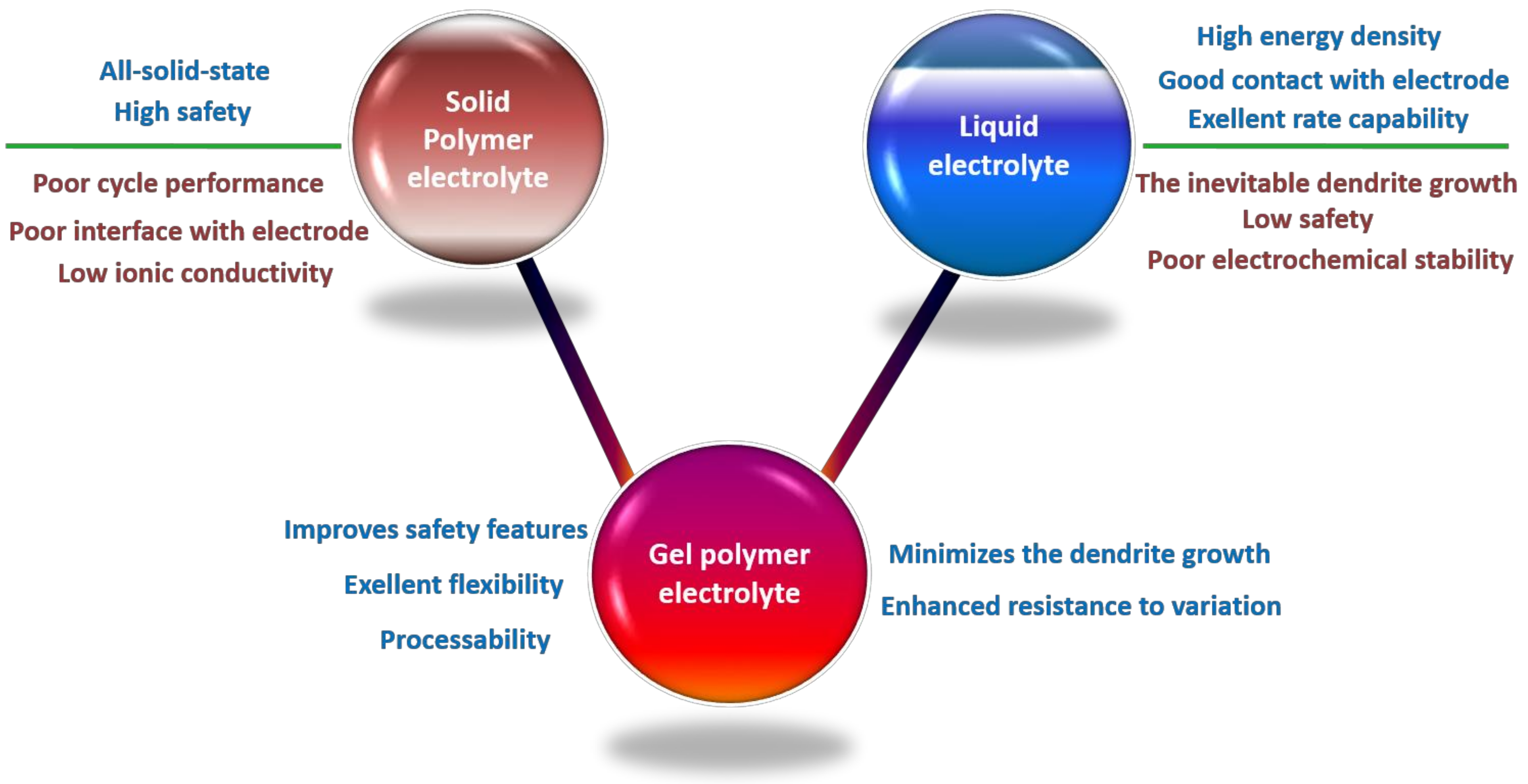
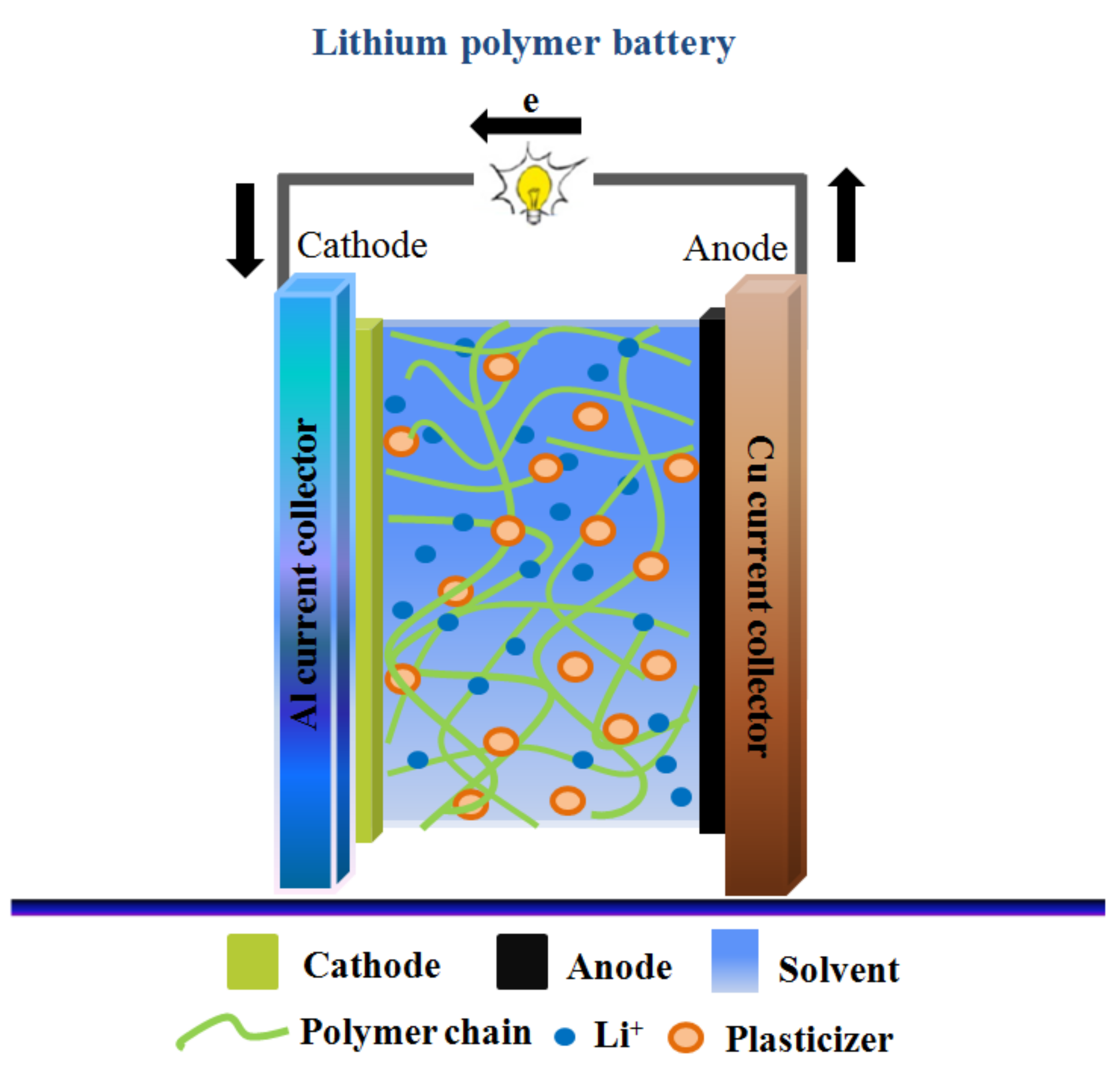
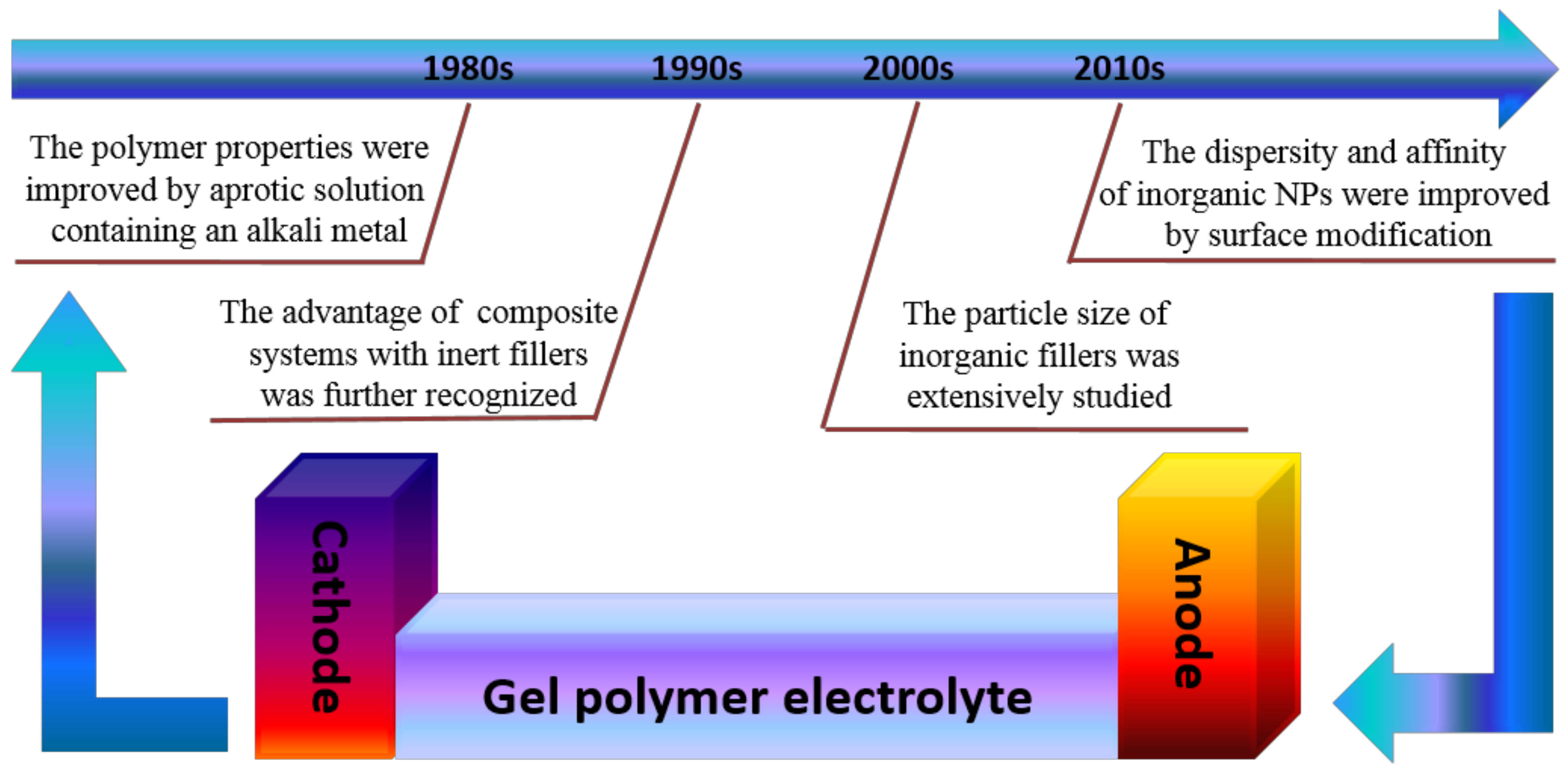
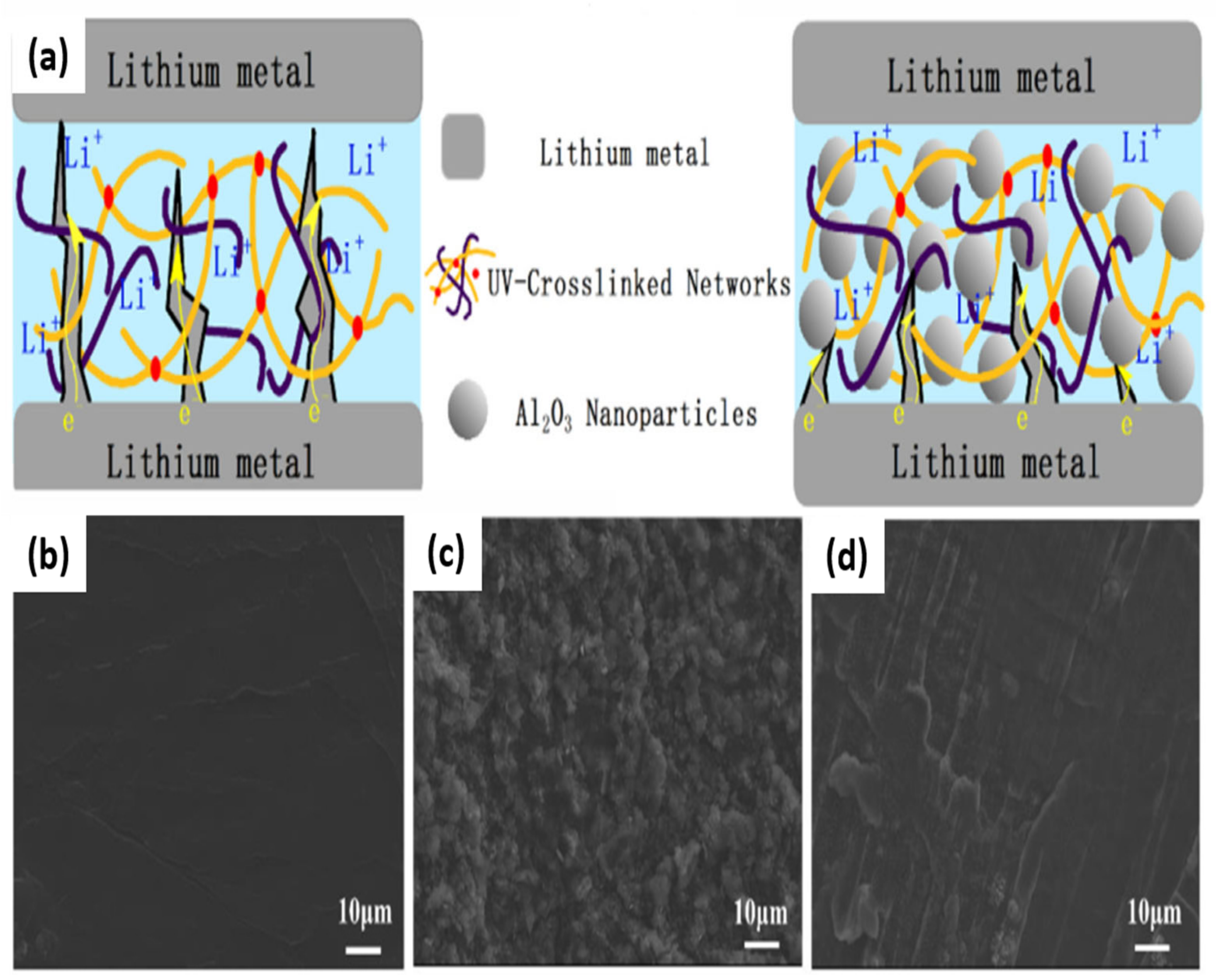
3.1. Titanium Dioxide (TiO2)
Chung et al. [
73] investigated the effect of TiO
2 nanoparticle (NP) addition in (PEO)-LiClO
4 for the improvement of electrochemical performance of GPEs. This study provided a model for the effects of inorganic fillers on the overall Li
+ ion transport in nanocomposite electrolytes. In addition, the specific role of inorganic filler was interpreted in terms of Lewis acid-base interactions. Over a wide temperature range, two structural modifications occurred at the ceramic surface. First, the morphology of the polymer was modified by the structural groups on the surface of the particles, which provided crosslinking opportunities for the PEO segments and X-anion. This resulted in a reduction in the energy barrier of the reorganized PEOs, where appropriate Li
+ ion-conducting pathways were established at the ceramic surface. Second, an “ion-ceramic complex” was formed through the dissociation of salt due to the interaction between polar groups on the surface of the fillers and ions of the electrolyte. These two structural variations can account for the improvement in the electrolytic conductance of inorganic fillers in GPEs. Liu et al. [
67] reported that GPEs with a TiO
2 ceramic filler exhibited higher Li
+ ion transfer numbers than GPEs without TiO
2. The interaction between the fillers, anions, and polymer chains enhanced Li
+ ion transfer. Kim et al. [
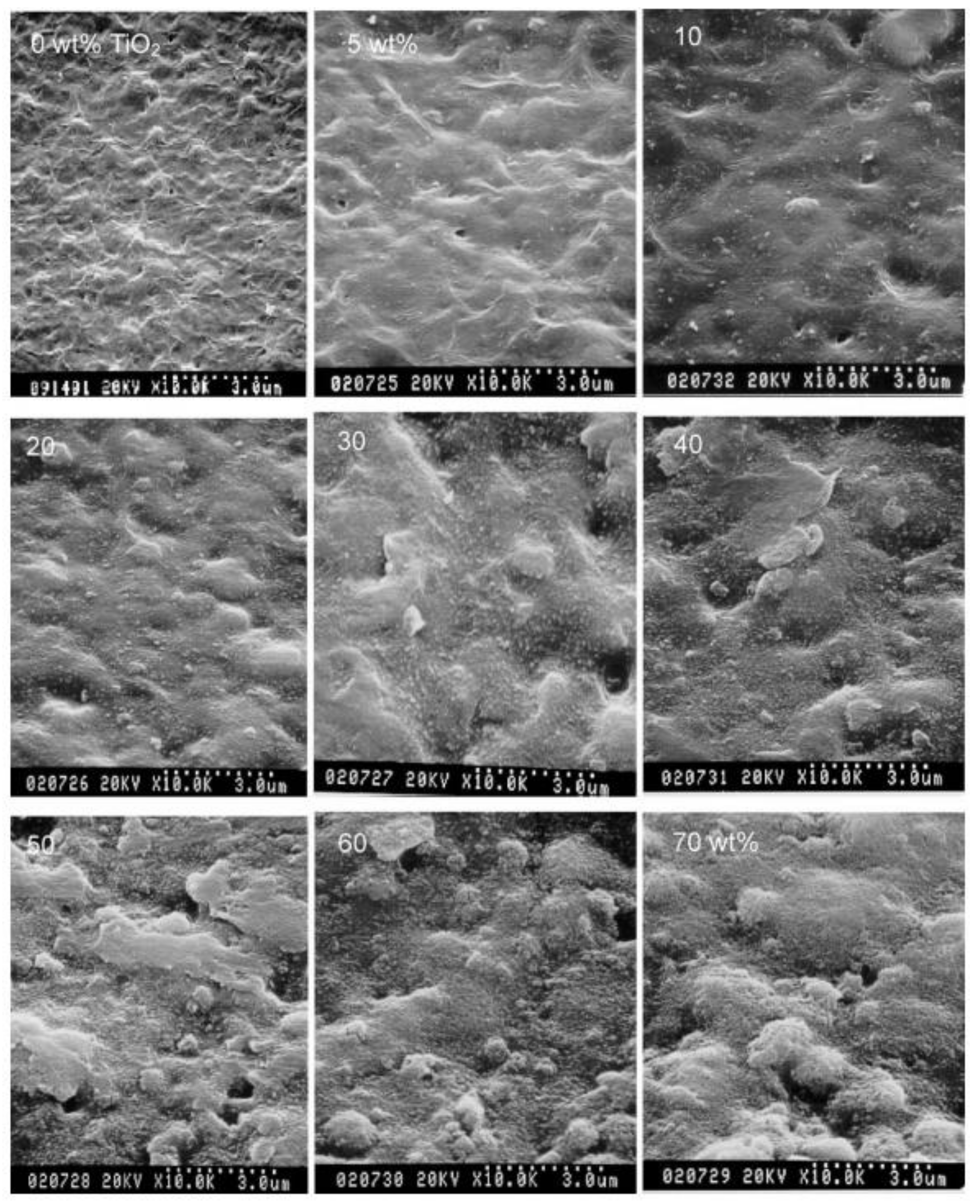
74] investigated the influence of filler content on the morphology of GPE membranes. As the TiO
2 content increased, the surface of the membrane coarsened, and small aggregates appeared; however, the TiO
