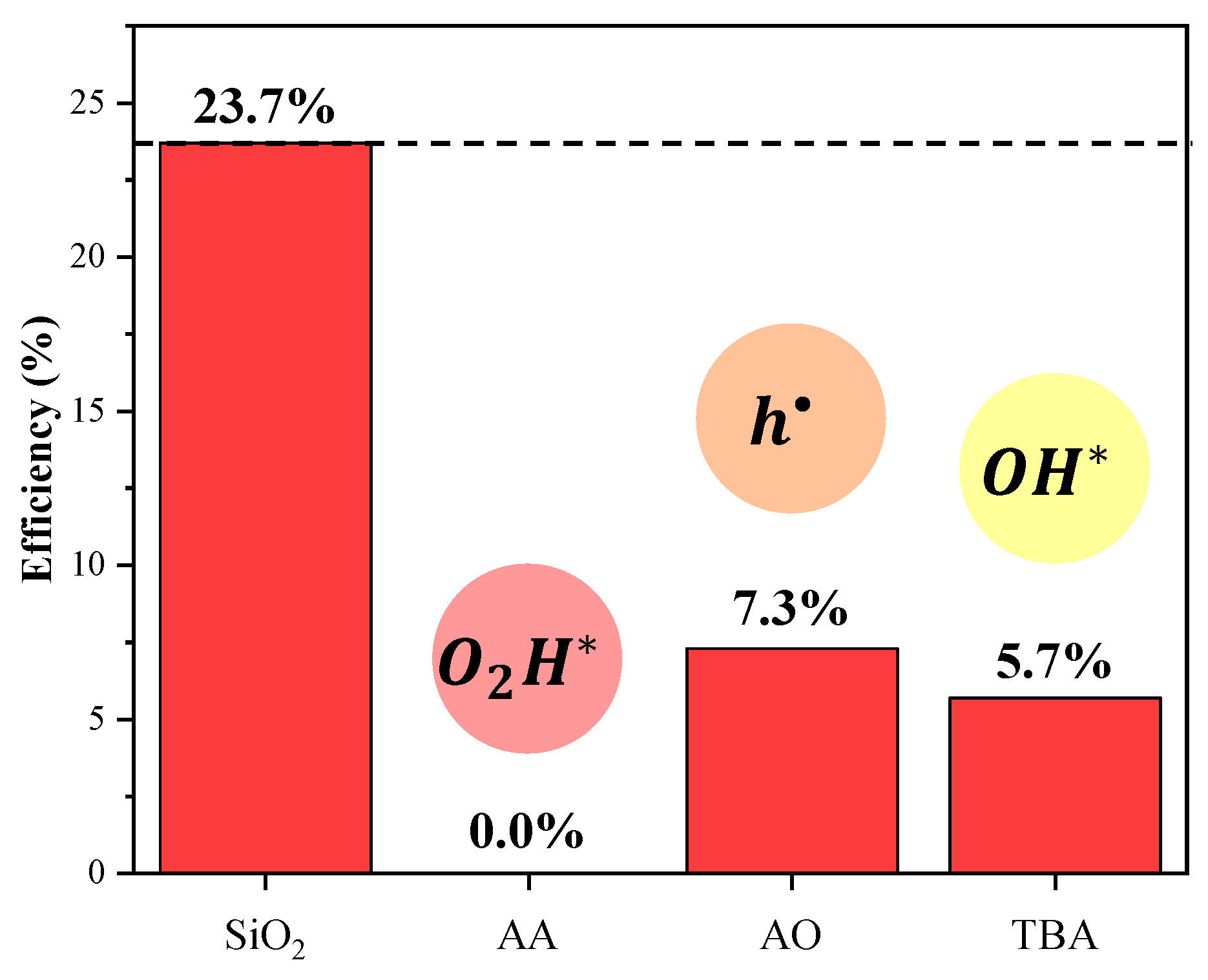

| EVA | Eva-SiO2-Ag | Reduction in Relation to Control | ||||
|---|---|---|---|---|---|---|
| CFU*/test piece (recovery) | Log10 of CFU*/test piece (recovery) | CFU*/test piece (recovery) | Log10 of CFU*/test piece (recovery) | Reduction in Log10 | Percentage reduction | |
| S. aureus | 5.53 × 105 | 5.74 | <1.0 × 10−1 | <1.0 | >4.74 | >99.99% |
| E. coli | 6.40 × 105 | 5.80 | <1.0 × 10−1 | <1.0 | >4.80 | >99.99% |
| Sample | Incubation Time | Day 1 | Day 2 | ||
|---|---|---|---|---|---|
| Copies/mL (SARS-CoV-2) | Viral Inactivation (%) | Copies/mL (SARS-CoV-2) | Viral Inactivation (%) | ||
| EVA | 2 min | 7.68 × 109 | − | 3.85 × 108 | − |
| EVA-SiO2-Ag | 2 min | 7.27 × 107 | 99.05 | 2.87 × 106 | 99.26 |
| EVA | 10 min | 2.21 × 109 | − | 5.21 × 108 | − |
| EVA-SiO2-Ag | 10 min | 3.28 × 106 | 99.85 | 1.98 × 106 | 99.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, M.; Simoes, L.G.P.; Tremiliosi, G.C.; Coelho, D.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.B.; Santos, J.R.d.; Ribeiro, L.K.; Rosa, I.L.V.; et al. SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap that Rapidly Eliminates SARS-CoV-2. Nanomaterials 2021, 11, 638. https://doi.org/10.3390/nano11030638
Assis M, Simoes LGP, Tremiliosi GC, Coelho D, Minozzi DT, Santos RI, Vilela DCB, Santos JRd, Ribeiro LK, Rosa ILV, et al. SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap that Rapidly Eliminates SARS-CoV-2. Nanomaterials. 2021; 11(3):638. https://doi.org/10.3390/nano11030638
Chicago/Turabian StyleAssis, Marcelo, Luiz Gustavo P. Simoes, Guilherme C. Tremiliosi, Dyovani Coelho, Daniel T. Minozzi, Renato I. Santos, Daiane C. B. Vilela, Jeziel Rodrigues do Santos, Lara Kelly Ribeiro, Ieda Lucia Viana Rosa, and et al. 2021. "SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap that Rapidly Eliminates SARS-CoV-2" Nanomaterials 11, no. 3: 638. https://doi.org/10.3390/nano11030638
APA StyleAssis, M., Simoes, L. G. P., Tremiliosi, G. C., Coelho, D., Minozzi, D. T., Santos, R. I., Vilela, D. C. B., Santos, J. R. d., Ribeiro, L. K., Rosa, I. L. V., Mascaro, L. H., Andrés, J., & Longo, E. (2021). SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap that Rapidly Eliminates SARS-CoV-2. Nanomaterials, 11(3), 638. https://doi.org/10.3390/nano11030638