2 NPs remained well distributed over the entire surface area of the membrane with an increase in the content to 60 wt% (
Figure 4). In addition, the ionic conductivity was enhanced owing to the nanopores in the liquid medium, as well as the effective ion transport achieved by the presence of TiO
2. Therefore, the addition of rutile TiO
2 NPs not only enhanced the dispersion of the constituents but also improved the physical and electrochemical properties of the GPE. Karlsson et al. [
75] studied polymer kinetics using quasi-elastic neutron scattering experiments to observe the effect of the filler on the crystallinity and structural changes in the polymer. The results showed that the improvement in ionic conductivity was because of the filler rather than the enhanced polymer dynamics based on the presence of a 5-nm-thick immobilized polymer layer around the filler particles. Kwak et al. [
76] prepared a viscous P(EO-EC)/LiCF
3SO
3/TiO
2 polymer electrolyte mixture with a porous P(VdF-HFP)/P(EO-EC)/TiO
2 membrane. The TiO
2 content used was 0.0, 0.5, 1.0, 1.5, 2.0, 5.0, and 10.0 wt% in the polymer electrolyte and 0.0, 10.0, 20.0, 30.0, and 40.0 wt% in the porous membrane with a blend composition of 6:4 P(VdF-HFP) to P(EO-EC). The stress and tensile modulus values showed an increase for the membrane up to 2.0 and 41.0 MPa, respectively, using 30 wt% TiO
2, and a decrease up to 1.2 and 34.5 MPa, respectively, when adding 40 wt% TiO
2. These results indicated that the presence of high concentrations of TiO
2 up to 20 wt% improved the mechanical properties of the membrane owing to the interaction between the NPs and the host polymer. In addition, an increase in ionic conductivity was observed up to 4.7 × 10
−2 mS cm
−1 at 25 °C for the GPEs containing 1.5 wt% TiO
2 owing to the interaction between the oxide groups of the polymer and hydroxyl groups of the TiO
2 filler.
Hwang et al. [
77] reported that the electrolytic conductance of GPEs was increased when reducing the particle size of the TiO
2. The ionic conductivity of a GPE containing TiO
2 NPs was higher than that of a GPE without TiO
2 at 30 °C. Agnihotry et al. [
78] examined the effects of different concentrations of nanosized TiO
2 in a PMMA-based GPE. This study demonstrated that the ionic conductivity was enhanced when using an optimum TiO
2 loading (2 wt%) in the GPE. Byung et al. [
79] synthesized a GPE by blending poly(acrylonitrile)-poly(ethylene glycol diacrylate) (PAN-PEGDA) with Li-salt and TiO
2 NPs. The high surface area of inorganic fillers can improve the interfacial resistance and ionic conductivity of Li metal owing to an increase in chemical stability and possible retention of organic solvents in the micropores; this assists in the regulation of possible side reactions associated with Li metal. In addition, nanosized inorganic fillers were evenly dispersed and increased the mechanical stability of the polymer matrix. Walkowial et al. [
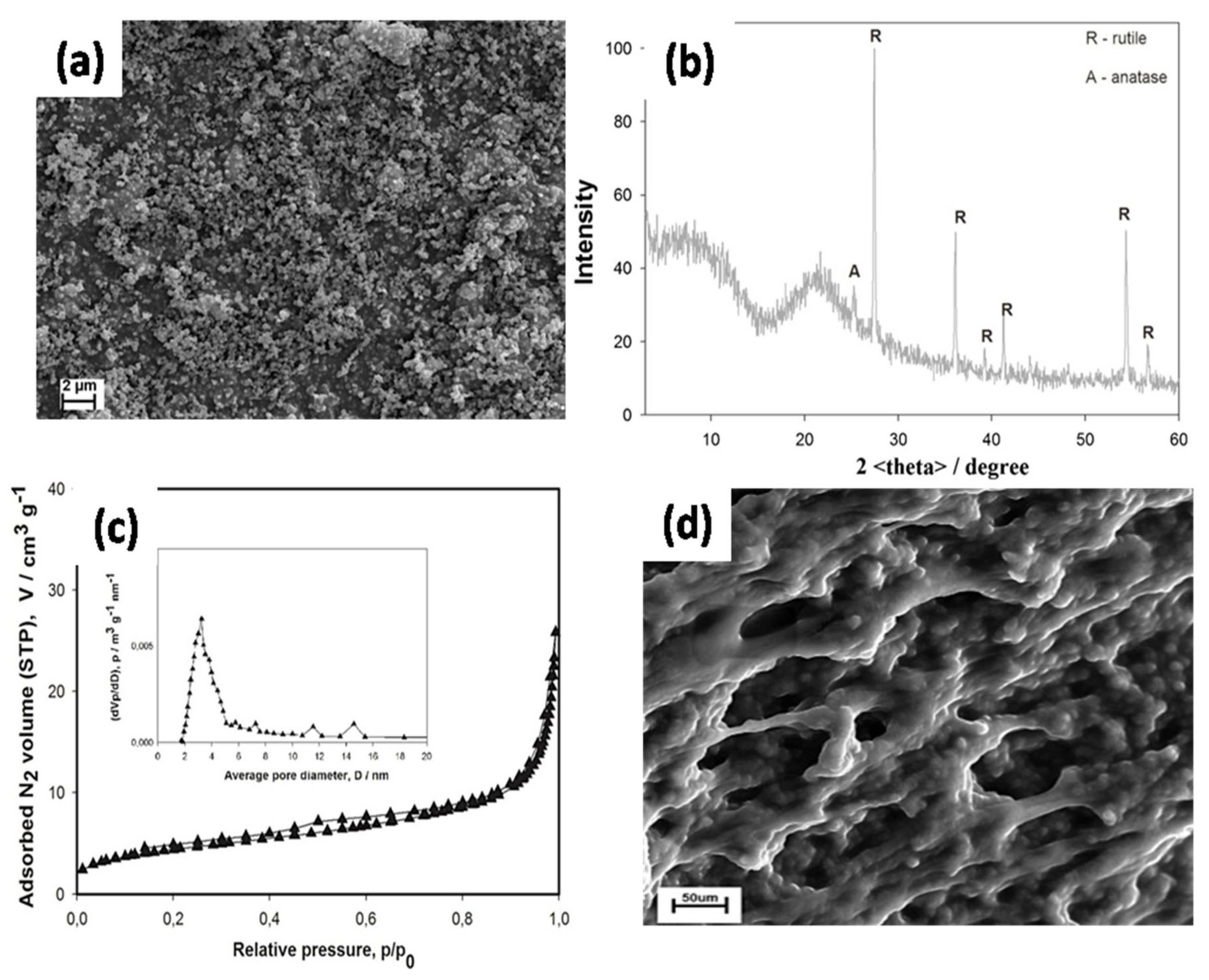
80] and Kurc et al. [
81,
82] modified the surface of a new hybrid TiO
2/SiO
2 filler for GPEs. The original hybrid fillers were modified by grafting functional groups, such as methacryloxy or vinyl groups, on the surface of the fillers. The surface modification chemistry of the filler seemed to be another factor affecting the overall performance of the GPE in terms of solvent absorption, specific conductivity, and intercalation of lithium on graphite. As shown in the SEM images (
Figure 5a), the hybrid TiO
2/SiO
2 spherical NPs are homogeneously dispersed.
Figure 5b shows that the hybrid TiO
2-SiO
2 predominantly contains rutile TiO
2.
Figure 5c indicates that the mean pore diameter of the TiO
2-SiO
2 hybrid is 3.8 nm, which represents the mesoporous components, and the surface area is 12.5 m
2/g, which suggests an intermediate surface activity level. Hybrid TiO
2/SiO
2 powder with a moderate degree of surface functionalization was considered as a potential candidate for GPE applications in LIBs. The SEM image of the GPE without functionalized fillers typically exhibits a porous structure (
Figure 5d).
An experimental investigation by Yahya et al. was performed on proton-conducting GPE nanocomposites based on TiO
2 NP-dispersed cellulose acetate (CA) [
83]. The increase in the ionic conductivity of the GPE nanocomposite could be explained by the addition of TiO
2, which increased the total solution/solvent dielectric constant. The dielectric constant of TiO
2 was higher than that of N,N-dimethylformamide (DMF), leading to a reduced Coulombic interaction between the ion aggregates and dissociation of ions from the salt resulting in free NH
4+ ions. The GPE prepared by adding NH
4BF
4 and TiO
2 to CA was considered a promising material for proton batteries.
Cao et al. [
84] demonstrated improvement in the GPE by incorporating TiO
2 in PVDF/PMMA via electrospinning for practical applications in LIBs. The GPE containing 3 wt% TiO
2 showed a highest electrolytic conductance of 3.9 × 10
−1 mS cm
−1 with an electrochemical stability up to 5.1 V vs. Li
+/Li at room temperature. The increase in electrolytic conductance with the addition of TiO
2 particles was due to better dispersion through a Lewis acid–base interaction between the polar groups of the electrolytes and the filler and a decrease in the crystallinity of the polymer. Hong Chen et al. [
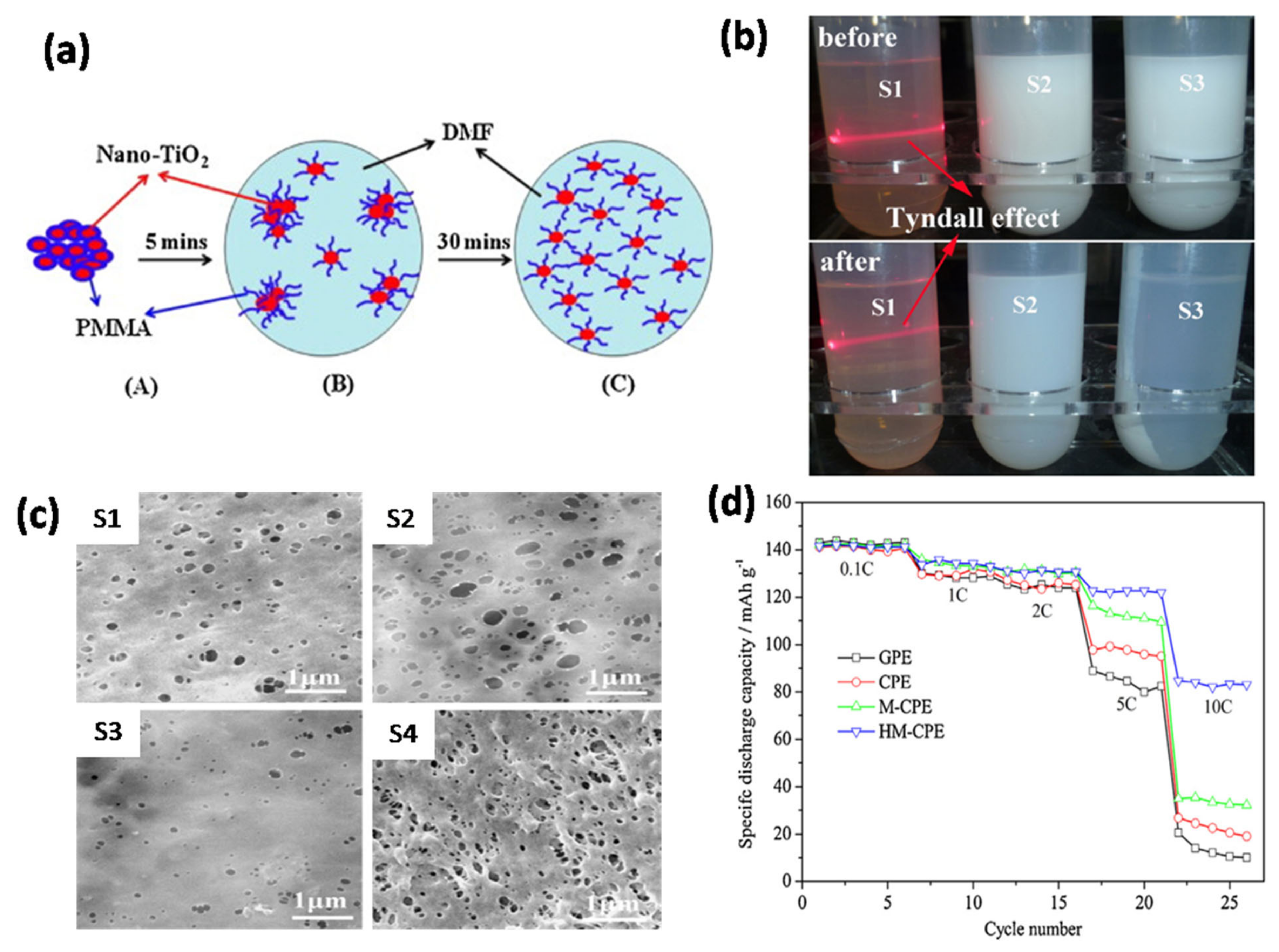
85] investigated the role of nano-TiO
2 dispersibility in a GPE based on a PVDF-HFP polymer for LIBs (schematic is shown in
Figure 6a).
Figure 6b shows the dispersion of the nanoparticles (pristine, commercial, and modified TiO
2) in DMF. The morphology of nano-TiO
2-PMMA doped PVDF-HFP membrane exhibits a smoother surface with fewer pores compared to the pristine PVDF-HFP membrane and nano-TiO
2 doped PVDF-HFP membrane. Interestingly, the highly dispersed TiO
2-PMMA hybrid membrane has a rougher surface, and the pore size is more uniform (
Figure 6c). The effect of TiO
2 dispersion on the C-rate discharge performance is shown in
Figure 6d. The addition of nanosized TiO
2 to the PVDF-HFP-based GPE significantly improved the discharge capacity of the cells. In addition, the highly dispersed nano-TiO
2-PMMA doped GPE showed the highest discharge capacity compared to the other electrolytes. This study demonstrated that the dispersion of nanosized TiO
2 is an important factor that influences the performance of the PVDF-HFP-based polymer electrolyte. Sankaranarayanan et al. [
86] explained the effect of TiO
2 on the electrochemical performance based on the distribution and aggregation of the fillers, Lewis acid–base interactions, and polymer segment-ion coupling. The addition of TiO
2 to the GPE nanocomposite promoted Lewis acid–base interactions, thereby facilitating the dissolution of LiClO
4 salts and increasing the amount of free Li
+ ions. Bozkurt et al. [
87] produced polymer electrolyte nanocomposites based on borate ester graft copolymer PVA, poly(ethylene glycol) methyl ether (PEGME)), nano-TiO
2, and trifluoromethane sulfonate (CF
3SO
3Li). The conduction pathway for ion transport was improved by the boron-containing PVA backbone and flexible side chains. This study suggested that the presence of inorganic fillers resulted in an increase in ionic conductivity, Li
+ ion transfer, interfacial stability between the electrode and electrolyte, and the mechanical strength of the GPEs. Chen et al. [
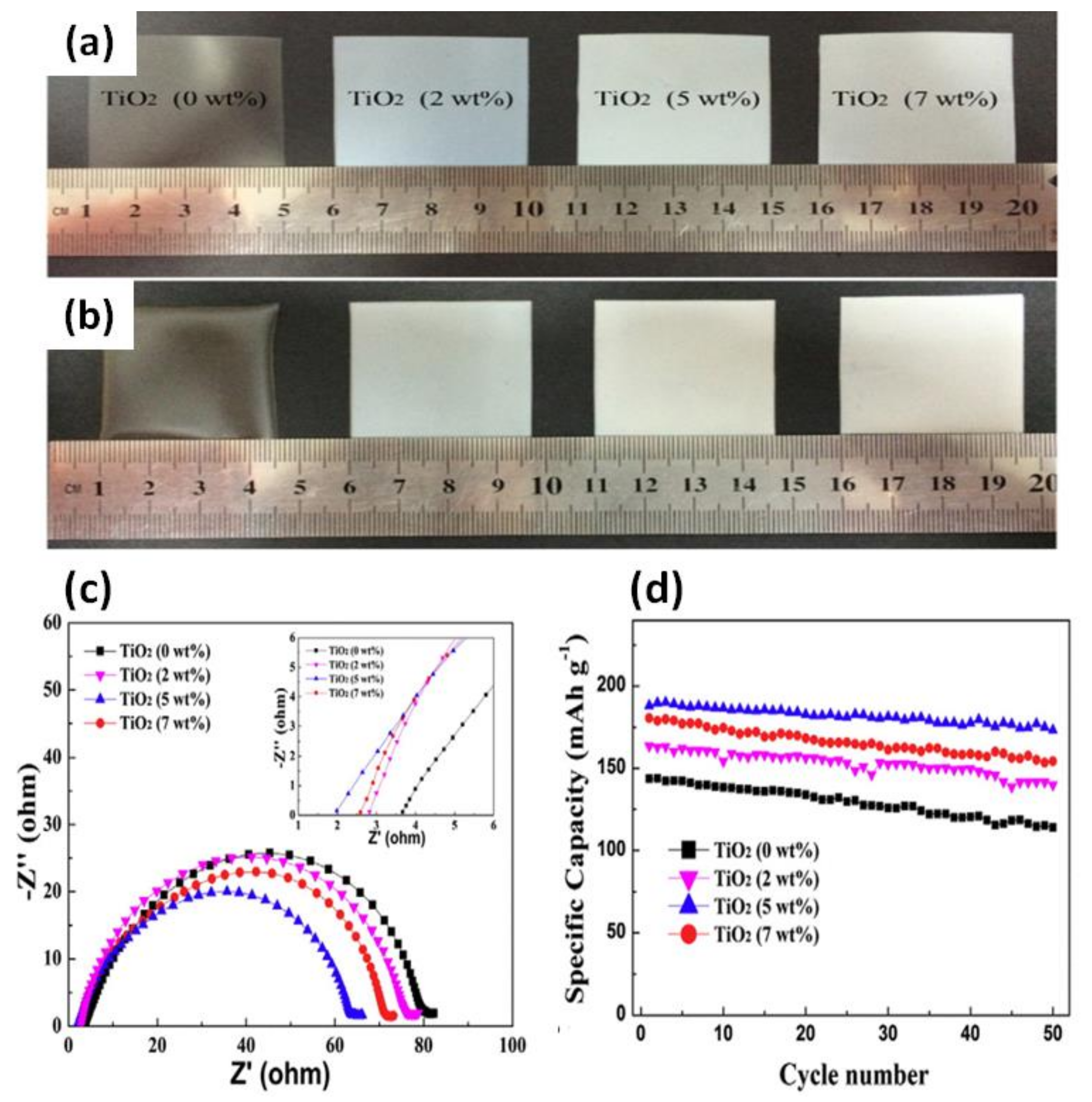
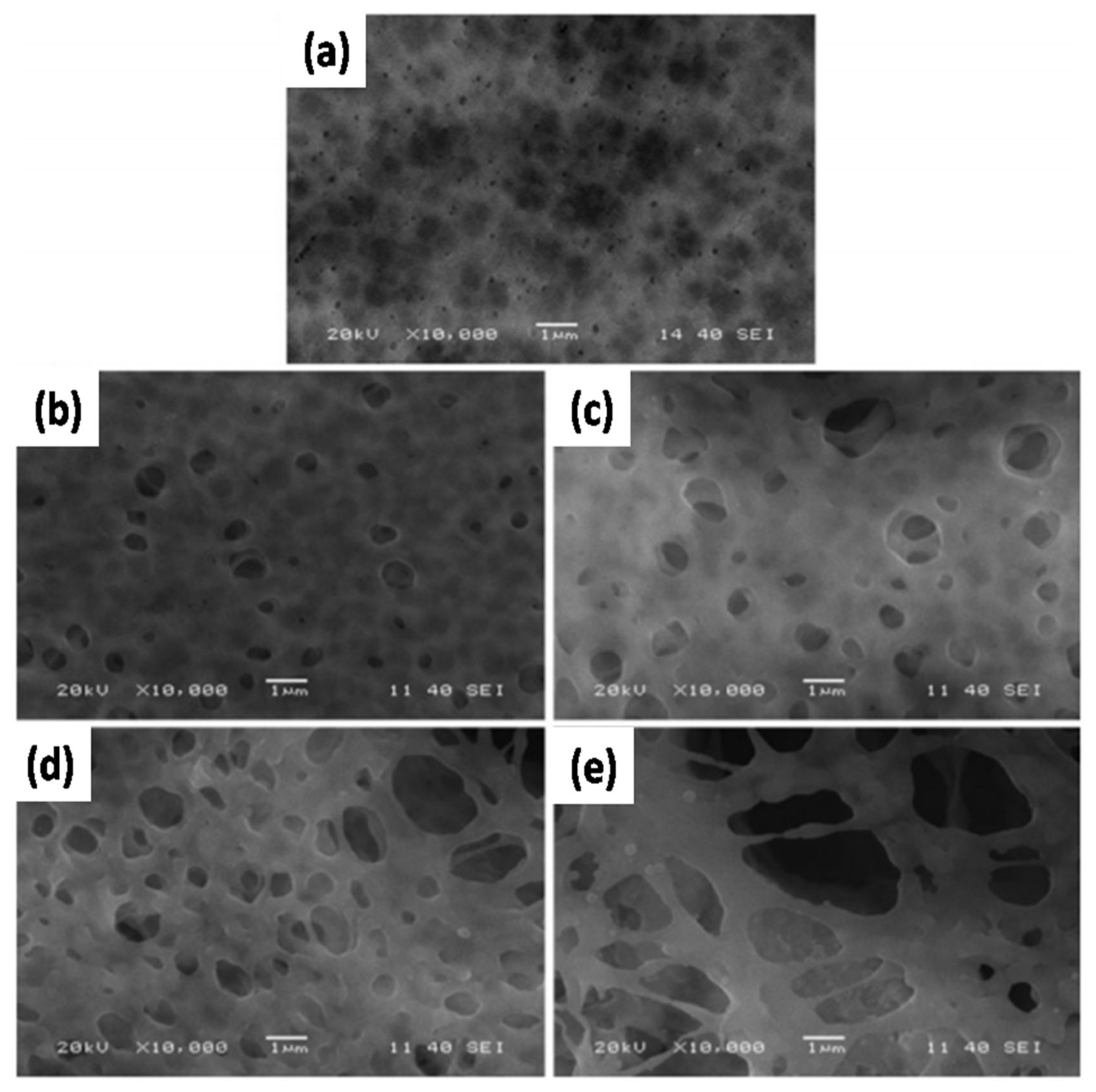
88] showed that after adding TiO
2 NPs, the PVDF-HFP/PMMA/TiO
2 membrane exhibited enhanced thermal stability and electrolytic conductance. Based on the images of the GPE membrane, its thermal stability was significantly improved after the addition of TiO
2 NPs (
Figure 7a,b). In particular, the GPE containing 5 wt% TiO
2 NPs showed homogeneously interconnected pores which resulted in an excellent performance (
Figure 7c,d). The EIS analysis of GPE containing varying concentrations of TiO
2, shown in
Figure 7c, shows that after the introduction of TiO
2, the bulk resistance (R
b) of the GPEs significantly decreases for the sample containing the lowest TiO
2 content of 5 wt%. The initial discharge capacities were 143.6, 180.5, 188.1, and 163.6 mAh g
−1 for the different TiO
2 contents (0, 2, 5, and 7 wt%), respectively. After 50 cycles, the capacity decreased to 114, 154.3, 173.2, and 139.9 mAh g
−1, with a capacity retention of 79.4%, 85.5%, 92.1%, and 85.5%, respectively (
Figure 7d). This study showed that the incorporation of TiO
2 NPs to the GPE improved its electrochemical stability and ionic conductivity in LIBs. Yamolenko et al. [
89] extended this study on the effect of NPs to a polyester-diacrylate (PEDAC)-based network polymer electrolyte. The mechanical strength of the GPE was enhanced after improving the distribution of the NPs in the polymer electrolyte by ultrasonic treatment compared to simple mechanical stirring.
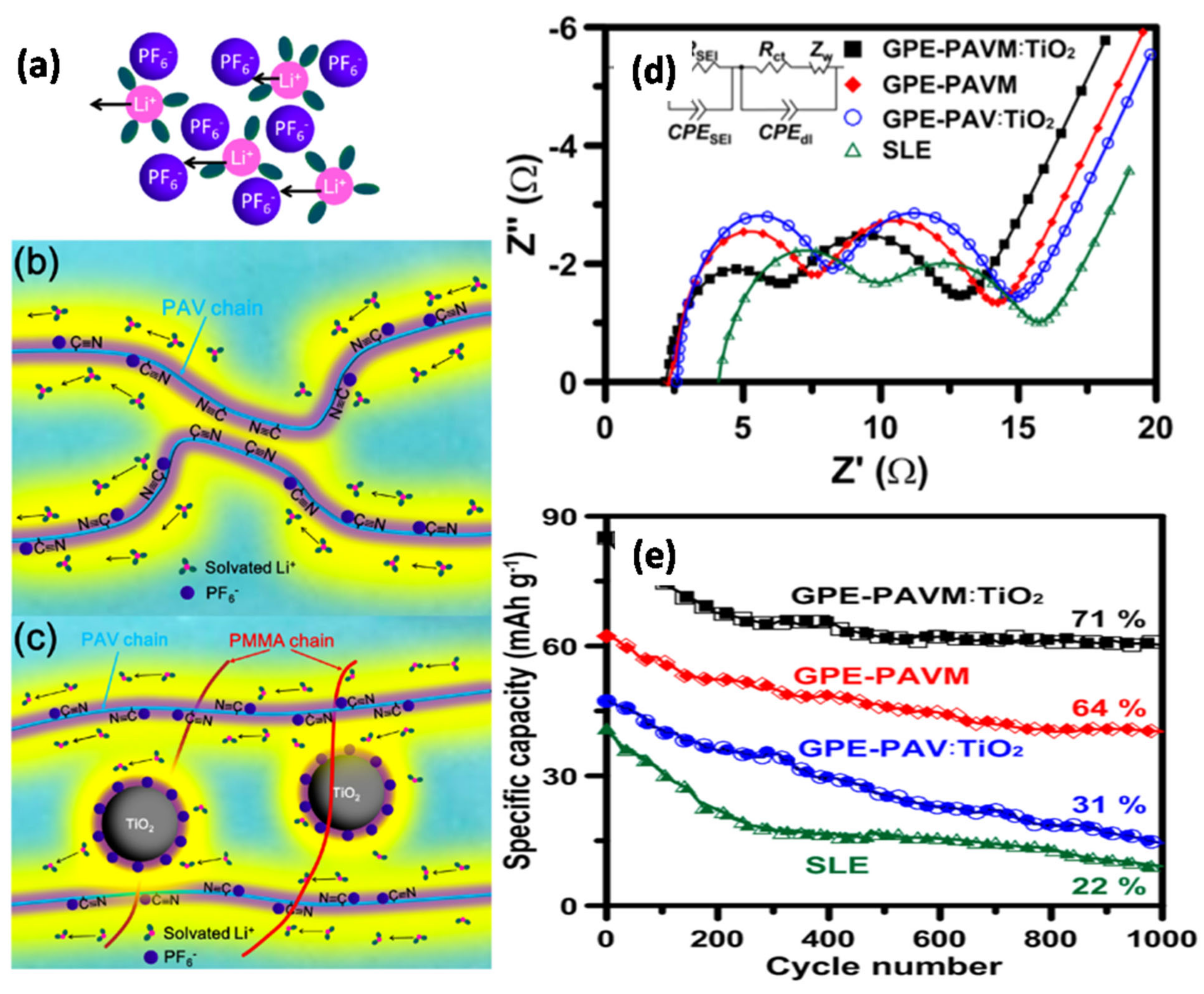
Teng et al. [
90] reported a GPE prepared by combining poly(acrylonitrile-co-vinyl acetate) (PAV) with PMMA and TiO
2 NPs, i.e., PAVM:TiO
2 (
Figure 8). This study introduced a new concept concerning the association of oxide NPs with the space-charge regimes around the polymer’s functional groups to induce 3D conduction pathways for Li
+ ions in GPEs applied in LIBs (
Figure 8a–c). Based on the impedance spectra shown in
Figure 8d, the high-frequency semicircle represents the movement of charge carriers through the SEI layer, middle-frequency semicircle shows charge-transfer resistance, and sloping line is related to the Warburg impedance. The results revealed the superiority of GPE-PAVM:TiO
2 in enhancing Li
+ ion transport.
Figure 8e presents the discharge capacities of the full-cell batteries at a high rate of 20 C. The cell with the GPE-PAVM:TiO
2 electrolyte delivered discharge capacities of 152 and 84 mAh g
−1 at 0.1 and 20 C, respectively, thus outperforming the cell with the SLE electrolyte with capacities of 146 and 40 mAh g
−1, respectively. The Li
+ ion transport efficiency in the bulk solution as well as at the electrode–electrolyte interface was enhanced through the 3D percolation pathway by immobilizing the PF
6− anions in the oxide NP framework. Sakunthala et al. performed a comparative study between single-crystalline TiO
2 nanorods and submicronsized TiO
2 fillers in PVDF-HFP/EC/LiClO
4. The Li
+ ion transfer and tensile strength of the membrane containing 5 wt% TiO
2 nanorods were higher than those of the membrane containing 5 wt% submicronsized TiO
2. This can be explained by the improved interaction of the rod-shaped morphology of the single-crystalline TiO
2 filler with the polymer/salt/plasticizer matrix in the GPE [
91].
Sivakumar et al. [
92] fabricated a GPE containing a PVC-PEMA blend using hydrothermally derived TiO
2 NPs as an inorganic filler. The influence of the filler NPs on the surface morphology, thermal stability, and electrochemical properties were studied. The addition of TiO
2 NPs reduced the crystallinity of the polymer and enhanced the Li
+ ion transport pathways. Similarly, Singh et al. [
93] modified the structural properties of PEMA-based plasticized polymer electrolytes by incorporating TiO
2 NPs. The addition of TiO
2 NPs in the plasticized polymer electrolyte suppressed the crystallinity and enhanced the amorphous nature. Hence, the addition of TiO
2 NPs could be a novel approach to enhance the electrolytic conductance in GPEs. The ionic conductivity and temperatures of some significant GPEs containing TiO
2 fillers are listed in
Table 1.
3.2. Aluminum Oxide (Al2O3)
Li et al. [
94] prepared a GPE by combining porous P(VDF-co-HFP) with alumina (Al
2O
3) NPs as the filler. An increase in the Al
2O
3 NP content reduced the level of crystallization in the polymer, thereby increasing the amorphous phase of the membrane. Piotrowska et al. [
95] described the effect of oxide fillers on the properties of GPEs with a PVDF-HFP polymer matrix. Modification of the PVDF-HFP membranes with Al
2O
3 NPs led to a decrease in the liquid phase uptake ability owing to the reduction of accessible pore spaces. Egshira et al. [
96] assessed the availability of the alumina filler in an imidazolium-based gel electrolyte. The Al
2O
3 filler enhanced Li
+ ion mobility by providing alternative pathways for Li
+ ion movement and changing the interaction between the Li
+ ions and the EO chain. Rai et al. [
97] prepared nano-Al
2O
3-filled PVA composite gel electrolytes. As shown in
Figure 9a, the PVA membrane exhibits a porous structure, while the addition of 2 wt% Al
2O
3 NPs reduces the porosity of the PVA composite electrolyte as the Al
2O
3 NPs are trapped among the chains in the pores (
Figure 9b). Upon the addition of 6 wt% Al
2O
3 NPs (
Figure 9c), the PVA chains are fully covered with Al
2O
3 NPs. This indicates complete dispersion of the Al
2O
3 nanofiller in the electrolyte film. Upon further addition of Al
2O
3 NPs (10 wt%), the grain sizes and shapes become irregular resulting in a partially crystalline structure containing Al
2O
3 NPs and PVA electrolyte (
Figure 9d). An increase in the Al
2O
3 NP content increased the amorphous phase of pristine PVA. The Li
+ ion transfer capacity of the GPE reached its maximum with the addition of 6 wt% Al
2O
3 NPs.
A novel GPE was prepared by combining a poly(methyl methacrylate-acrylonitrile-ethyl acrylate) (P(MMA-AN-EA)-based polymer electrolyte with nano-SiO
2 and Al
2O
3 as inorganic fillers [
98]. The maximum electrolytic conductance of the GPE was achieved when 5 wt% nano-SiO
2 and nano-Al
2O
3 were used. This study showed the different roles of the inorganic fillers: SiO
2 contributed to the enhanced ion conduction due to strong Lewis acid–base interactions, whereas Al
2O
3 improved the structural and thermal stability of the GPE owing to its high stiffness. Wen et al. [
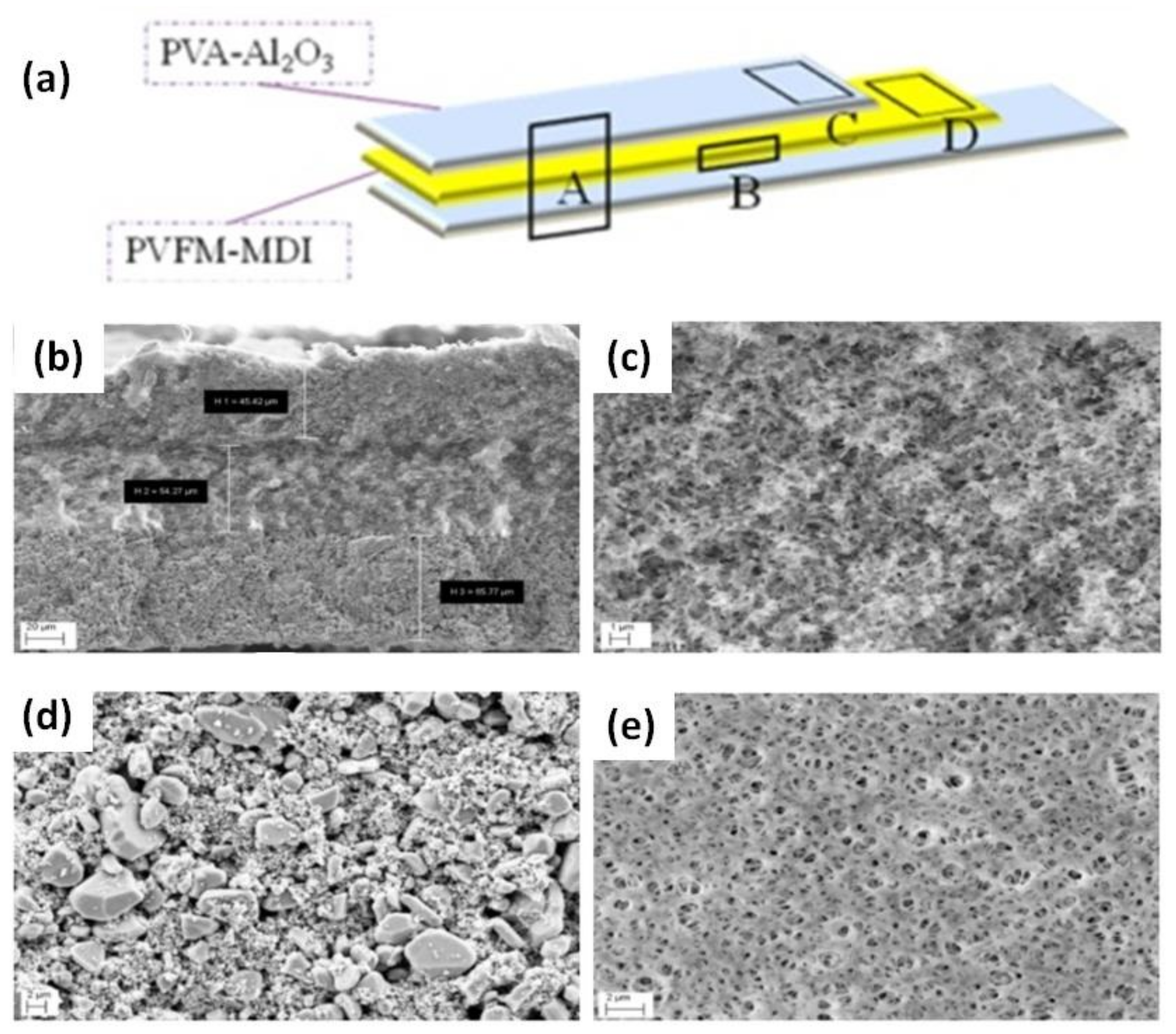
99] synthesized a novel GPE with a trilayer structure consisting of polyvinyl formal (PVFM)-4,4-diphenyl-methane diisocyanate (MDI) covered by a PVA-Al
2O
3 solution on both sides to achieve synergistic effects for each layer (
Figure 10a). The morphology of the Al
2O
3/PVFM/Al
2O
3 trilayer membrane was determined by FESEM (
Figure 10b–e). The thicknesses of the layers were 45.42, 54.27, and 65.77 µm, respectively (
Figure 10b). The morphology of the PVFM membrane exhibits sponge-like pores, which are expected to enhance the transport of Li
+ ions (
Figure 10c). The porous structure of the inorganic particulate films did in fact increase Li
+ ion transfer (
Figure 10d). The surface morphology of the PVFM membrane shows evenly distributed pores, as shown in
Figure 10e. This study indicated that the inorganic layers enhanced the thermal integrity and mechanical properties of the membrane. This multilayer polymer membrane could be a potential material for application in LIBs.
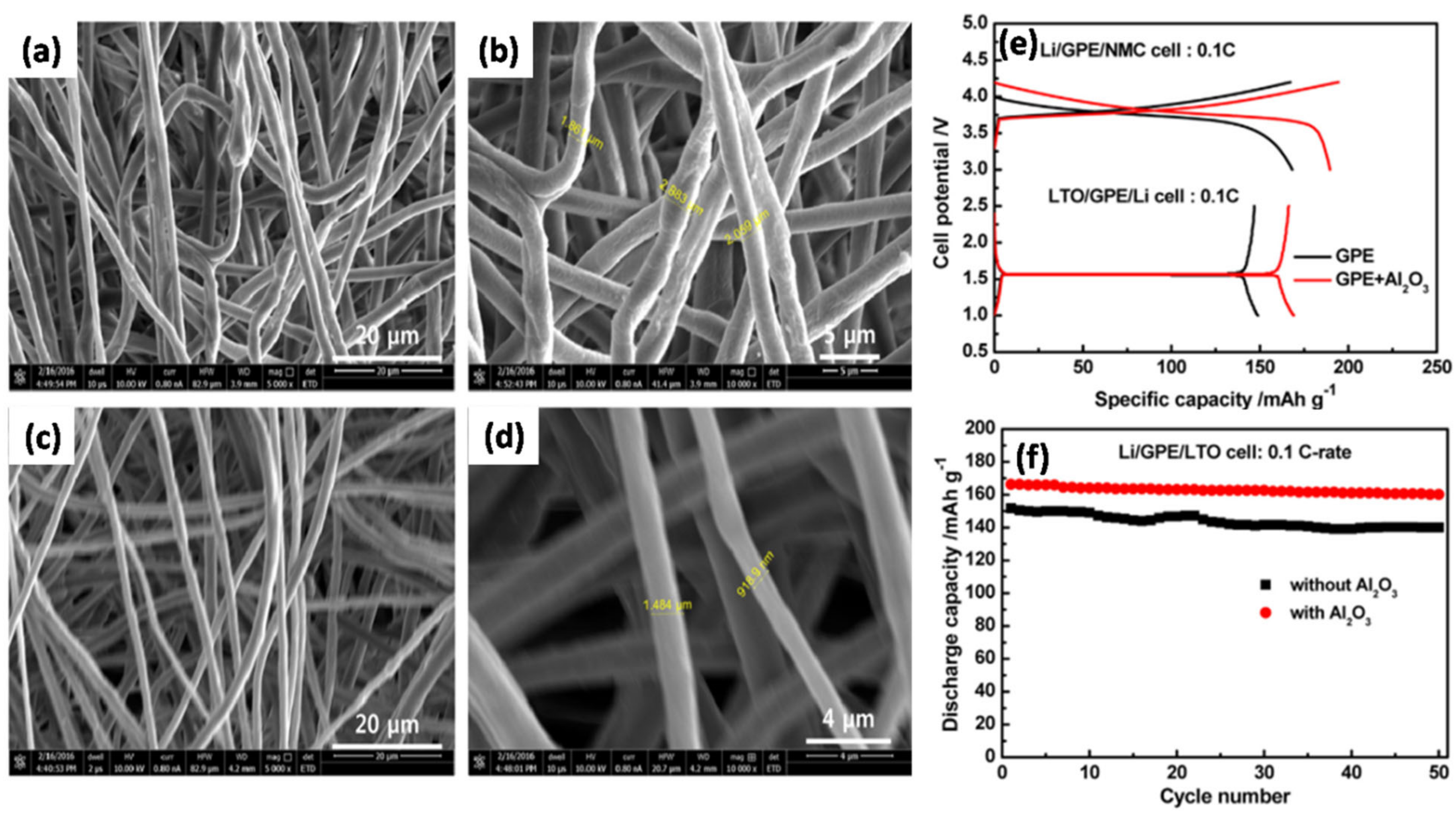
Kim et al. [
100] prepared a GPE containing a PVDF-HFP fibrous matrix with Al
2O
3 as the inorganic filler by electrospinning. The morphologies of polymer fibrous matrices exhibiting different diameters are shown in
Figure 11a–d. The surface morphology of the pure polymer is rougher than that of the Al
2O
3-composite polymers and exhibits an average diameter of 2.3 µm (
Figure 11a,b) compared to 1.2 µm for the Al
2O
3-composite polymer matrix (
Figure 11c,d). The presence of inorganic fillers in the fibrous polymer matrices prevented polymer shrinkage and agglomeration, which was beneficial for the formation of homogeneous pores.
Figure 11e shows that the discharge capacities of the NMC and LTO half cells of the GPE-Al
2O
3 composite are 189.6 and 166.3 mAh g
−1, respectively, which are higher than those of the GPE without Al
2O
3 (168.2 and 146.8 mAh g
−1, respectively). The presence of Al
2O
3 NPs increased the porosity and absorption of free ions, thereby enhancing the electrolytic conductance and electrochemical stability compared to a pure GPE. The Al
2O
3-composite GPE also showed a better discharge capacity retention of ~96% (initial and final specific capacities of 166.3 and 160.2 mAh g
−1, respectively) (
Figure 11f). Jain et al. [
101] presented efficient conduction pathways constructed in PVdF-based GPEs with Al
2O
3 and boron nitride (BN) ceramic nano/microparticles. The high dielectric constants of Al
2O
3 and BN facilitated anion capture in the GPE and the transfer of Li
+ without coordination to the anions.
Delgado-Rosero et al. [
102] synthesized a GPE containing PEO and sodium trifluoroacetate (CF
3COONa) with different contents of Al
2O
3. The addition of inorganic fillers increased the amorphous phase portion surrounding the filler of the (PEO)
10CF
3COONa + x wt% Al
2O
3 composite, thereby improving Na
+ ion transport through the pathways of the amorphous phase. Maragani et al. [
103] reported a GPE containing a combination of PAN and sodium fluoride (NaF) with Al
2O
3 nanofibers formed through a solution casting technique. With an increase in the Al
2O
3 nanofiber content, the amorphous phase of the GPE increased, resulting in an improvement in ion conduction. Yang et al. [
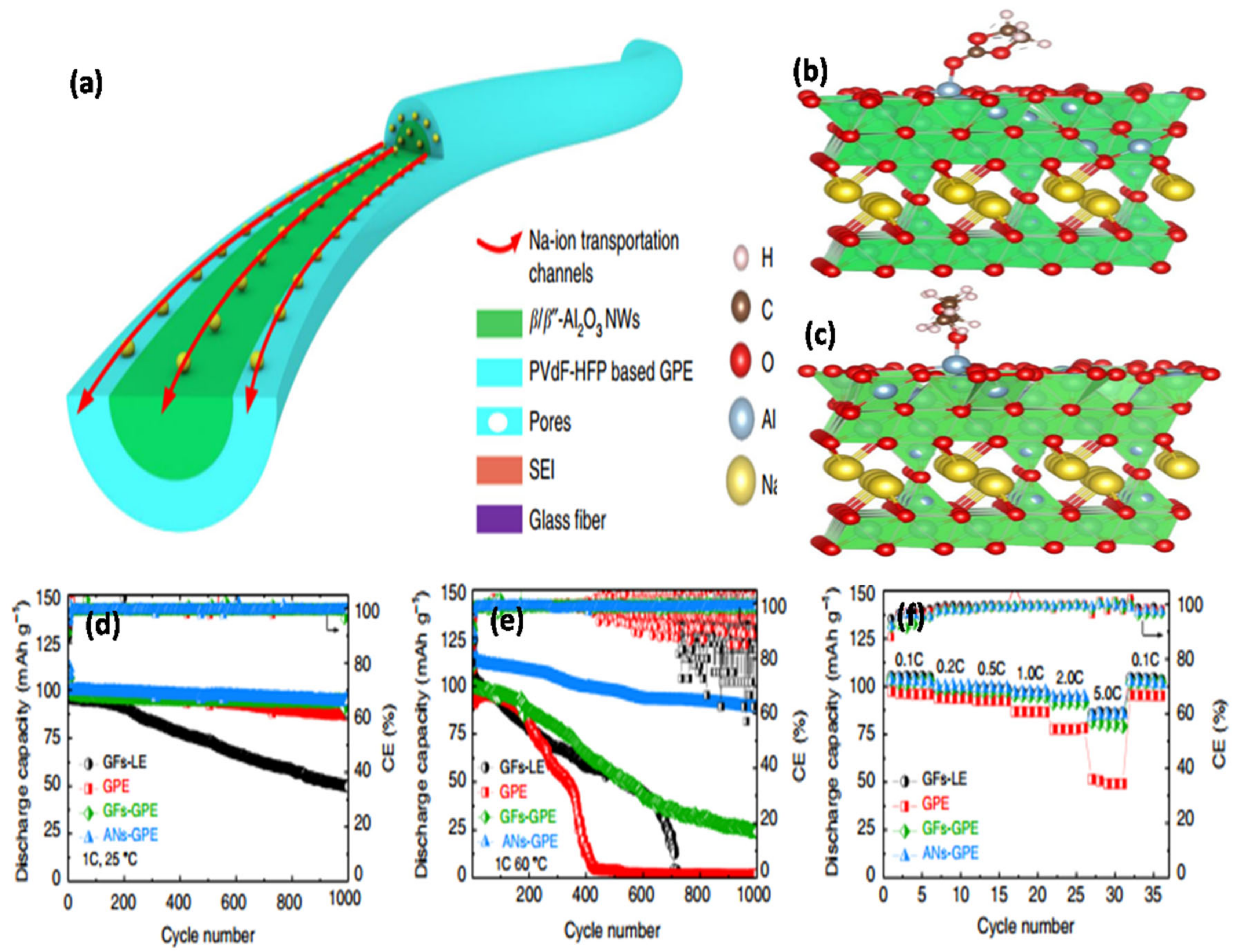
104] designed a novel GPE with uniformly cross-linked β-Al
2O
3 nanowires that compactly covered a P(VDF-co-HFP)-GPE through strong molecular interactions (
Figure 12a–c). In this innovative structure, the LEs were immobilized through bonding between the cross-linked Al
2O
3 nanowires and PVDF-HFP (ANs-GPE), thereby creating uniform and continuous Na
+ ion transport channels along the Al
2O
3 nanowires (
Figure 12a–c). This innovative structure can significantly improve the density and homogeneity of the Na
+ ion transport channel, resulting in superior electrochemical performance (
Figure 12d–f). Mishra et al. [
105] studied the effect of Al
2O
3 NP dispersion on a PVdF-HFP/PMMA blend-based nanocomposite GPE system. The electrolytic conductance changed significantly depending on the Al
2O
3 concentration in the PVdF-HFP/PMMA membrane. The maximum electrolytic conductance achieved was ~1.5 × 10
0 mS cm
−1 when 6 wt% Al
2O
3 NPs were added to the GPE. The ionic conductivities and temperatures of the GPEs containing Al
2O
3 fillers are summarized in
Table 2.
3.3. Silicon Dioxide (SiO2)
Wieczorek et al. [
106] designed a novel model GPE using a combination of amorphous poly(ethylene oxide) dimethyl ether (PEODME) and LiClO
4 with fumed nano-silica. The presence of nanosized fumed silica in the GPE was promising because of the reduction in ion association. Wu et al. [
107] prepared a hybrid polymer electrolyte film consisting of PMMA, LiClO
4, propylene carbonate (PC), and SiO
2 filler using a solvent casting technique. The conductivity was not positively correlated with the increased concentration of SiO
2 owing to the aggregation of SiO
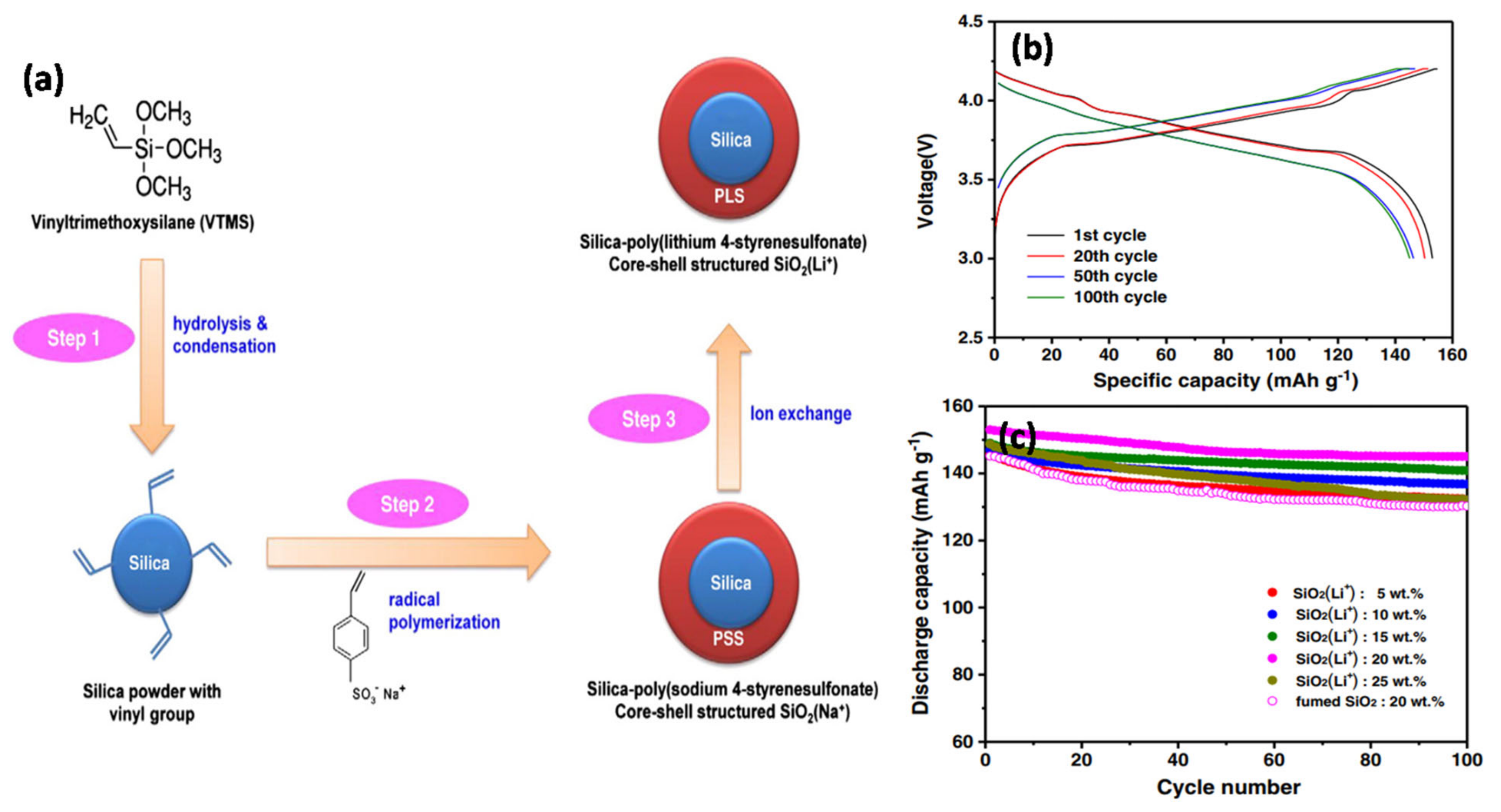
2, which led to the formation of crystal-like particles on the surface of the membrane. Kim et al. [
108] reported novel homogeneous spherical core-shell structured SiO
2(Li
+) NP fillers that were applied as functional fillers in GPEs (
Figure 13). SiO
2(Li
+) was synthesized by dispersing Li
+ ions in the core-shell structure of the SiO
2 particles (
Figure 13a).
Figure 13b shows the charge–discharge curves of the GPE containing 20 wt% SiO
2(Li
+). The first discharge capacity with LiCoO
2 in the cathode was 153 mAh g
−1. The GPEs containing the novel filler exhibited unique Li
+ ion transport and mechanical strength. Consequently, the battery exhibited low internal resistance, high capacity, and a stable cyclic performance. In addition, the capacity retention was enhanced by increasing the SiO
2(Li
+) content in the GPEs up to 20 wt% (
Figure 13c). The addition of SiO
2(Li
+) particles resulted in the retention of more LEs in the GPE, thereby improving the electrochemical performance during cycling.
Li et al. [
109] obtained similar results and proposed that the addition of SiO
2(Li
+) increases the amorphous phase and porosity of the polymer film, thus improving the adsorption and gelation of the LE. From the surface morphology of the membranes, as shown in
Figure 14, it was established that as the content of SiO
2(Li
+) increases, the pore size of the film also increases, resulting in a high uptake of liquid electrolyte. The electrolytic conductance of the GPE was improved because of the large number of Li
+ ions in SiO
2(Li
+). However, the GPE became very fragile when the SiO
2(Li
+) content reached 10 wt%. Manisankar et al. [
110] synthesized superhydrophobic PVDF-SiO
2 films with different SiO
2 contents by electrospinning. When the SiO
2 content increased, the surface roughness of the membrane also increased, but the average diameter of the nanofibers was not affected. Kim et al. [
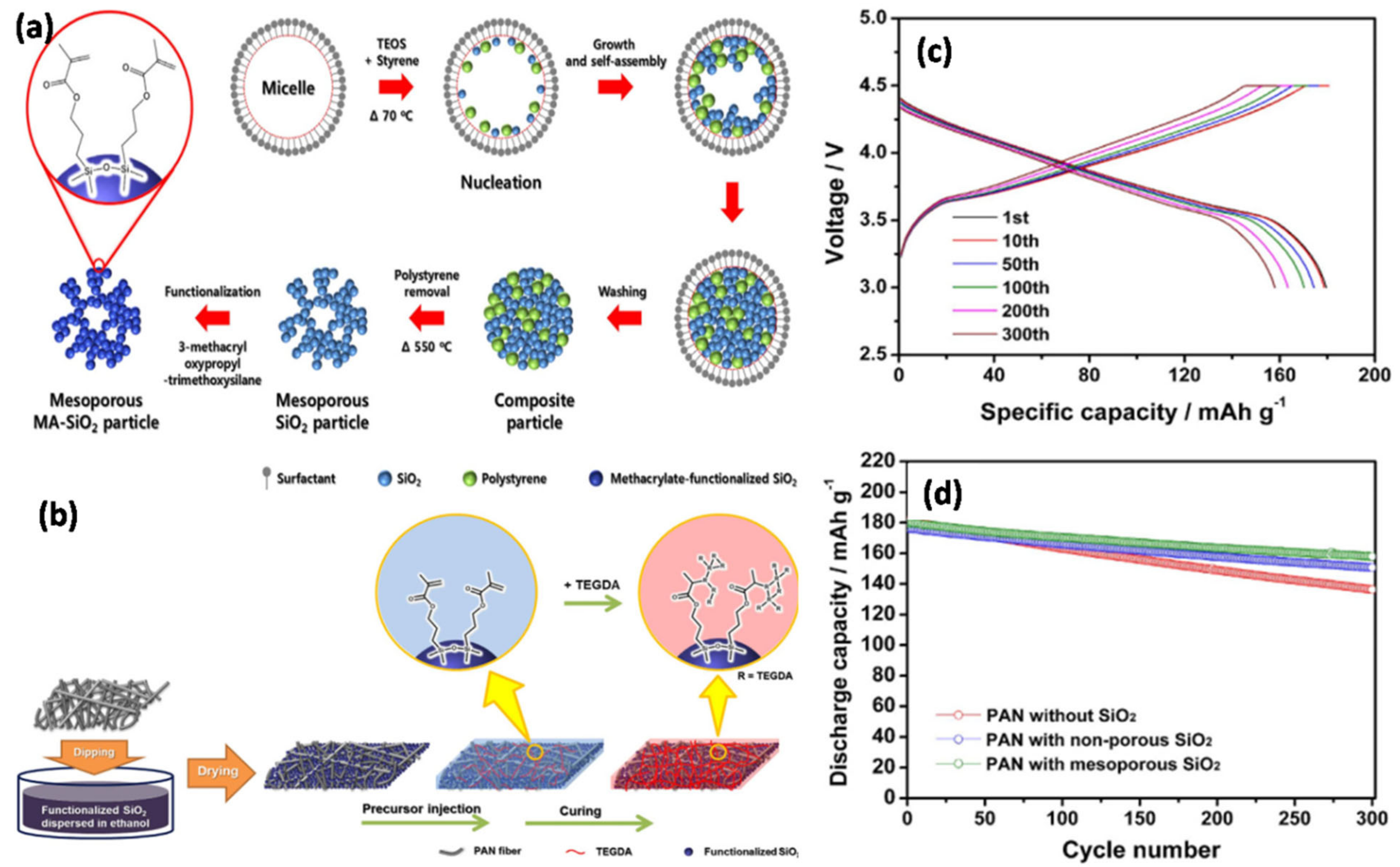
111] synthesized a secure and flexible electrolyte through the combination of mesoporous SiO
2 NPs containing methacrylate groups and fibrous PAN membrane (
Figure 15a,b). The initial discharge capacity delivered was 157.9 mAh g
−1 after 300 cycles with a capacity retention of 88.0%. In addition, the GPE containing the mesoporous SiO
2 particles exhibits better Li
+ ion transfer than the GPE containing non-porous SiO
2 particles (
Figure 15c,d). This study emphasized the role of SiO
2 mesoporous NPs compared with non-porous SiO
2 NPs when attempting to achieve good electrochemical properties in terms of discharge capacity, capacity retention, rate capability, and cycling stability. GPEs containing a combination of PEO/LiClO
4 complex and 1,3 dioxolane (DIOX)/tetraethyleneglycol dimethylether (TEGDME) as plasticizer with a SiO
2 filler has also been synthesized [
112]. An increase in SiO
2 filler content and plasticizer reduced the degree of crystallization of the polymer membrane, thus increasing the ionic conductivity.
Wu et al. [
22] studied the influence of SiO
2 NP content on PVdF-HFP/IL membranes in terms of their ion conduction and discharge capacity in LIBs. The crystallization phase of the membrane was reduced due to the dispersion of SiO
2 NPs, which hindered the structural stability of the polymer but improved ion transport because of their interaction with the amorphous phase of the host polymer. The SiO
2 NPs acted as multifunctional inorganic fillers with good interfacial stability, which increased the ion conduction and Li
+ ion transfer number for the poly(propylene carbonate)-based GPE in Li-S batteries [
113].
Hu et al. [
114] studied a GPE containing dispersed SiO
2 NPs in a PEO matrix. Uniformly dispersed SiO
2 NPs were obtained in the polymer matrix owing to the high miscibility of all the precursors. The synthesized GPE nanocomposite membrane significantly improved the electrochemical performance, which suggests a promising strategy for the development of safer and more flexible Li-metal batteries. The ionic conductivities and temperatures of the significant GPEs containing SiO
2 fillers are listed in
Table 3.
3.4. Zirconium Dioxide (ZrO2)
Vickraman et al. [
115] studied a novel GPE containing lithium bis(oxalato)borate (LiBOB) as the Li salt and PVdF-PVC as the polymer matrix with varying contents of ZrO
2. The high ionic mobility of the GPE is related to the large amorphous phase of the polymer host and its large free volumes that enhanced Li
+ ion transfer. Suthanthiraraj et al. [
116] investigated the effect of ZrO
2 NPs in a new PPG-silver triflate (AgCF
3SO
3) system in terms of the improvement to ion transport and electrochemical behavior. This study showed that structural modification of the polymer matrix by the addition of ZrO
2 NPs increased the ionic mobility and physicochemical properties of the GPE. Sivakumar et al. [
117] reported the effect of the different concentrations of dispersed ZrO
2 from the perspective of its enhanced ionic conductivity. The ionic conductivity increased upon the introduction of ZrO
2 in the bare gel polymer system up to a loading of 6 wt%. However, a further increase in the ZrO
2 content reduced the conductivity because of the larger crystalline region present in the matrix, which hindered the ionic mobility. Similar results were observed by Sivakumar et al. [
118] demonstrating that the introduction of ZrO
2 into a PVDF-HFP-(PC+DEC)-LiClO
4 system significantly improved the electrolytic conductance of the GPE. The addition of ZrO
2 NPs restricted the reorganization of the polymer chain structure, thereby increasing the amorphous phase of the polymer and improving the electrolytic conductance. Chen et al. [
119] synthesized a novel GPE by in situ immobilization of ionic liquids (ILs) and nanoporous ZrO
2 in a polymer matrix. This study showed that the ZrO
2 skeleton cooperates with Li salts, resulting in improved dissociation of the Li salts and Li
+ ion transfer. Therefore, a discharge capacity of 135.9 mAh g
−1 was obtained after 200 cycles at 30 °C. In addition, the cell operated well in the temperature range of −10 to 90 °C. The good contact and stable interface between the Li-metal electrode and GPE can be attributed to its effective electrochemical performance in Li-metal batteries.
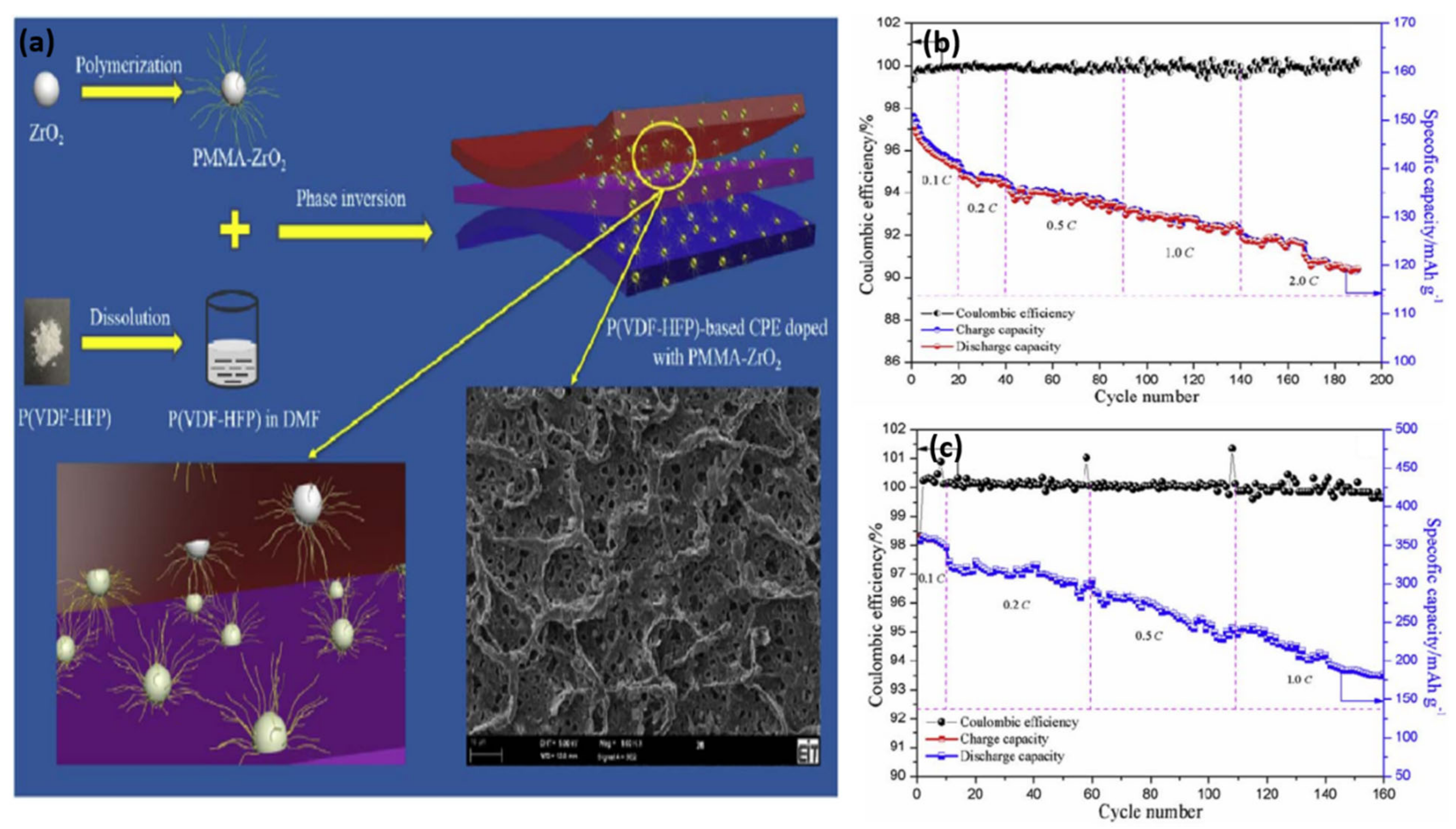
Xiao et al. [
120] synthesized a novel GPE by combining synthesized PMMA-ZrO
2 (sPZ) hybrid particles with a P(VDF-HFP) polymer matrix. The morphology of the GPE membrane containing homogeneously interconnected micropores is shown in
Figure 16a. As shown in
Figure 16b, the cell delivers 126.4 mAh g
−1 at 2.0 C after 150 cycles, corresponding to a capacity retention of 85.2% at 0.1 C. The cell containing a graphite electrode delivers 288.5 mAh g
−1 at 0.5 C after 80 cycles (
Figure 16c). The modified ZrO
2 significantly enhanced the properties of the GPE in terms of mechanical strength, ion conduction, and thermal stability.
Khoon et al. [
121] obtained similar results and proposed that the ZrO
2-based GPE has the potential to be applied in lithium polymer batteries owing to the improved Li
+ ion transfer. The incorporation of ZrO
2 NPs into the polymer-salt system resulted in a higher electrolytic conductance than that for the GPE without ZrO
2 NPs. Prasanna et al. [
122] prepared a nanocomposite GPE comprising a solution of zinc trifluoromethanesulfonate in a 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide IL entrapped in a PVC/PEMA blend and dispersed ZrO
2 nanofillers via a solution casting method. The GPE film showed the highest ionic conductivity of 3.63 × 10
−1 mS cm
−1 at room temperature when 3 wt% ZrO
2 nanofiller was added. A list of significant GPEs containing ZrO
2 fillers is provided in
Table 4 with their ionic conductivities and temperatures.